Effects of Iodine Doping on Carrier Behavior at the Interface of Perovskite Crystals: Efficiency and Stability
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication
2.2. Characterization
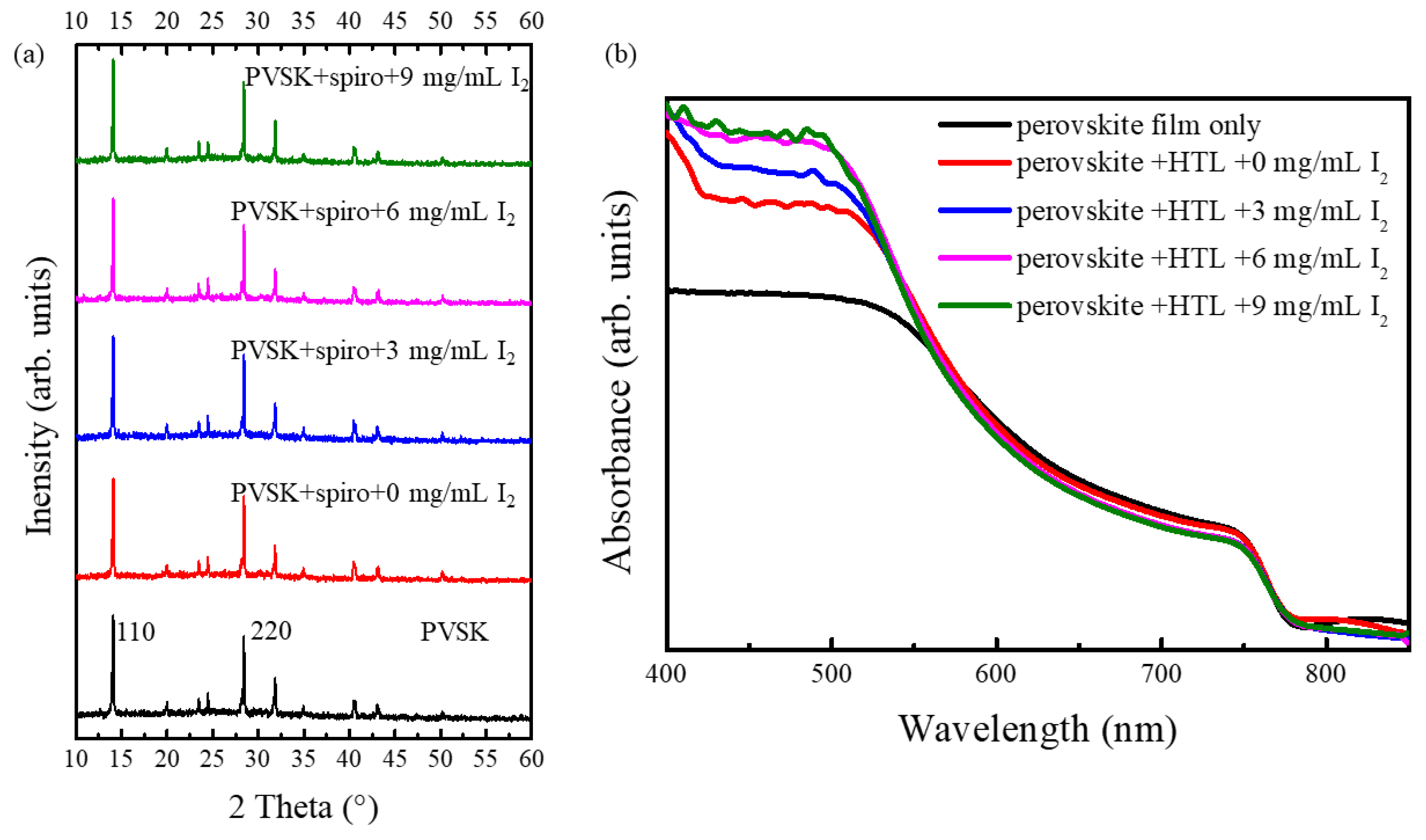
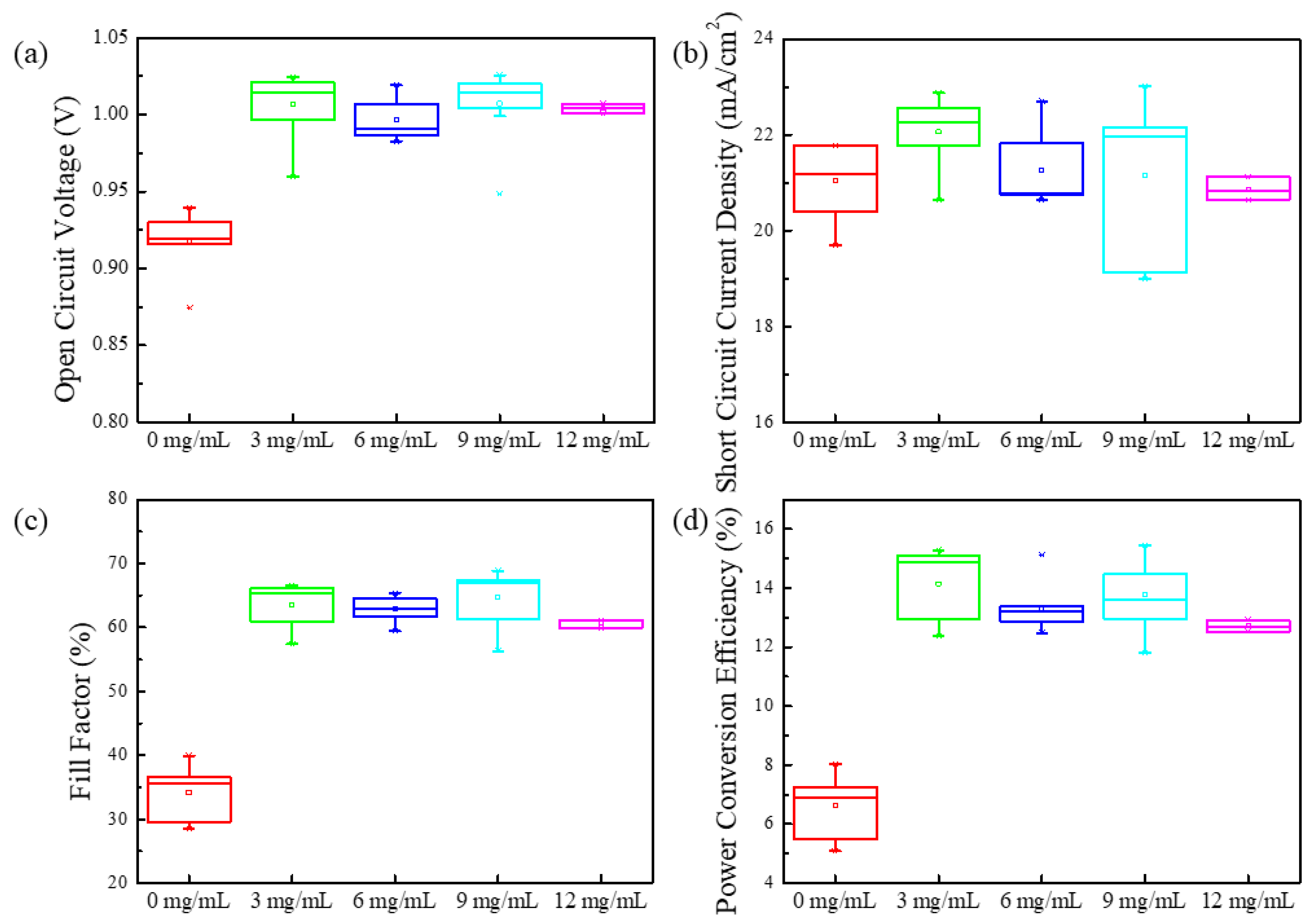
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, Y.; Xiao, Y.; Zhao, Z.; Ye, S.; Rao, H.; Ting, H.; Bian, Z.; Xiao, L.; Huang, C.; et al. An ammonia modified PEDOT: PSS for interfacial engineering in inverted planar perovskite solar cells. Org. Electron. 2017, 46, 22–27. [Google Scholar] [CrossRef]
- Burschka, J.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Shin, D.H.; Jang, M.H.; Lee, M.L.; Kang, M.G.; Im, S.H. Highly flexible, high-performance perovskite solar cells with adhesion promoted AuCl3-doped graphene electrodes. J. Mater. Chem. A 2017, 5, 21146–21152. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, L.; Lin, Z.; Ni, J.; Yi, P.; Lai, X.; He, X.; Lei, Z. Flexible semiconductor technologies with nanohole-provided high area coverages and their application in plasmonic-enhanced thin film photovoltaics. Sci. Rep. 2017, 7, 13155. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, X.; Liu, Z.; Lee, E.C. A transparent poly(3,4-ethylenedioxylenethiophene): Poly (styrene sulfonate) cathode for low temperature processed, metal-oxide free perovskite solar cells. J. Mater. Chem. A 2017, 5, 6974–6980. [Google Scholar] [CrossRef]
- Qiao, B.; Song, P.; Cao, J.; Zhao, S.; Shen, Z.; Gao, D.; Liang, Z.; Xu, Z.; Song, D.; Xu, X. Water-resistant, monodispersed and stably luminescent CsPbBr3/CsPb2Br5 core-shell-like structure lead halide perovskite nanocrystals. Nanotechnology 2017, 28, 445602. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Huang, S.; Ou-Yang, W.; Pan, L.; Sun, Z.; Chen, X. Constructing efficient and stable perovskite solar cells via interconnecting perovskite grains. ACS Appl. Mater. Interfaces 2017, 9, 35200–35208. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Shukla, S.; Haur, L.J.; Dintakurti, S.S.; Han, G.; Priyadarshi, A.; Baikie, T.; Mhaisalkar, S.G.; Mathews, N. Effect of formamidinium/Cesium substitution and PbI2 on the long-term stability of triple-cation perovskites. ChemSusChem 2017, 10, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Moumni, B.; Jaballah, A.B. Correlation between oxidant concentrations, morphological aspects and etching kinetics of silicon nanowires during sliver-assist electroless etching. Appl. Surf. Sci. 2017, 425, 1–7. [Google Scholar] [CrossRef]
- Jin, J.; Shen, H.; Zheng, P.; Chan, K.S.; Zhang, X.; Jin, H. >20.5% diamond wire sawn multicrystalline silicon solar cells with maskless inverted pyramid like texturing. IEEE J. Photovolt. 2017, 7, 1264–1269. [Google Scholar] [CrossRef]
- Dullweber, T.; Hannebauer, H.; Dorn, S.; Schimanke, S.; Merkle, A.; Hampe, C.; Brendel, R. Emitter saturation current densities of 22fA/cm2 applied to industrial PERC solar cells approaching 22% conversion efficiency. Prog. Photovolt. 2017, 25, 509–514. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W. Solar cell efficiency tables (version 51). Prog. Photovolt. 2017. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.S.; Wang, H.H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Conings, B.; Baeten, L.; De Dobbelaere, C.; D’Haen, J.; Manca, J.; Boyen, H.G. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach. Adv. Mater. 2014, 26, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Tan, K.W.; Sai, H.; Moore, D.T.; Scott, T.; Zhang, W.; Estroff, L.A.; Wiesner, U.; Snaith, H.J. Influence of thermal processing protocol upon the crystallization and photovoltaic performance of organic-inorganic lead trihalide perovskites. J. Phys. Chem. C 2014, 118, 17171–17177. [Google Scholar] [CrossRef]
- Xiao, M.; Huang, F.; Huang, W.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. 2014, 126, 10056–10061. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chiang, Y.F.; Lee, M.H.; Peng, S.R.; Guo, T.F.; Chen, P.; Wen, T.C. CH3NH3PbI3 perovskite/fullerene planar heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Xuan, Y.M.; Li, Q. Quantifying energy losses in planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 174, 206–213. [Google Scholar] [CrossRef]
- Si, H.; Liao, Q.; Zhang, Z.; Li, Y.; Yang, X.; Zhang, G.; Kang, Z.; Zhang, Y. An innovative design of perovskite solar cells with Al2O3 inserting at ZnO/perovskite interface for improving the performance and stability. Nano Energy 2016, 22, 223–231. [Google Scholar] [CrossRef]
- Zheng, K.; Žídek, K.; Abdellah, M.; Chen, J.; Chábera, P.; Zhang, W.; Al-Marri, M.J.; Pullerits, T. High excitation intensity opens a new trapping channel in organic-inorganic hybrid perovskite nanoparticles. ACS Energy Lett. 2016, 1, 1154–1161. [Google Scholar] [CrossRef]
- Sun, X.; Ji, L.Y.; Chen, W.W.; Guo, X.; Wang, H.H.; Lei, M.; Wang, Q.; Li, Y.F. Halide anion-fullerene pi noncovalent interactions: N-doping and a halide anion migration mechanism in p-i-n perovskite solar cells. J. Mater. Chem. A 2017, 5, 20720–20728. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; Xu, Z.; Chen, Q.; Wang, X.; Zhou, H. Precise Composition Tailoring of Mixed-Cation Hybrid Perovskites for Efficient Solar Cells by Mixture Design Methods. ACS Nano 2017, 11, 8804–8813. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Seok, S.I. Iodine management in formamidinium-ldea-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Guerrero, A.; Rahimnejad, S.; Almora, O.; Zarazua, I.; Mas-Marza, E.; Bisquert, J.; Garcia-Belmonte, G. Ionic reactivity at contacts and aging of methylammonium lead triiodode perovskite solar cells. Adv. Energy Mater. 2016, 6, 1502246. [Google Scholar] [CrossRef]
- Tsai, H.; Nie, W.; Cheruku, P.; Mack, N.H.; Xu, P.; Gupta, G.; Mohite, A.D.; Wang, H.L. Optimizing composition and morphology for large-grain perovskite solar cells, via chemical control. Chem. Mater. 2015, 27, 5570–5576. [Google Scholar] [CrossRef]
- Park, I.J.; Seo, S.; Park, M.A.; Lee, S.; Kim, D.H.; Zhu, K.; Shin, H.; Kim, J.Y. Effect of rubidium incorporation on the structural, electrical, and photovoltaic properties of Methylammonium lead iodide-based perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 41898–41905. [Google Scholar] [CrossRef] [PubMed]
- Shlenskaya, N.N.; Belich, N.A.; Grätzel, M.; Goodilin, E.A.; Tarasov, A.B. Light-induced reactivity of gold and hybrid perovskite as a new possible degradation mechanism in perovskite solar cells. J. Mater. Chem. A 2018, 6, 1780–1786. [Google Scholar] [CrossRef]
- Cao, J.; Jing, X.; Yan, J.; Hu, C.; Chen, R.; Yin, J.; Li, J.; Zheng, N. Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films. J. Am. Chem. Soc. 2016, 138, 9919–9926. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, L.; Niu, X.; Zhou, H.; Chen, Q. Effects of Iodine Doping on Carrier Behavior at the Interface of Perovskite Crystals: Efficiency and Stability. Crystals 2018, 8, 185. https://doi.org/10.3390/cryst8050185
Liu G, Liu L, Niu X, Zhou H, Chen Q. Effects of Iodine Doping on Carrier Behavior at the Interface of Perovskite Crystals: Efficiency and Stability. Crystals. 2018; 8(5):185. https://doi.org/10.3390/cryst8050185
Chicago/Turabian StyleLiu, Guilin, Lang Liu, Xiuxiu Niu, Huanping Zhou, and Qi Chen. 2018. "Effects of Iodine Doping on Carrier Behavior at the Interface of Perovskite Crystals: Efficiency and Stability" Crystals 8, no. 5: 185. https://doi.org/10.3390/cryst8050185
APA StyleLiu, G., Liu, L., Niu, X., Zhou, H., & Chen, Q. (2018). Effects of Iodine Doping on Carrier Behavior at the Interface of Perovskite Crystals: Efficiency and Stability. Crystals, 8(5), 185. https://doi.org/10.3390/cryst8050185



