Structural Identification of Binary Tetrahydrofuran + O2 and 3-Hydroxytetrahydrofuran + O2 Clathrate Hydrates by Rietveld Analysis with Direct Space Method
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeffrey, G.A. Inclusion Compounds; Atwood, J.L., Davies, J.E.D., MacNicol, D.D., Eds.; Academic Press: London, UK, 1984; Volume 1, pp. 135–190. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wang, W.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane Storage in Dry Water Gas Hydrates. J. Am. Chem. Soc. 2008, 130, 11608–11609. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.P.; Lee, H. Recovery of CO2 from Flue Gas Using Gas Hydrate: Thermodynamic Verification through Phase Equilibrium Measurements. Environ. Sci. Technol. 2000, 34, 4397–4400. [Google Scholar] [CrossRef]
- Stern, L.A.; Circone, S.; Kirby, S.H.; Durham, W.B. Temperature, Pressure, and Compositional Effects on Anomalous or “Self” Preservation of Gas Hydrates. Can. J. Phys. 2003, 81, 271–283. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Pre-Combustion Capture of Carbon Dioxide in a Fixed Bed Reactor Using the Clathrate Hydrate Process. Energy 2013, 50, 364–373. [Google Scholar] [CrossRef]
- Koh, C.A.; Sum, A.K.; Sloan, E.D. State of the Art: Natural Gas Hydrates as a Natural Resource. J. Nat. Gas Sci. Eng. 2012, 8, 132–138. [Google Scholar] [CrossRef]
- Ohgaki, K.; Takano, K.; Sangawa, H.; Matsubara, T.; Nakano, S. Methane Exploitation by Carbon Dioxide from Gas Hydrates Phase Equilibria for CO2-CH4 Mixed Hydrate System. J. Chem. Eng. Jpn. 1996, 29, 478–483. [Google Scholar] [CrossRef]
- Lee, H.; Seo, Y.; Seo, Y.T.; Moudrakovski, I.L.; Ripmeester, J.A. Recovering Methane from Solid Methane Hydrate with Carbon Dioxide. Angew. Chem. Int. Ed. 2003, 42, 5048–5051. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Zhang, X.; Chen, G.; Wang, W. Replacement Mechanism of Methane Hydrate with Carbon Dioxide from Microsecond Molecular Dynamic Simulations. Energy Environ. Sci. 2012, 5, 7033–7041. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of Natural Gas Hydrates as an Energy Resource: Prospects and Chanllenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Takeya, S.; Udachin, K.A.; Moudrakovski, I.L.; Susilo, R.; Ripmeester, J.A. Direct Space Methods for Powder X-ray Diffraction for Guest-Host Materials: Applications to Cage Occupancies and Guest Distributions in Clathrate Hydrates. J. Am. Chem. Soc. 2010, 132, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Cha, M.; Lee, W.; Seo, Y.; Lee, H. Abnormal Proton positioning of Water Framework in the Presence of Paramagnetic Guest within Ion-Doped Clathrate Hydrate Host. J. Phys. Chem. C 2014, 118, 15193–15199. [Google Scholar] [CrossRef]
- Susilo, R.; Alavi, S.; Moudrakovski, I.L.; Englezos, P.; Ripmeester, J.A. Guest-Host Hydrogen Bonding in Structure H Clathrate Hydrates. ChemPhysChem 2009, 10, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Udachin, K.; Ripmeester, J.A. Effect of Guest-Host Hydrogen Bonding on the Structures and Properties of Clathrate Hydrates. Chem. Eur. J. 2010, 16, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Udachin, K.A.; Alavi, S.; Ripmeester, J.A. Water-Halogen Interactions in Chlorine and Bromine Clathrate Hydrates: An Example of Multidirectional Halogen Bonding. J. Phys. Chem. C 2013, 117, 14176–14182. [Google Scholar] [CrossRef]
- Alavi, S.; Takeya, S.; Ohmura, R.; Woo, T.K.; Ripmeester, J.A. Hydrogen-bonding alcohol-water interactions in binary ethanol, 1-propanol, and 2-propanol + methane structure II clathrate hydrates. J. Chem. Phys. 2010, 133, 074505. [Google Scholar] [CrossRef] [PubMed]
- Udachin, K.; Alavi, S.; Ripmeester, J.A. Communication: Single Crystal X-ray Diffraction Observation of Hydrogen Bonding between 1-Propanol and Water in a Structure Ii Clathrate Hydrate. J. Chem. Phys. 2011, 134, 121104. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kumar, R.; Udachin, K.A.; Alavi, S.; Ripmeester, J.A. Ammonia Clathrate Hydrates as New Solid Phases for Titan, Enceladus, and other Planetary Systems. Proc. Natl. Acad. Sci. USA 2012, 109, 14785–14790. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Udachin, K.A.; Moudrakovski, I.L.; Leek, D.M.; Alavi, S.; Ratcliffe, C.I.; Ripmeester, J.A. Methanol Incorporation in Clathrate Hydrates and the Implications for Oil and Gas Pipeline Flow Assurance and Icy Planetary Bodies. Proc. Natl. Acad. Sci. USA 2013, 110, 8437–8442. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.Y.; Marshall, S.L.; Chakoumakos, B.C.; Rawn, C.J.; Ishii, Y. Structure and Thermal Expansivity of Tetrahydrofuran Deuterate Determined by Neutron Powder Diffraction. J. Phys. Chem. B 2003, 107, 6026–6031. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.-W.; Kim, D.Y.; Park, J.; Seo, Y.-T.; Zeng, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Tuning Clathrate Hydrates for Hydrogen Storage. Nature 2005, 434, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Florusse, L.J.; Peters, C.J.; Schoonman, J.; Hester, K.C.; Koh, C.A.; Dec, S.F.; Marsh, K.N.; Sloan, E.D. Stable Low-Pressure Hydrogen Clusters Stored in a Binary Clathrate Hydrate. Science 2004, 306, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-H.; Kang, H.; Koh, D.-Y.; Park, Y.; Lee, H. Gas hydrate Inhibition by 3-Hydroxytetrahydrofuran: Spectroscopic Identifications and Hydrate Phase Equilibria. Fluid Phase Equilib. 2016, 413, 65–70. [Google Scholar] [CrossRef]
- Favre-Nicolin, F.; Cerny, R. ‘Free objects for crystallography’: A Modular Approach to Ab Initio Structure Determination from Powder Diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Cerny, R.; Favre-Nicolin, F. Direct Space Methods of Structure Determination from Powder Diffraction: Principles, Guidelines and Perspectives. Z. Kristallogr. 2007, 222, 105–113. [Google Scholar]
- Rodriguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Massa, W. Crystal Structure Determination, 2nd ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Kirchner, M.T.; Boese, R.; Billups, W.E.; Norman, L.R. Gas Hydrate Single-Crystal Structure Analyses. J. Am. Chem. Soc. 2004, 126, 9407–9412. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Susilo, R.; Ripmeester, J.A. Linking Microscopic Guest Properties to Macroscopic Observables in Clathrate Hydrates: Guest-Host Hydrogen Bonding. J. Chem. Phys. 2009, 130, 174501. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Shin, K.; Ripmeester, J.A. Molecular Dynamics Simulations of Hydrogen Bonding in Clathrate Hydrates with Ammonia and Methanol Guest Molecules. J. Chem. Eng. Data 2015, 60, 389–397. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lu, H.; Moudrakovski, I.L.; Ratcliffe, C.I.; Ripmeester, J.A. Thermodynamic and molecular-scale analysis of new systems of water-soluble hydrate formers+ CH4. J. Phys. Chem. B 2010, 114, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
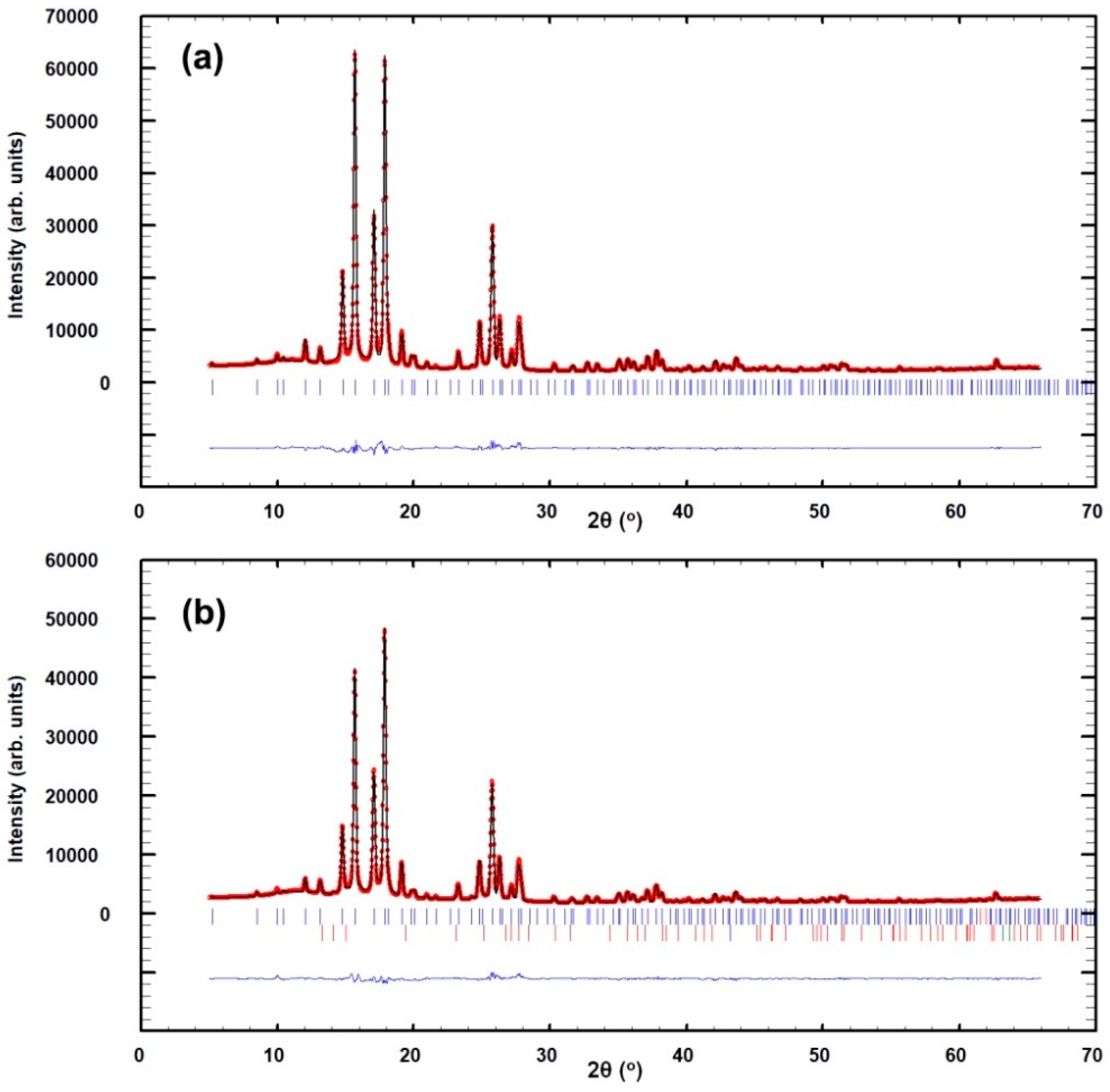
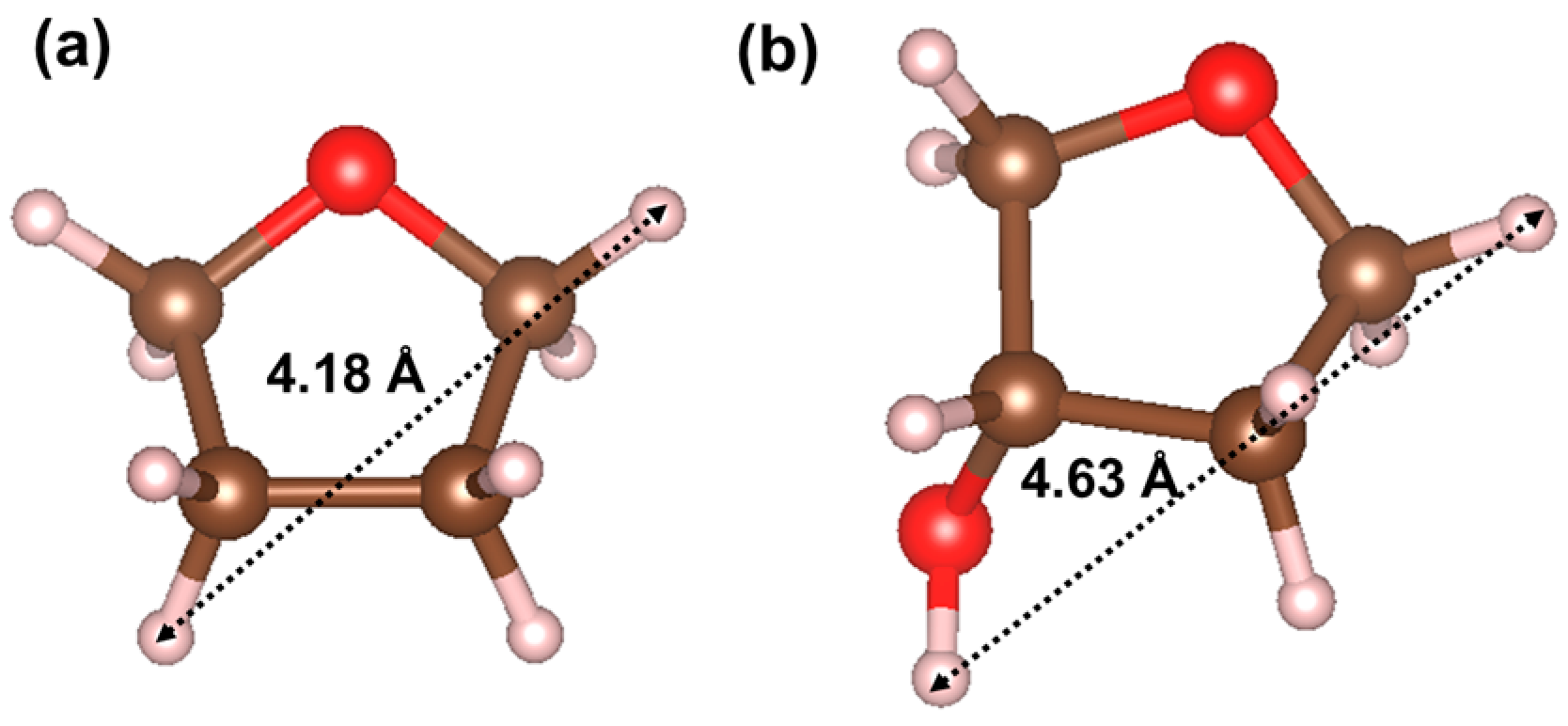

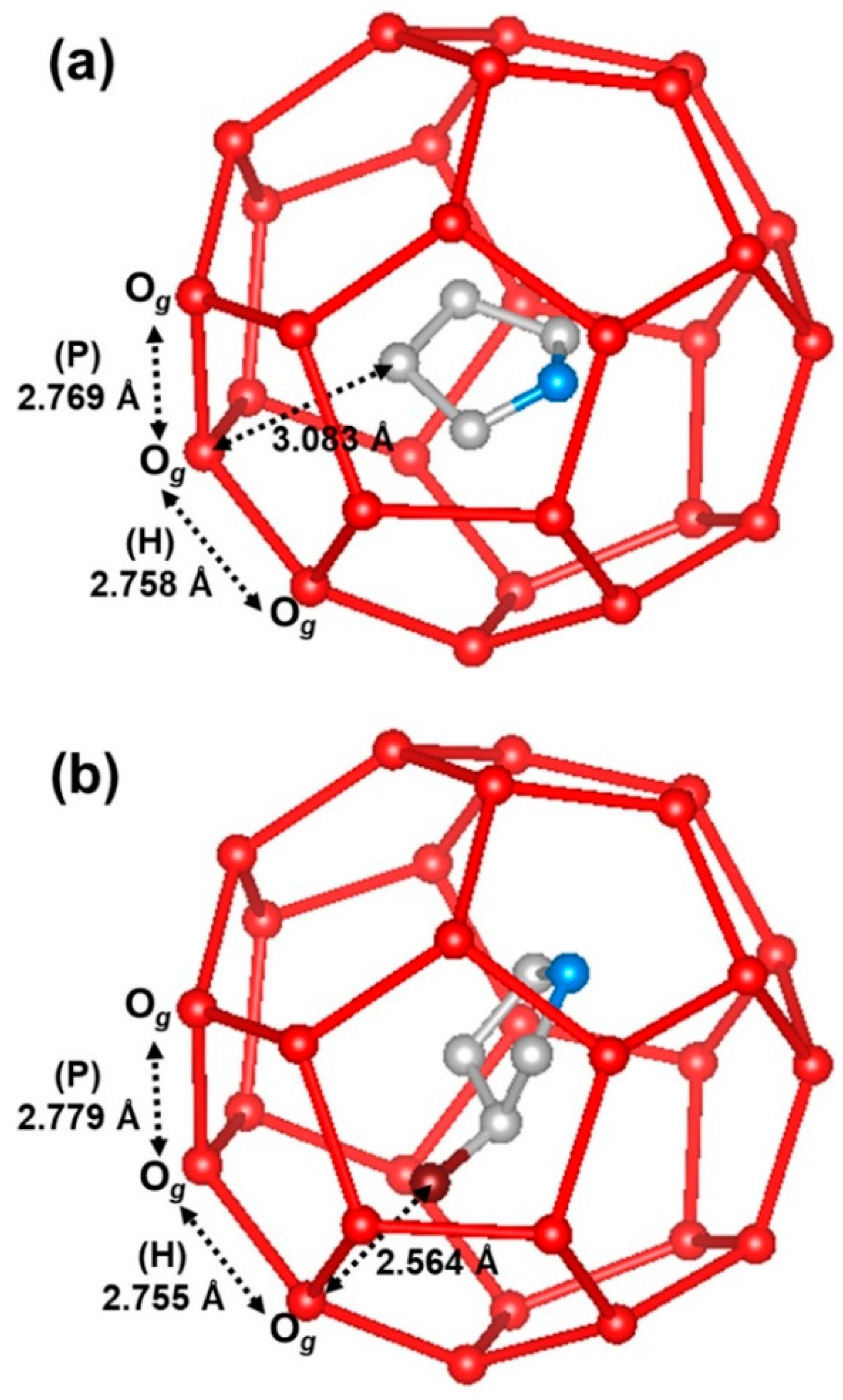
| Atom | x | y | z | B (Å2) | g | Site |
|---|---|---|---|---|---|---|
| Oa | 0.125 | 0.125 | 0.125 | 1.84 (10) | 1 | 8 a |
| Oe | 0.2166 (1) | 0.2166 | 0.2166 | 1.56 (6) | 1 | 32 e |
| Og | 0.1822 (1) | 0.1822 | 0.3706 (1) | 1.80 (3) | 1 | 96 g |
| Hea | 0.1842 (2) | 0.1842 | 0.1842 | 2.34 | 0.5 | 32 e |
| Hae | 0.1581 (2) | 0.1581 | 0.1581 | 2.76 | 0.5 | 32 e |
| Heg | 0.2106 (10) | 0.2106 | 0.2731 (6) | 2.34 | 0.5 | 96 g |
| Hge | 0.1827 (10) | 0.1827 | 0.3110 (5) | 2.70 | 0.5 | 96 g |
| Hgg(p) | 0.1414 (3) | 0.1414 | 0.3640 (19) | 2.70 | 0.5 | 96 g |
| Hgg(h) | 0.2373 (7) | 0.1807 (8) | 0.3912 (11) | 2.70 | 0.5 | 192 i |
| CL1 | 0.9314 | 0.9142 | 0.3679 | 3.43 (29) | 0.0415 (2) | 192 i |
| OL2 | 0.9143 | 0.8484 | 0.3197 | 3.43 | 0.0415 | 192 i |
| CL3 | 0.8364 | 0.8600 | 0.2924 | 3.43 | 0.0415 | 192 i |
| CL4 | 0.7909 | 0.8937 | 0.3624 | 3.43 | 0.0415 | 192 i |
| CL5 | 0.8557 | 0.9311 | 0.4138 | 3.43 | 0.0415 | 192 i |
| HL6 | 0.9811 | 0.8990 | 0.4045 | 5.14 | 0.0415 | 192 i |
| HL7 | 0.9474 | 0.9644 | 0.3312 | 5.14 | 0.0415 | 192 i |
| HL8 | 0.8365 | 0.9012 | 0.2433 | 5.14 | 0.0415 | 192 i |
| HL9 | 0.8146 | 0.8039 | 0.2718 | 5.14 | 0.0415 | 192 i |
| HL10 | 0.7471 | 0.9359 | 0.3438 | 5.14 | 0.0415 | 192 i |
| HL11 | 0.7609 | 0.8473 | 0.3941 | 5.14 | 0.0415 | 192 i |
| HL12 | 0.8576 | 0.9038 | 0.4712 | 5.14 | 0.0415 | 192 i |
| HL13 | 0.8469 | 0.9936 | 0.4223 | 5.14 | 0.0415 | 192 i |
| OS1 | 0.2317 | 0.2285 | 0.9789 | 5.31 (19) | 0.0808 (4) | 192 i |
| OS2 | 0.2775 | 0.2695 | 1.0131 | 5.31 | 0.0808 | 192 i |
| Atom | x | y | z | B (Å2) | g | Site |
|---|---|---|---|---|---|---|
| Oa | 0.125 | 0.125 | 0.125 | 1.42 (9) | 1 | 8 a |
| Oe | 0.2166 (1) | 0.2166 | 0.2166 | 1.55 (6) | 1 | 32 e |
| Og | 0.1824 (1) | 0.1824 | 0.3708 (1) | 1.90 (3) | 1 | 96 g |
| Hea | 0.1842 (2) | 0.1842 | 0.1842 | 2.32 | 0.5 | 32 e |
| Hae | 0.1578 (2) | 0.1578 | 0.1578 | 2.13 | 0.5 | 32 e |
| Heg | 0.2156 (9) | 0.2156 | 0.2752 (5) | 2.32 | 0.5 | 96 g |
| Hge | 0.1857 (11) | 0.1857 | 0.3127 (6) | 2.85 | 0.5 | 96 g |
| Hgg(p) | 0.1416 (3) | 0.1416 | 0.3835 (15) | 2.85 | 0.5 | 96 g |
| Hgg(h) | 0.2372 (7) | 0.1782 (9) | 0.3878 (12) | 2.85 | 0.5 | 192 i |
| CL1 | 0.6740 | 0.1037 | 0.1417 | 1.89 (34) | 0.0387 (2) | 192 i |
| OL2 | 0.6493 | 0.0585 | 0.0732 | 1.89 | 0.0387 | 192 i |
| CL3 | 0.5662 | 0.0766 | 0.0571 | 1.89 | 0.0387 | 192 i |
| CL4 | 0.5426 | 0.1418 | 0.1146 | 1.89 | 0.0387 | 192 i |
| CL5 | 0.5986 | 0.1280 | 0.1841 | 1.89 | 0.0387 | 192 i |
| HL6 | 0.7121 | 0.0671 | 0.1774 | 2.83 | 0.0387 | 192 i |
| HL7 | 0.7047 | 0.1574 | 0.1252 | 2.83 | 0.0387 | 192 i |
| HL8 | 0.5601 | 0.0947 | −0.0039 | 2.83 | 0.0387 | 192 i |
| HL9 | 0.5315 | 0.0237 | 0.0668 | 2.83 | 0.0387 | 192 i |
| HL10 | 0.5564 | 0.1996 | 0.0909 | 2.83 | 0.0387 | 192 i |
| HL11 | 0.4809 | 0.1392 | 0.1307 | 2.83 | 0.0387 | 192 i |
| HL12 | 0.5771 | 0.0795 | 0.2203 | 2.83 | 0.0387 | 192 i |
| OL13 | 0.6164 | 0.1972 | 0.2297 | 1.89 | 0.0387 | 192 i |
| HL14 | 0.5686 | 0.2118 | 0.2593 | 2.83 | 0.0387 | 192 i |
| OS1 | 0.2829 | 0.2292 | 0.9757 | 4.27 (25) | 0.0785 (5) | 192 i |
| OS2 | 0.2440 | 0.2626 | 1.0236 | 4.27 | 0.0785 | 192 i |
| Hydrates | Oa-Oe (Å) | Oe-Og (Å) | Og-Og (p) 1 (Å) | Og-Og (h) 2 (Å) |
|---|---|---|---|---|
| THF + O2 | 2.714 (2) | 2.765 (2) | 2.769 (2) | 2.758 (3) |
| 3-OH THF + O2 | 2.716 (2) | 2.768 (3) | 2.779 (2) | 2.755 (3) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.-H.; Lee, B.; Shin, K. Structural Identification of Binary Tetrahydrofuran + O2 and 3-Hydroxytetrahydrofuran + O2 Clathrate Hydrates by Rietveld Analysis with Direct Space Method. Crystals 2018, 8, 328. https://doi.org/10.3390/cryst8080328
Ahn Y-H, Lee B, Shin K. Structural Identification of Binary Tetrahydrofuran + O2 and 3-Hydroxytetrahydrofuran + O2 Clathrate Hydrates by Rietveld Analysis with Direct Space Method. Crystals. 2018; 8(8):328. https://doi.org/10.3390/cryst8080328
Chicago/Turabian StyleAhn, Yun-Ho, Byeonggwan Lee, and Kyuchul Shin. 2018. "Structural Identification of Binary Tetrahydrofuran + O2 and 3-Hydroxytetrahydrofuran + O2 Clathrate Hydrates by Rietveld Analysis with Direct Space Method" Crystals 8, no. 8: 328. https://doi.org/10.3390/cryst8080328
APA StyleAhn, Y.-H., Lee, B., & Shin, K. (2018). Structural Identification of Binary Tetrahydrofuran + O2 and 3-Hydroxytetrahydrofuran + O2 Clathrate Hydrates by Rietveld Analysis with Direct Space Method. Crystals, 8(8), 328. https://doi.org/10.3390/cryst8080328




