Effect of the Ultrasonic Substrate Vibration on Nucleation and Crystallization of PbI2 Crystals and Thin Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christo, N.N.; Hodzhaoglu, F.V.; Ivaylo, L.D. Kinetics of insulin crystal nucleation, energy barrier, and nucleus size. Cryst. Growth Des. 2011, 11, 196–202. [Google Scholar] [CrossRef]
- Ihli, J.; Wong, W.C.; Noel, E.H.; Kim, Y.Y.; Kulak, A.N.; Christenson, H.K.; Duer, M.J.; Meldrum, F.C. Dehydration and crystallization of amorphous calcium carbonate in solution and in air. Nat. Commun. 2014, 5, 3169. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Koza, J.A.; Switzer, J.A. Eectrodeposition of epitaxial lead-iodide and conversion to textured methylammonium lead iodide perovskite. ACS App. Mater. Interfaces 2015, 7, 26012–26016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.Y.; Zhang, S.; Lu, T.M.; Shi, J. Band gap engineering of a soft inorganic compound PbI2 by incommensurate van der Waals epitaxy. App. Phys. Lett. 2016, 108, 013105. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, H.; Yang, D. Fabrication and characterization of X-ray array detector based on polycrystalline PbI2 thick films. J. Mat. Sci. Mat. Electron. 2014, 25, 3337–3343. [Google Scholar] [CrossRef]
- Ying, C.; Shi, C.; Wu, N.; Zhang, J.; Wang, M. Two-layer structured PbI2 thin film for efficient planar perovskite solar cells. Nanoscale 2015, 7, 12092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, D.W.; Zhu, Z.; Cheng, L. Enhancement of crystallization processes by power ultrasound: Current state-of-the-art. Compr. Rev. Food Sci. Food Saf. Res. Adv. 2015, 14, 303–316. [Google Scholar] [CrossRef]
- Cao, J.; Wang, F.; Yu, H.; Zhou, Y.; Lu, H.; Zhao, N.; Wong, C.P. Porous films for the fabrication of efficient, stable perovskite solar cells via sequential deposition. J. Mater. Chem. A 2016, 4, 10223–10230. [Google Scholar] [CrossRef]
- Acuna, D.; Krishna, B.; Shaji, S.; Sepolveda, S.; Menchaca, J.L. Growth and properties of lead iodide thin films by spin coating. Bull. Mater. Sci. 2016, 39, 1453–1460. [Google Scholar] [CrossRef]
- Zhang, J.; Song, T.; Zhang, Z.; Ding, K.; Huang, F.; Sun, B. Layered ultrathin PbI2 single crystals for high sensitivity flexible photodetectors. J. Mater. Chem. C 2015, 3, 4402–4406. [Google Scholar] [CrossRef]
- Habibi, M.; Zabihi, F.; Ahmadian-Yazdi, M.R.; Eslamian, M. Progress in emerging solution-processed thin film solar cells—Part II: Perovskite solar cells. Renew. Sustain. Energy Rev. 2016, 62, 1012–1031. [Google Scholar] [CrossRef]
- Xiong, H.; DeLucab, D.; Ruic, Y.; Li, Y.; Reichmanis, E.; Zhang, Q.; Wang, H. Solvent vapor annealing of oriented PbI2 films for improved crystallization of perovskite films in the air. Sol. Energy Mater. Sol. Cells 2017, 166, 167–175. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, M.; Zhao, Y.; Zhu, K. Controllable sequential deposition of planar CH₃NH₃PbI₃ perovskite films via adjustable volume expansion. Nano Lett. 2015, 15, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Kariper, A. Optical and structural properties of PbI2 thin film produced via chemical dipping method. Opt. Rev. 2016, 23, 401–408. [Google Scholar] [CrossRef]
- Chaudhary, S.K. Lead iodide crystals as input material for radiation detectors. Cryst. Struct. Theory Appl. (CSTA) 2012, 1, 21–24. [Google Scholar] [CrossRef]
- Eslamian, M. Inorganic and organic solution-processed thin film devices. Nano-Micro Lett. 2017, 9, 3. [Google Scholar] [CrossRef]
- Mousa, A.M.; Al-rubaie, N.J. The influence of deposition conditions on structural properties of PbI2. Texture Stress Microstruct. 2009, 494537. [Google Scholar] [CrossRef]
- Matuchov, M.; Zdansky, K.; Zavdil, J.; Tonn, J.; Mousa, M.; Jafar, A.-G.; Danilewsky, A.N.; Croll, A.; Maixner, J. Influence of doping and non-stoichiometry on the quality of lead iodide for use in X-Ray detection. J. Cryst. Growth 2010, 312, 1233–1239. [Google Scholar] [CrossRef]
- Habibi, M.; Rahimzadeh, A.; Eslamian, M. On dewetting of thin films due to crystallization (crystallization dewetting). Eur. Phys. J. E 2016, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Okerberg, B.C.; Berry, B.C.; Garvey, T.R.; Douglas, J.F.; Karim, A.; Soles, C.L. Competition between crystallization and dewetting fronts in tin polymer films. Soft Matter 2009, 5, 562–567. [Google Scholar] [CrossRef]
- Peron, N.; Brochard-Wyart, F.; Duval, H. Dewetting of low-viscosity films at solid/liquid interfaces. Langmuir 2012, 28, 15844–15852. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, F.; Xie, Y.; Gao, S.; Eslamian, M. Morphology, conductivity and wetting characteristics of PEDOT:PSS thin films deposited by spin and spray coating. Appl. Surf. Sci. 2015, 338, 163–177. [Google Scholar] [CrossRef]
- Shklyaev, S.; Khenner, M.; Alabuzhev, A.A. Enhanced stability of a dewetting thin liquid film in a single-frequency vibration field. Phys. Rev. E 2008, 77, 036320. [Google Scholar] [CrossRef] [PubMed]
- Benilov, E.S.; Chugunova, M. Waves in liquid films on vibrating substrates. Phys. Rev. E 2010, 81, 036302. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, A.; Eslamian, M. Stability of thin liquid films subjected to ultrasonic vibration and characteristics of the resulting thin solid films. Chem. Eng. Sci. 2017, 158, 587–598. [Google Scholar] [CrossRef]
- Rahimzadeh, A.; Eslamian, M. On evaporation of thin liquid films subjected to ultrasonic substrate vibration. Int. Commun. Heat Mass Transf. 2017, 83, 15–22. [Google Scholar] [CrossRef]
- Eslamian, M. Excitation by acoustic vibration as an effective tool for improving the characteristics of the solution-processed coatings and thin films. Prog. Org. Coat. 2017, 113, 60–73. [Google Scholar] [CrossRef]
- Hem, S.L. The effect of ultrasonic vibration on crystallization processes. Ultrasonics 1967, 5, 202–207. [Google Scholar] [CrossRef]
- Djordjevic, S.; Poinern, G.E.J.; Brundavanam, R.K.; Fawcett, D.; Nikoloski, A.; Prokic, M. Enhanced deposition and reflective properties of thin aluminum films by substrate vibration. Int. J. Sci. 2014, 3, 67–73. [Google Scholar]
- Ruirun, C.; Zheng, D.; Tengfei, M.; Hongsheng, D.; Yanqing, S.; Jingjie, G.; Hengzhi, F. Effects of ultrasonic vibration on the microstructure and mechanical properties of high alloying TiAl. Sci. Rep. 2017, 7, 41463. [Google Scholar] [CrossRef] [PubMed]
- Nahidul Islam, M.; Zhang, M.; Adhikari, B. Ultrasound-assisted freezing of fruits and vegetables: Design, development, and applications. Glob. Food Secur. Wellness 2017, 457–487. [Google Scholar] [CrossRef]
- Gielen, B.; Jordens, J.; Thomassen, L.C.J.; Braeken, L.; Gerven, T.V. Agglomeration control during ultrasonic crystallization of an active pharmaceutical ingredient. Crystals 2017, 7, 40. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, K.J.; Yoo, J.B.; Kim, D. Properties of cadmium sulfide thin films deposited by chemical bath deposition with ultrasonication. Sol. Energy 1998, 64, 41–47. [Google Scholar] [CrossRef]
- Diemer, P.J.; Lyle, C.R.; Mei, Y.; Sutton, C.; Payne, M.M.; Anthony, J.E.; Coropceanu, V.; Bredas, J.L.; Jurchescu, O.D. Vibration-assisted crystallization improves organic/dielectric interface in organic thin-film transistors. Adv. Mater. 2013, 25, 6956–6962. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, F.; Ahmadian-Yazdi, M.R.; Eslamian, M. Photocatalytic Graphene-TiO2 thin films fabricated by low-temperature ultrasonic vibration-assisted spin and spray coating in a sol-gel process. Catalysts 2017, 7, 136. [Google Scholar] [CrossRef]
- Zabihi, F.; Eslamian, M. Substrate vibration-assisted spray coating (SVASC): Significant improvement in nano-structure, uniformity, and conductivity of PEDOT:PSS thin films for organic solar cells. J. Coat. Technol. Res. 2015, 12, 711–719. [Google Scholar] [CrossRef]
- Wang, Q.; Eslamian, M. Improving uniformity and nanostructure of solution-processed thin films using ultrasonic substrate vibration post treatment (SVPT). Ultrasonics 2016, 67, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, F.; Chen, Q.; Xie, Y.; Eslamian, M. Fabrication of efficient graphene-doped polymer/fullerene bilayer organic solar cells in air using spin coating followed by ultrasonic vibration post treatment. Superlattices Microstruct. 2016, 100, 1177–1192. [Google Scholar] [CrossRef]
- Zabihi, F.; Ahmadian-Yazdi, M.R.; Eslamian, M. Fundamental study on the fabrication of inverted planar perovskite solar cells using two-step sequential substrate vibration-assisted spray coating (2S-SVASC). Nanoscale Res. Lett. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, M.; Zabihi, F. Ultrasonic substrate vibration-assisted drop casting (SVADC) for the fabrication of solar cell arrays and thin film devices. Nanoscale Res. Lett. 2015, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Yazdi, M.R.; Eslamian, M. Toward scale-up of perovskite solar cells: Annealing-free perovskite layer by low-cost ultrasonic substrate vibration of wet films. Mater. Today Commun. 2018, 14, 151–159. [Google Scholar] [CrossRef]
- Asha, K.S.; Kavyasree, P.R.; George, A.; Mandal, S. The role of solvents in framework dimensionality and their effect on band gap energy. Dalton Trans. 2015, 44, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Birnie, P.; Dunbar, A. Model for drying control co-solvent selection for spin coating uniformity: The thin film limit. Langmuir 2013, 29, 9072–9078. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Kranz, L.; Yoon, S.; Lockinger, J.; Jager, T.; Perrenoud, J.; Feurer, T.; Gretener, C.; Bucheler, S.; Tiwari, A.N. Controlled growth of PbI2 nanoplates for rapid preparation of CH3NH3PbI3 in planar perovskite solar cells. Phys. Status Solids A 2015, 212, 2708–2717. [Google Scholar] [CrossRef]
- Sumari, S.; Roesyadi, A.; Sumarno, S. Effects of ultrasound on the morphology particle size, crystallinity and crystalline size of cellulose. Chem. Chem. Eng. Biotechnol. Food Ind. 2013, 14, 229–239. [Google Scholar]
- Wu, X.; Wang, Y.; Zhou, S.; Yuan, X.Y.; Gao, T.; Wang, K.; Lou, S.; Liu, Y.; Shi, X. Morphology control, crystal growth, and growth mechanism of hierarchical tellurium (Te) microstructures. Cryst. Growth Des. 2013, 13, 136–142. [Google Scholar] [CrossRef]
- Tidhar, Y.; Edri, E.; Weissman, H.; Zohar, D.; Hodes, G.; Cahen, D.; Rybtchinski, B.; Kirmayer, S. Crystallization of methyl ammonium lead halide perovskites: Implications for photovoltaic applications. J. Am. Chem. Soc. 2014, 136, 13249–13256. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.R.G.; Zeiger, B.W.; Suslick, K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014, 21, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef] [PubMed]
- Kolgomorov, A.N. A statistical theory for the recrystallization of metals. Izv. Akad. Nauk SSSR Ser. Mat. 1937, 3, 355–359. [Google Scholar]
- Johnson, W.A.; Meh, R.F. Reaction kinetics in processes of nucleation and growth. Trans. Metall. Soc. AIME 1939, 135, 416–458. [Google Scholar]
- Avrami, M. Kinetics of phase change, I. General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change, II. General Theory. J. Chem. Phys. 1940, 8, 212. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change, III. General Theory. J. Chem. Phys. 1941, 9, 177–181. [Google Scholar] [CrossRef]
- Kaswan, A.; Kumari, V.; Patidar, D.; Shai Saxena, N.; Sharma, K. Kinetics of crystallization of Ge30-xSe70Sbx (x = 15, 20, 25) chalcogenide glasses. Proc. Appl. Ceram. 2014, 8. [Google Scholar] [CrossRef]
- Linnikov, O.D. Relation between activation energy for nucleation and of growth of crystals. Nanosyst. Phys. Chem. Math. 2014, 5, 546–552. [Google Scholar]
- Miller, R.M.; Poulos, A.S.; Robles, E.S.J.; Brooks, N.J.; Ces, O.; Cabral, J.T. Isothermal crystallization kinetics of sodium dodecyl sulfate-water micellar solutions. Cryst. Growth Des. 2016, 16, 3379–3388. [Google Scholar] [CrossRef]
- Fidalgo-Marijuan, A.; Amayuelas, E.; Barandika, G.; Bazán, B.; Urtiaga, M.K.; Arriortua, M.I. Coordination and crystallization molecules: Their interactions affecting the dimensionality of metalloporphyrinic SCFs. Molecules 2015, 20, 6683–6699. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.H.; Rogers, M.A. Experimental validation of the modified Avrami model for non-isothermal crystallization, conditions. Cryst. Eng. Commun. 2011, 13, 866–875. [Google Scholar] [CrossRef]
- Muhammed Shafi, P.; Chandra Bose, A. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Bushroa, A.R.; Rahbari, R.G.; Masjuki, H.H.; Muhamad, M.R. Approximation of crystallite size and microstrain via XRD line broadening analysis in TiSiN thin films. Vacuum 2012, 86, 1107–1112. [Google Scholar] [CrossRef]
- Halder, N.C.; Wagner, C.N.J. Separation of particle size and lattice strain in integral breadth measurements. Acta Crystallogr. 1966, 20, 312–313. [Google Scholar] [CrossRef]
- Taraka Prabhu, Y.; Venkateswara Rao, K.; Sai Kumar, V.-S.; Siva Kumari, B. X-Ray analysis by williamson-hall and size-strain plot methods of ZnO nanoparticles with fuel variation. World J. Nano Sci. Eng. (WJNSE) 2014, 4, 21–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J. Microstrain and grain-size analysis from diffraction peak width and graphical derivation of high pressure thermomechanics. J. Appl. Cryst. 2008, 41, 1095–1108. [Google Scholar] [CrossRef]
- Groma, I.; Monnet, G. Analysis of asymmetric broadening of X-ray diffraction peak profiles caused by randomly distributed polarized dislocation dipoles and dislocation walls. J. Appl. Crystallogr. 2002, 35, 589–593. [Google Scholar] [CrossRef]
- Choquette, S.J.; Etz, E.S.; Hurst, W.S.; Blackburn, D.H.; Leigh, S.D. Relative intensity correction of Raman spectrometers: NIST SRMs 2241 through 2243 for 785 nm, 532 nm, 488 nm/514.5 nm excitation. Appl. Spectrosc. 2007, 61, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Slodczyk, A. Raman Intensity: An important tool in the study of nanomaterials and nanostructures. Acta Phys. Pol. A 2009, 116. [Google Scholar] [CrossRef]
- Ahlawat, D.S. Study of band gap energy and thermal properties of PbI2 by photoacoustic spectroscopy. Mod. Phys. Lett. B 2012, 26, 1250098. [Google Scholar] [CrossRef]
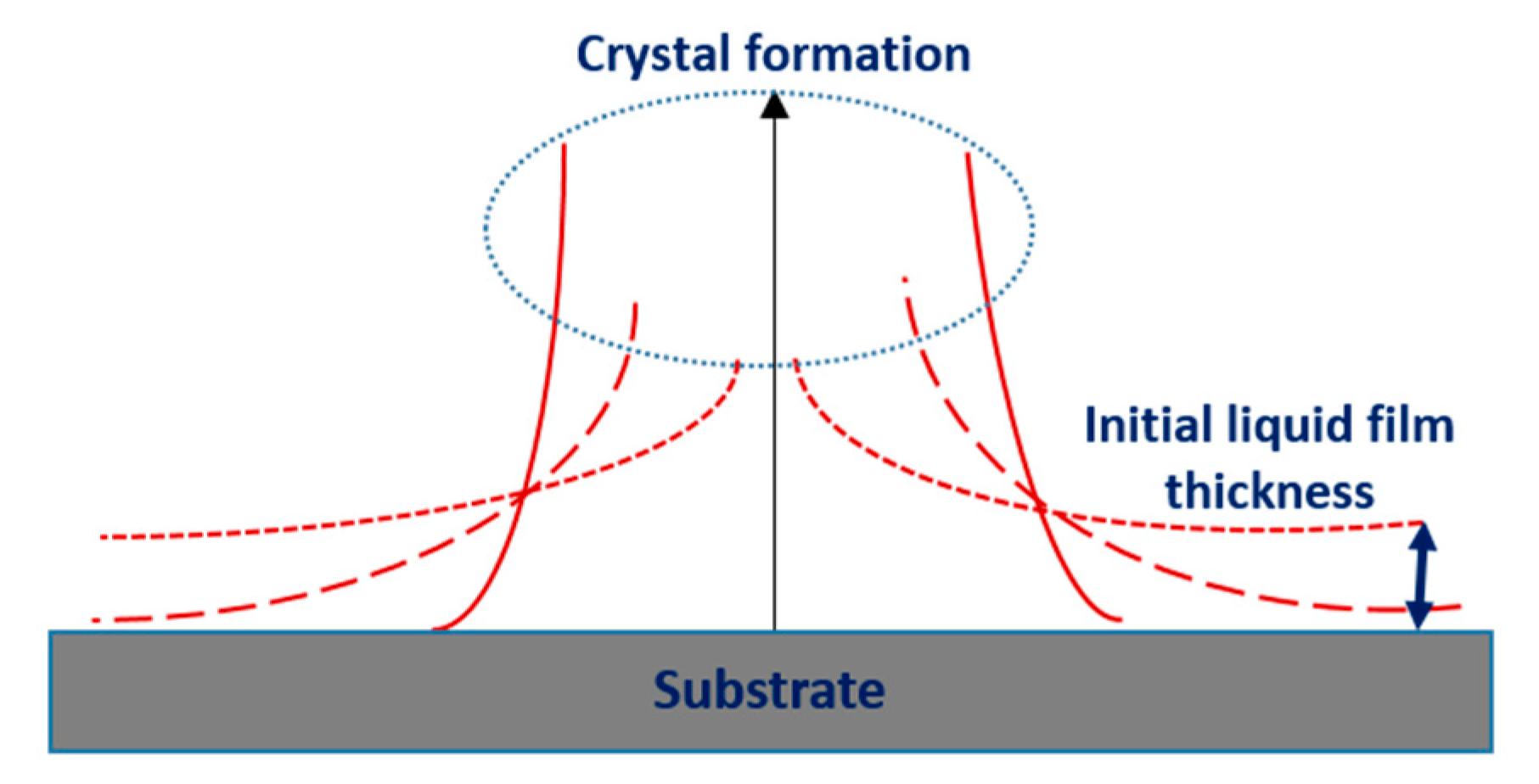
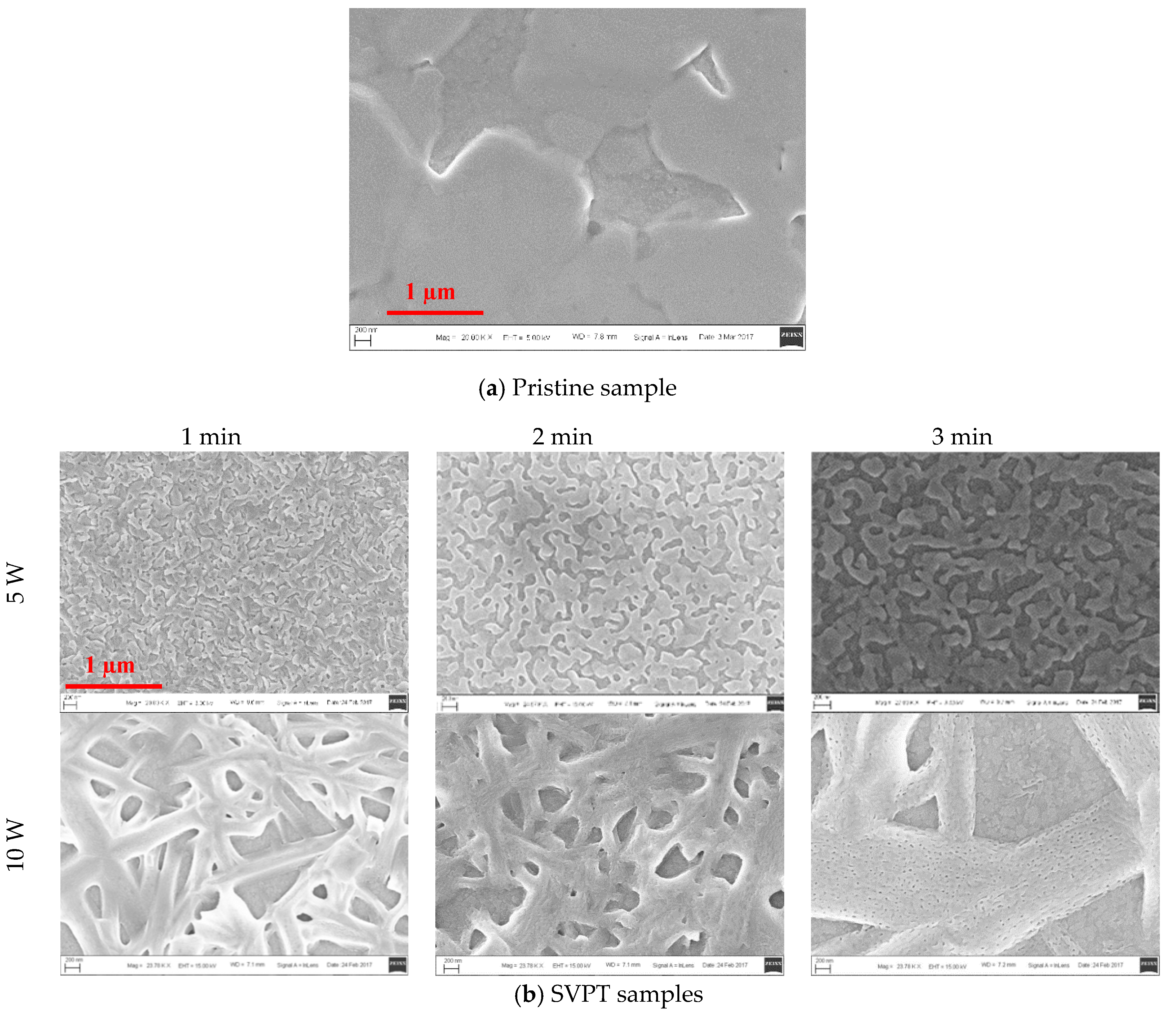
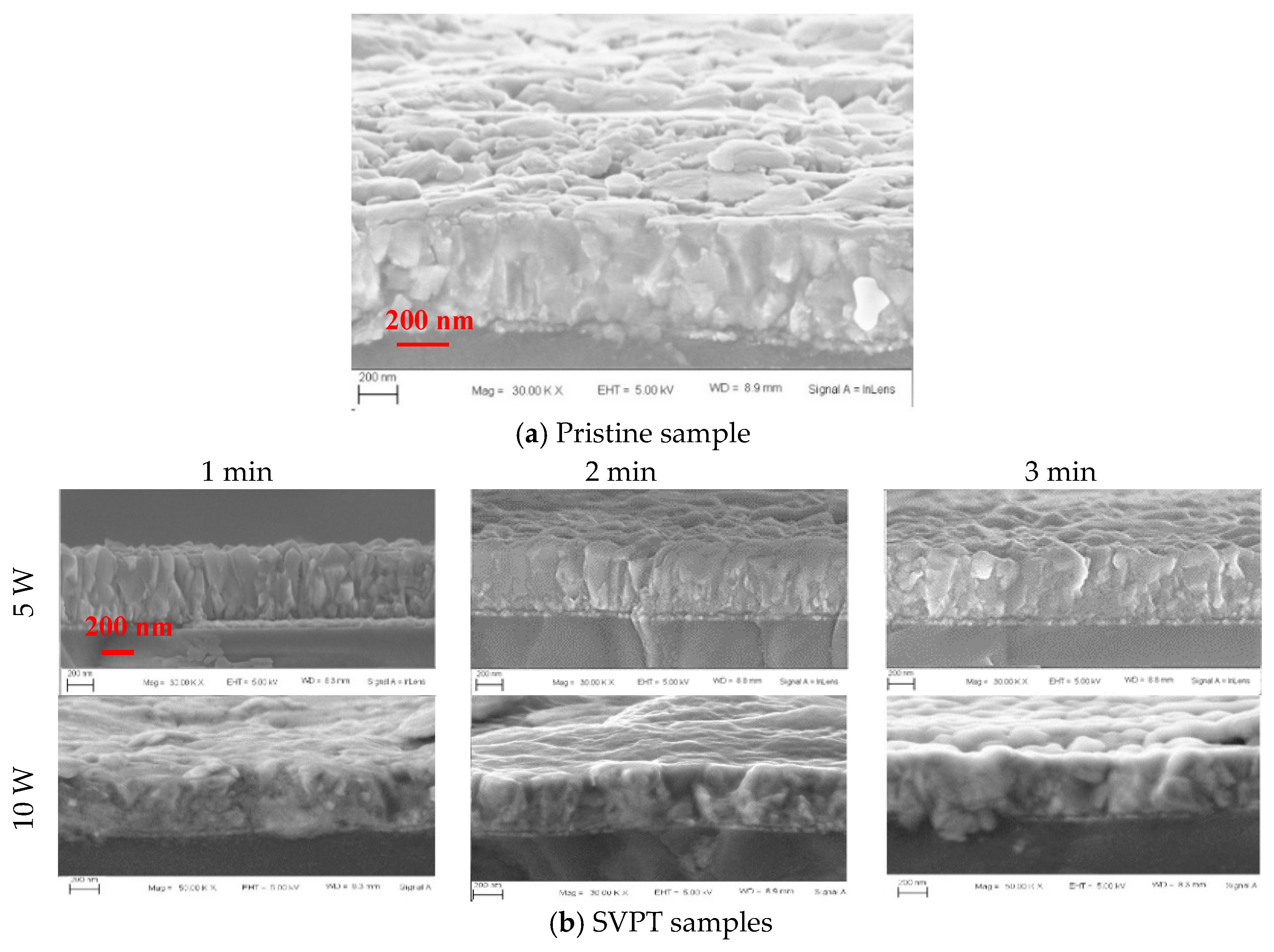
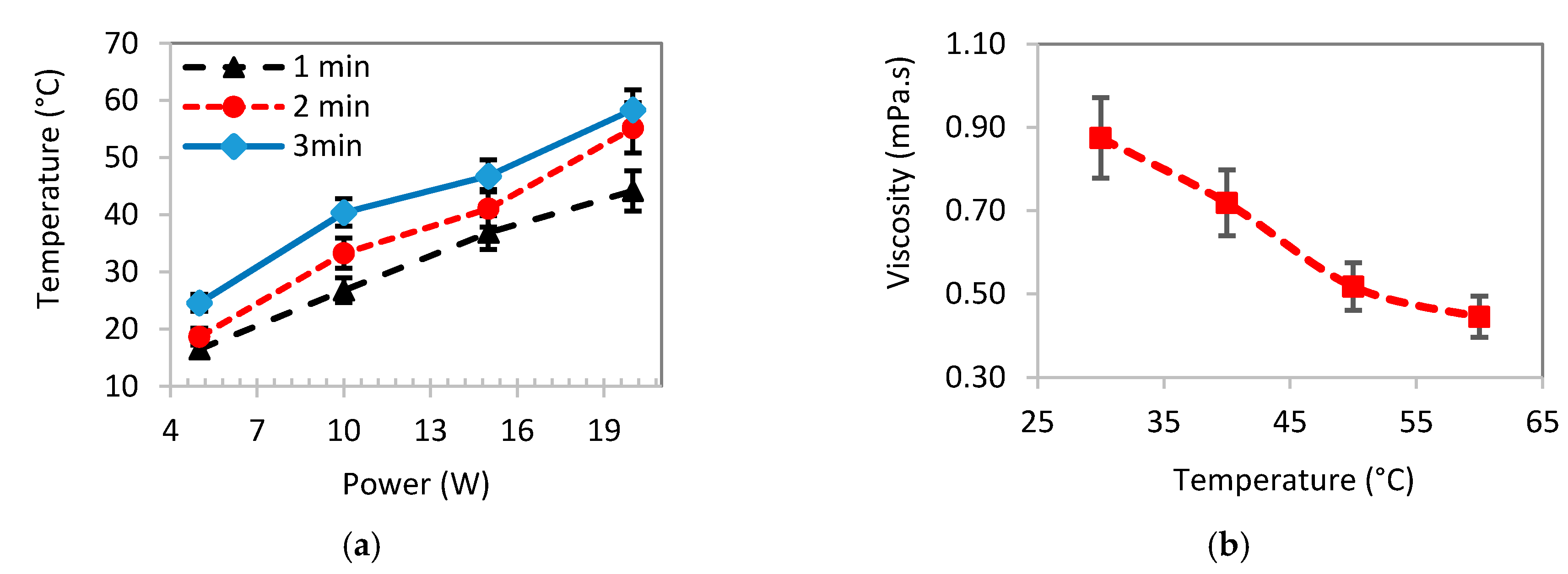
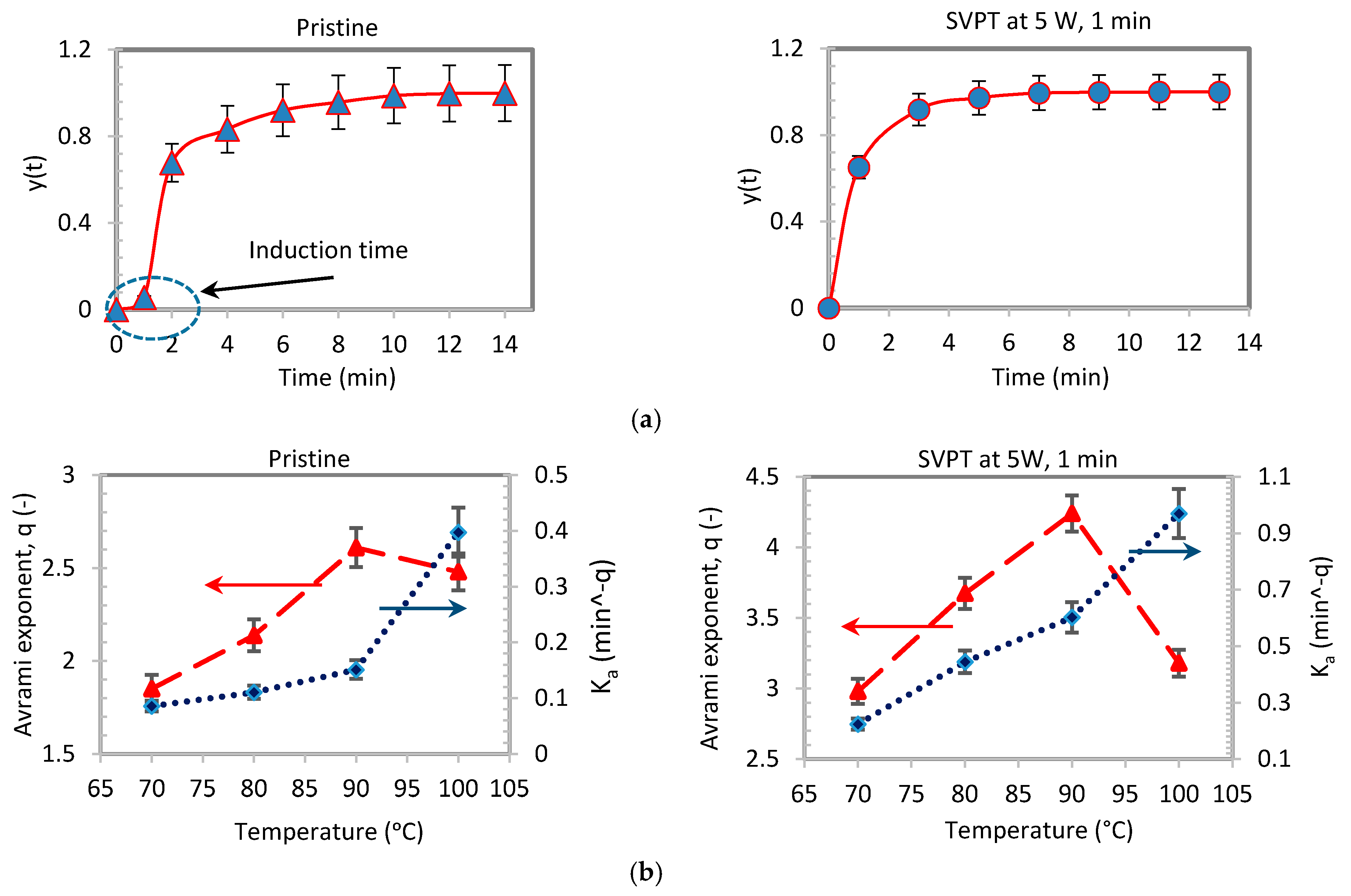
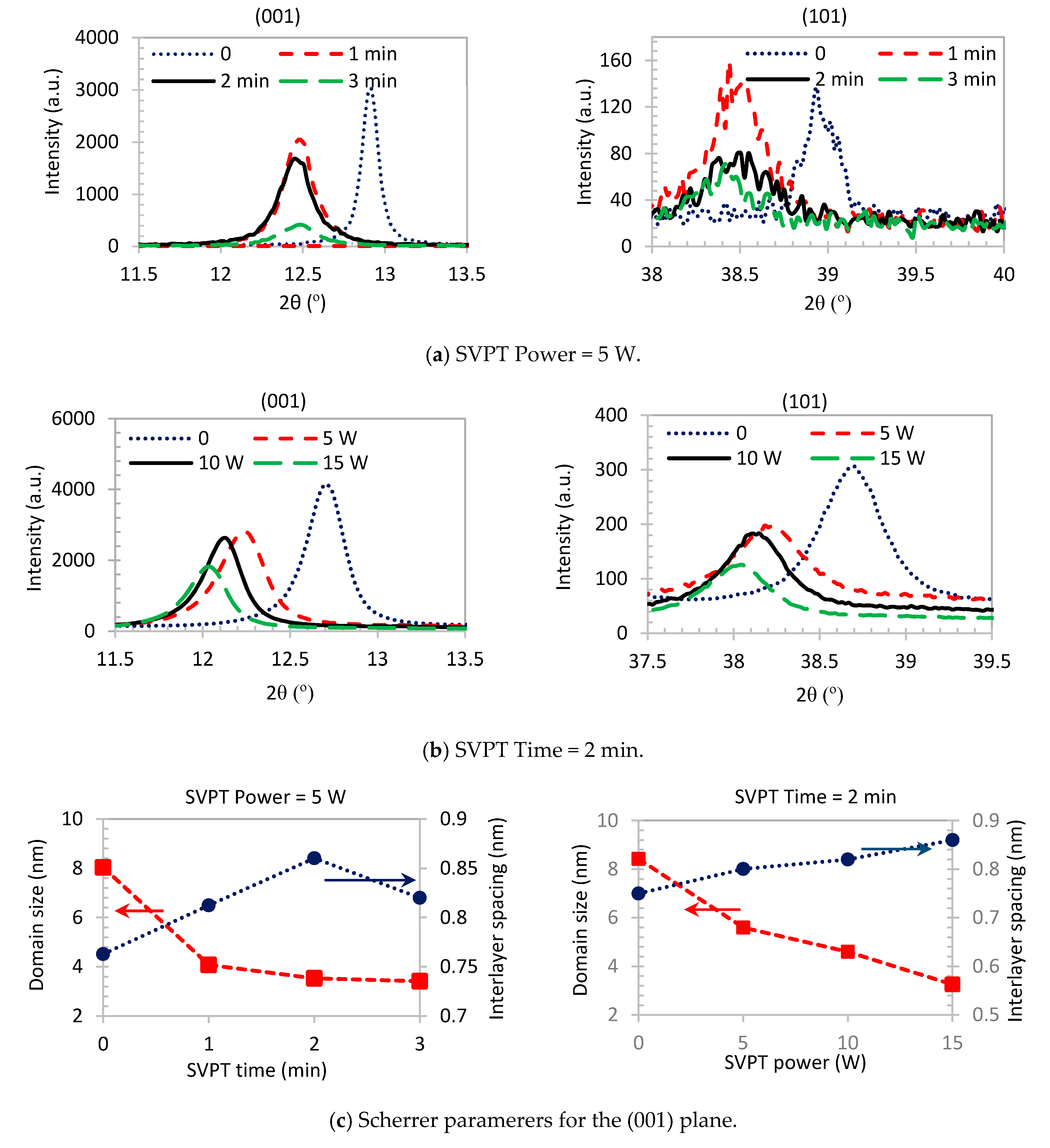
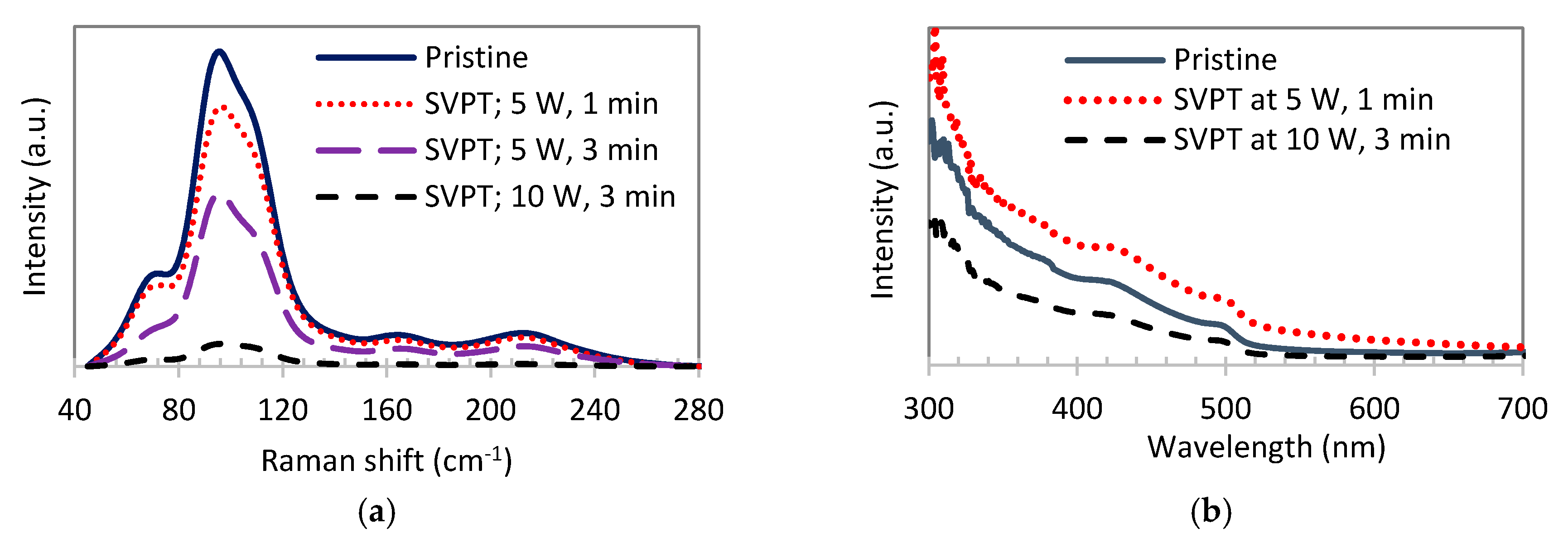
| Pristine Samples | SVPT Samples | ||||||
|---|---|---|---|---|---|---|---|
| (min−q) | Ea (kJ·mol−1) | Z0 (min−q) | (min−q) | Ea (kJ·mol−1) | Z0 (min−q) | ||
| 2.27 | 0.15 | 52.46 | 3.20 × 106 | 3.52 | 0.56 | 45.71 | 9.82 × 106 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabihi, F.; Eslamian, M. Effect of the Ultrasonic Substrate Vibration on Nucleation and Crystallization of PbI2 Crystals and Thin Films. Crystals 2018, 8, 60. https://doi.org/10.3390/cryst8020060
Zabihi F, Eslamian M. Effect of the Ultrasonic Substrate Vibration on Nucleation and Crystallization of PbI2 Crystals and Thin Films. Crystals. 2018; 8(2):60. https://doi.org/10.3390/cryst8020060
Chicago/Turabian StyleZabihi, Fatemeh, and Morteza Eslamian. 2018. "Effect of the Ultrasonic Substrate Vibration on Nucleation and Crystallization of PbI2 Crystals and Thin Films" Crystals 8, no. 2: 60. https://doi.org/10.3390/cryst8020060
APA StyleZabihi, F., & Eslamian, M. (2018). Effect of the Ultrasonic Substrate Vibration on Nucleation and Crystallization of PbI2 Crystals and Thin Films. Crystals, 8(2), 60. https://doi.org/10.3390/cryst8020060




