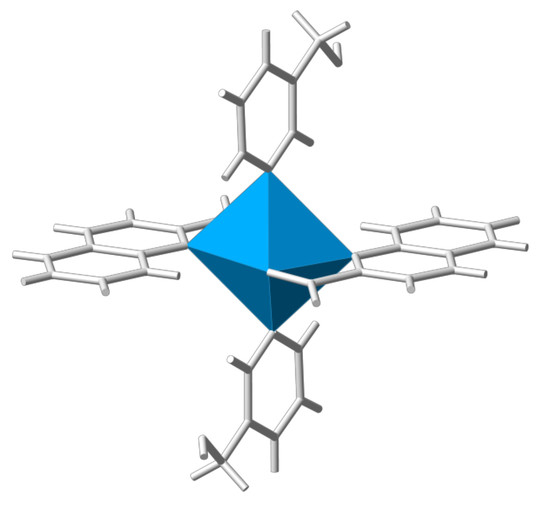
Crystal Chemistry of Zinc Quinaldinate Complexes with Pyridine-Based Ligands
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Preparation Procedures
2.3. X-ray Structure Determinations
3. Results and Discussion
3.1. Synthetic Aspects
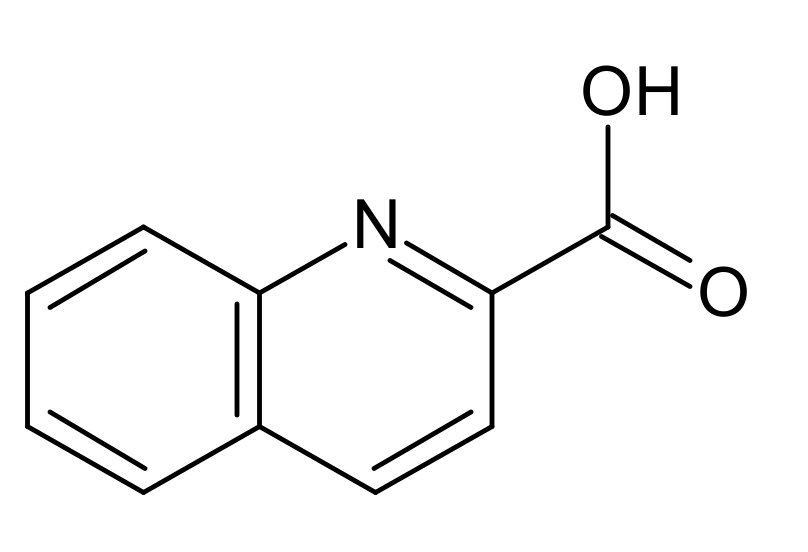
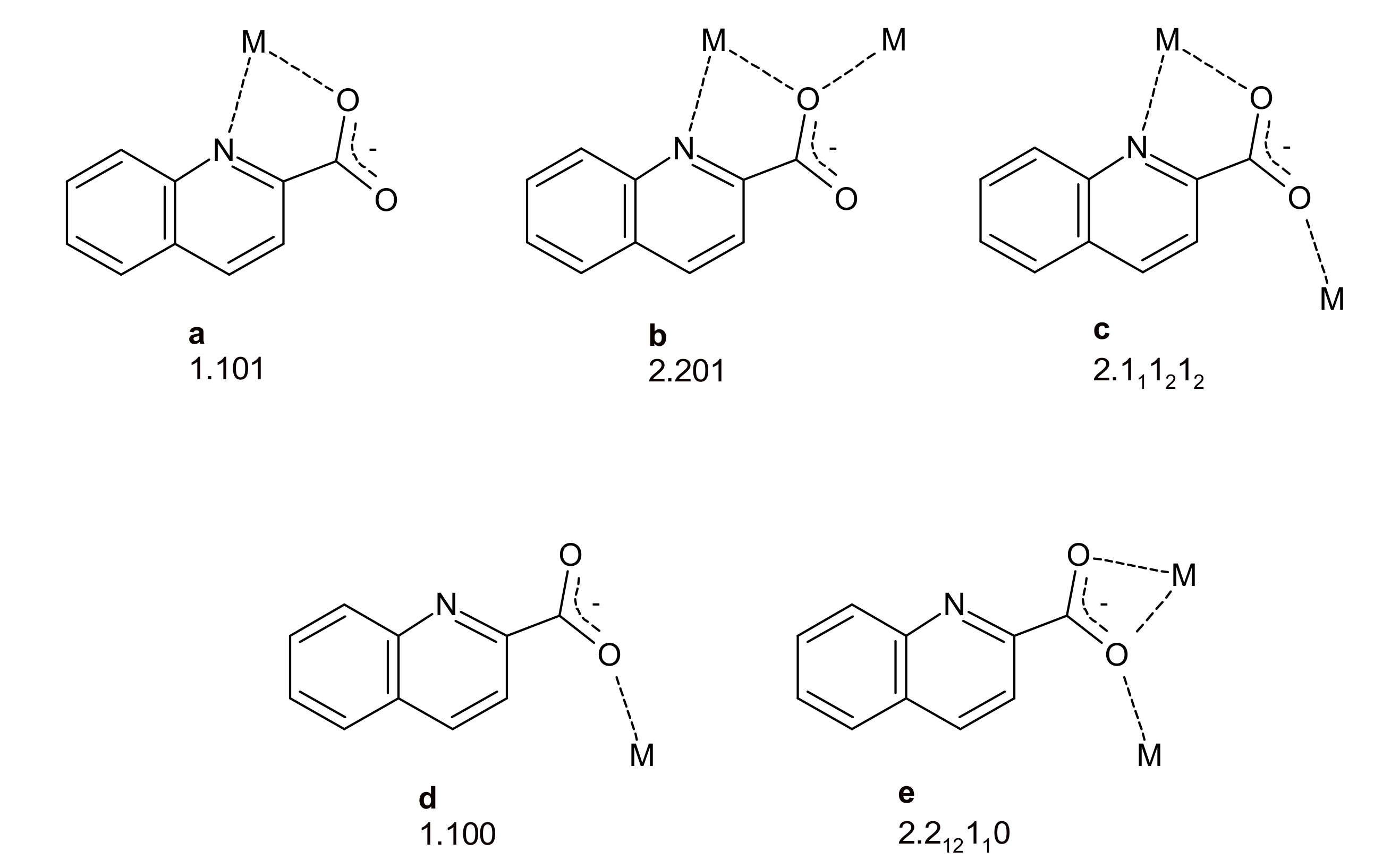
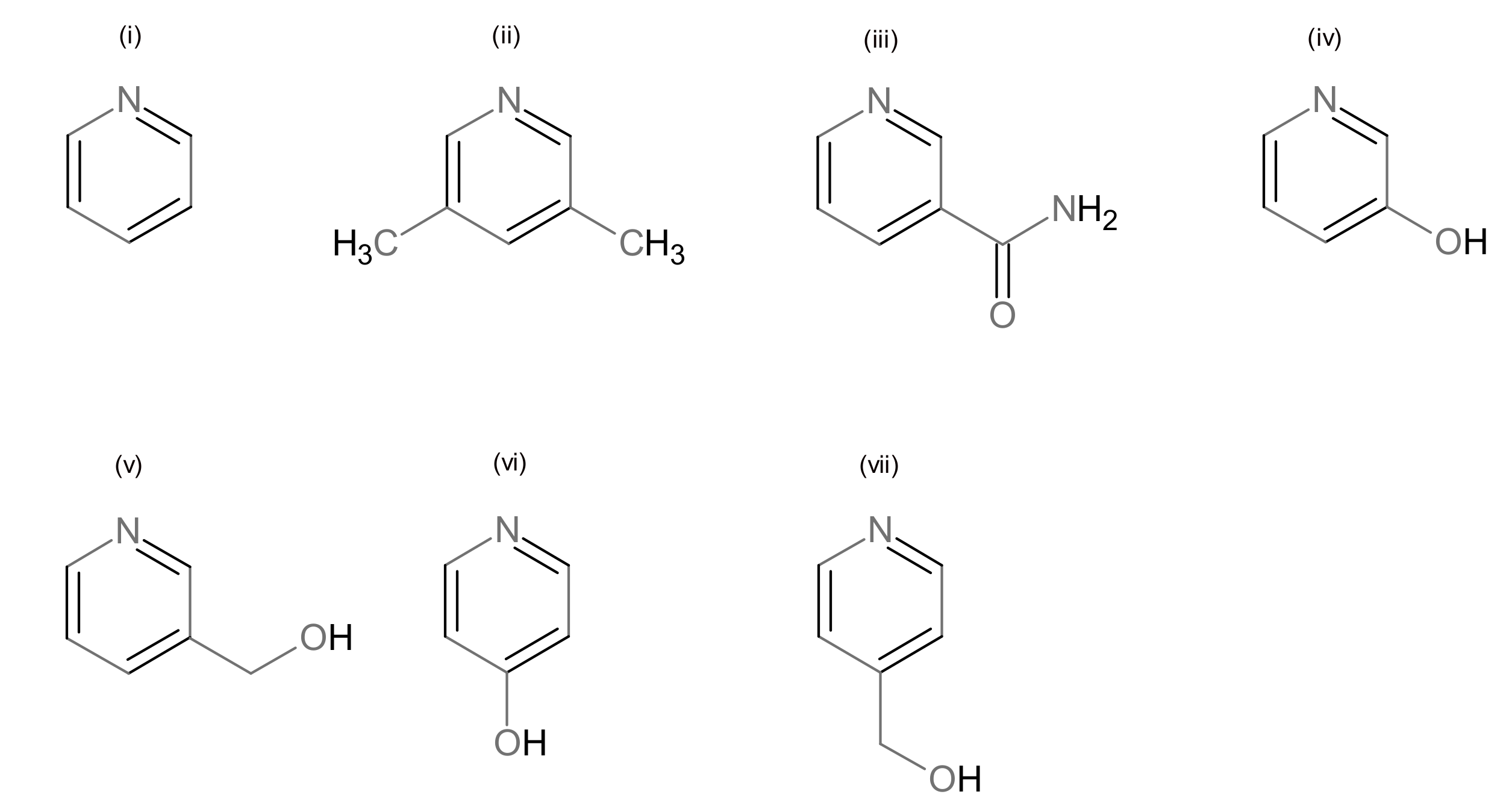
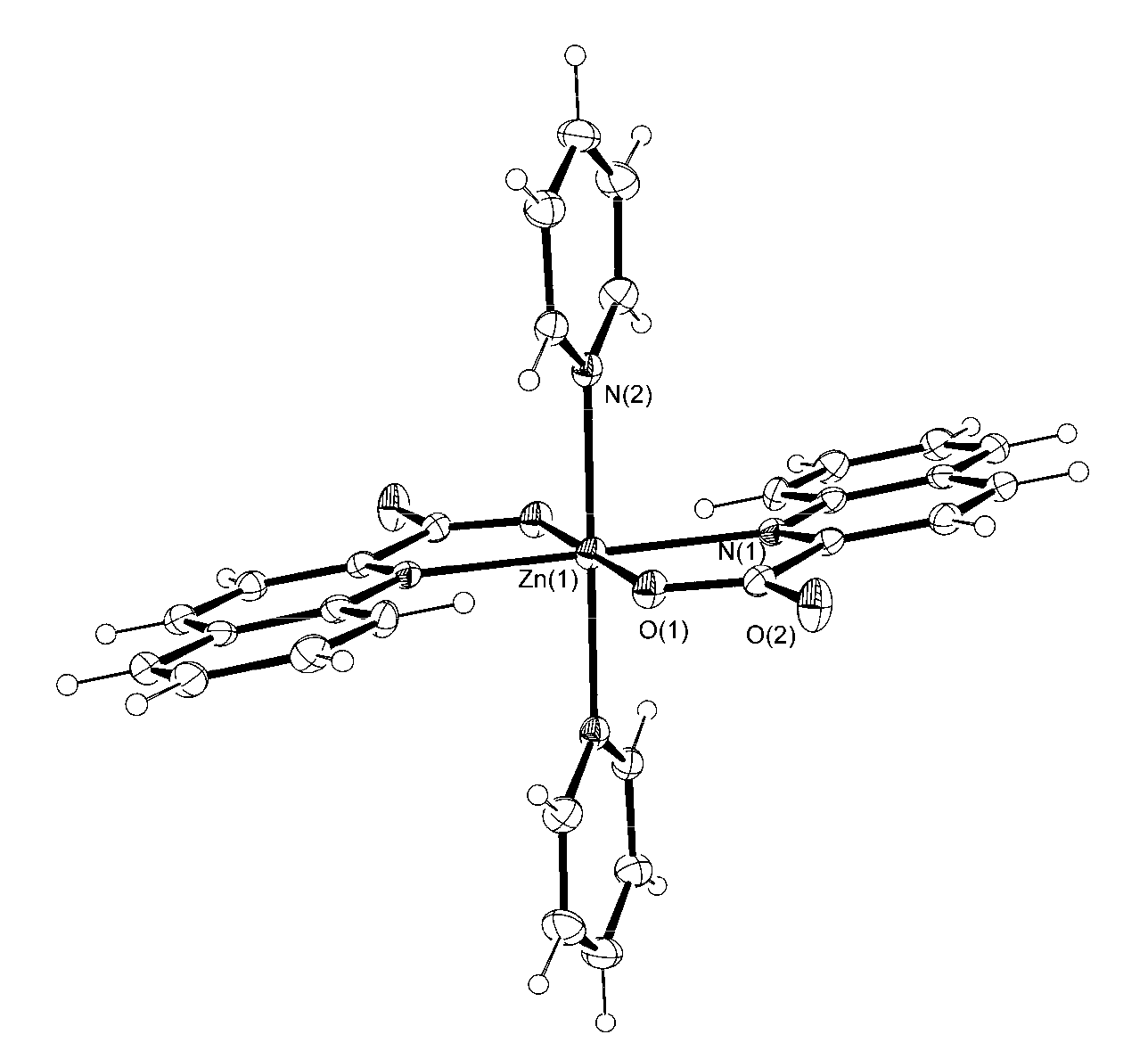
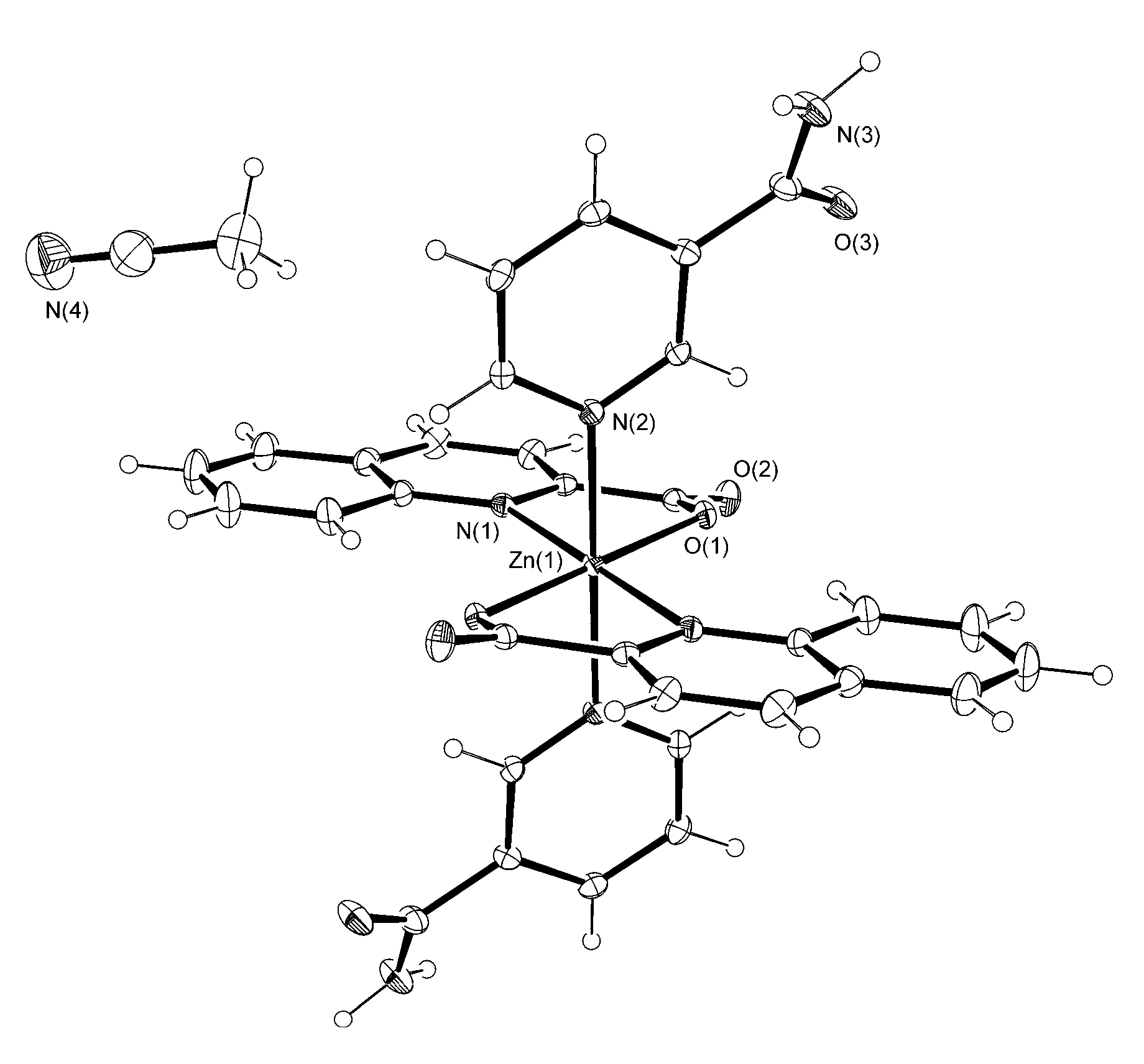
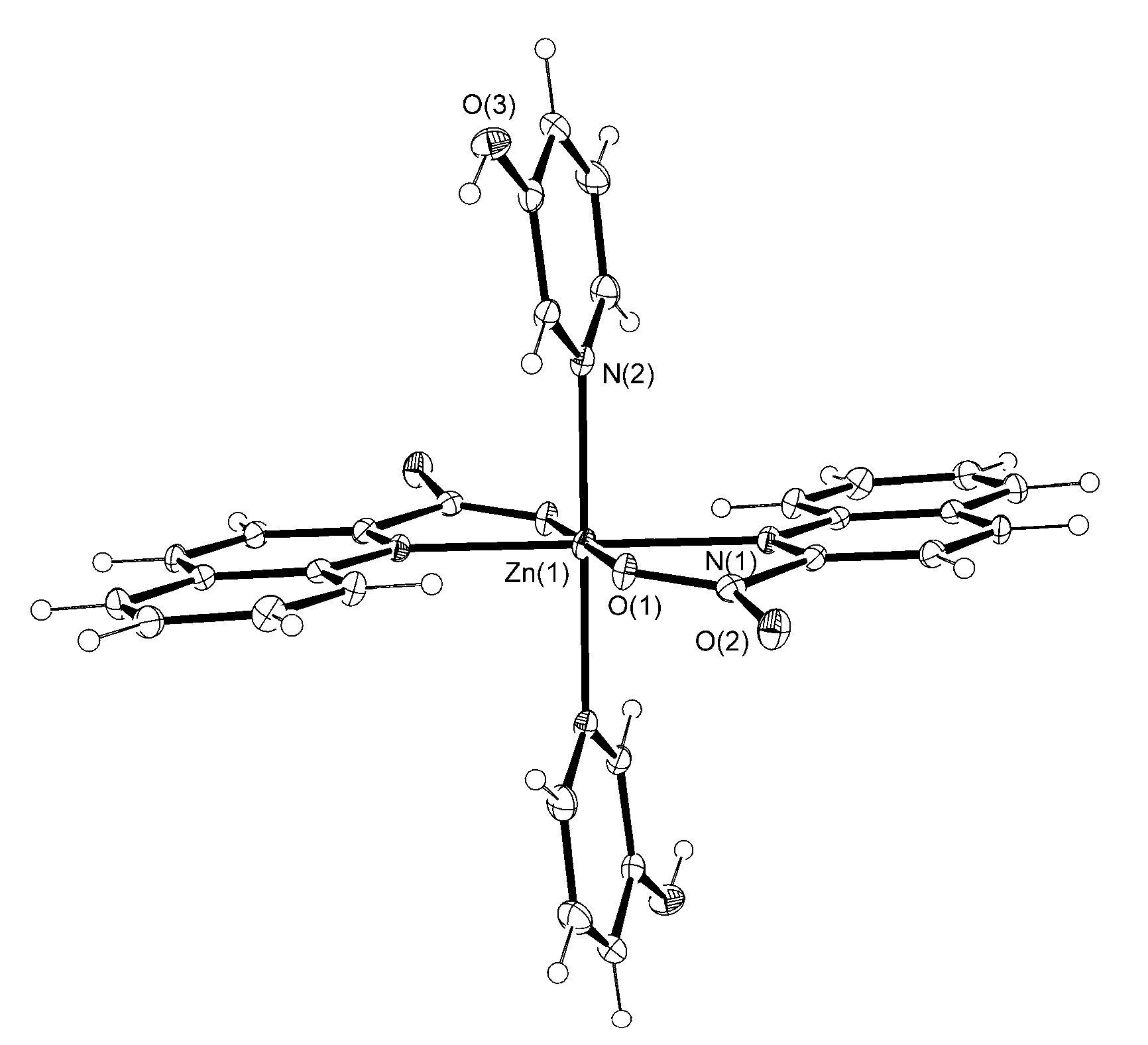
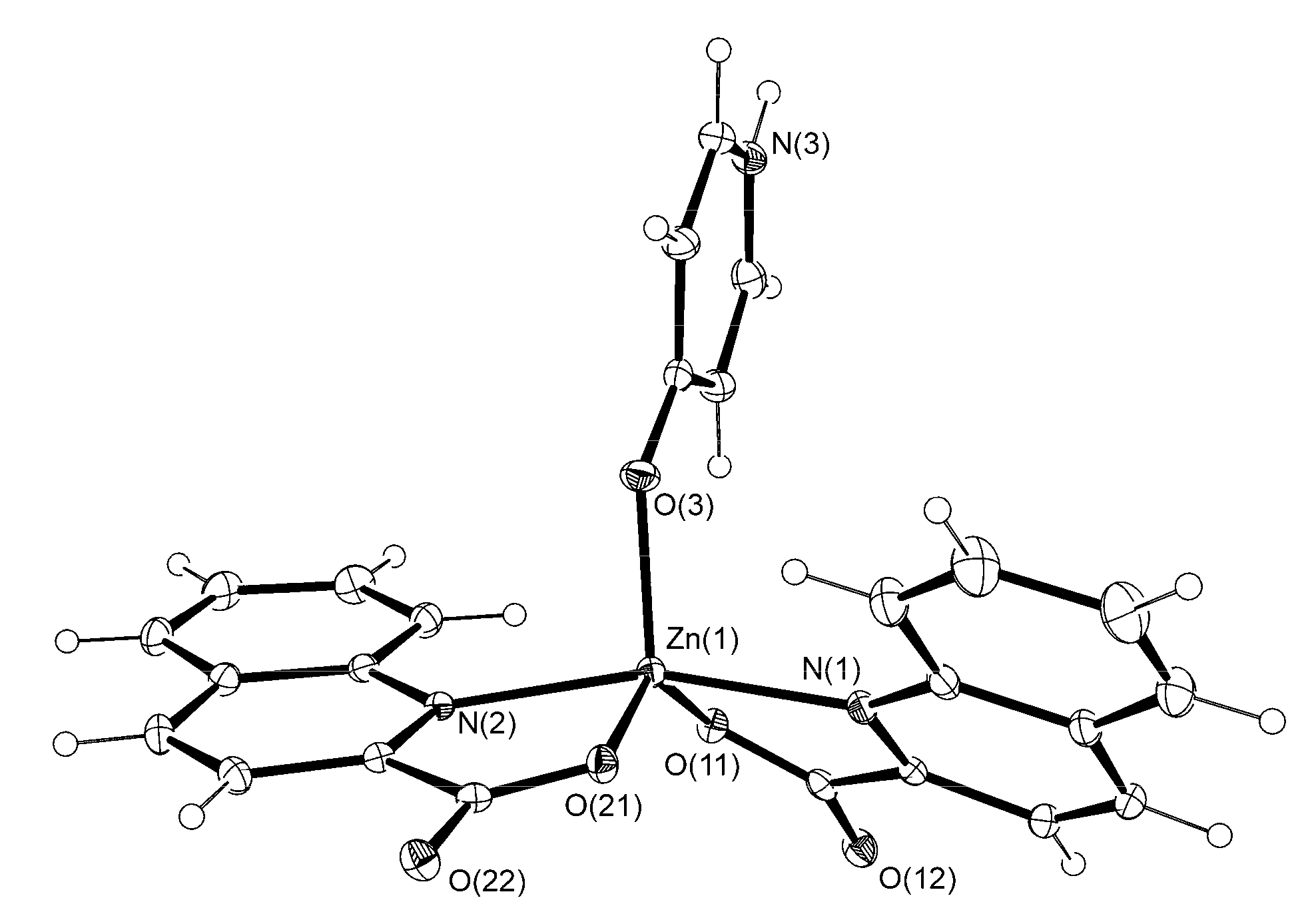
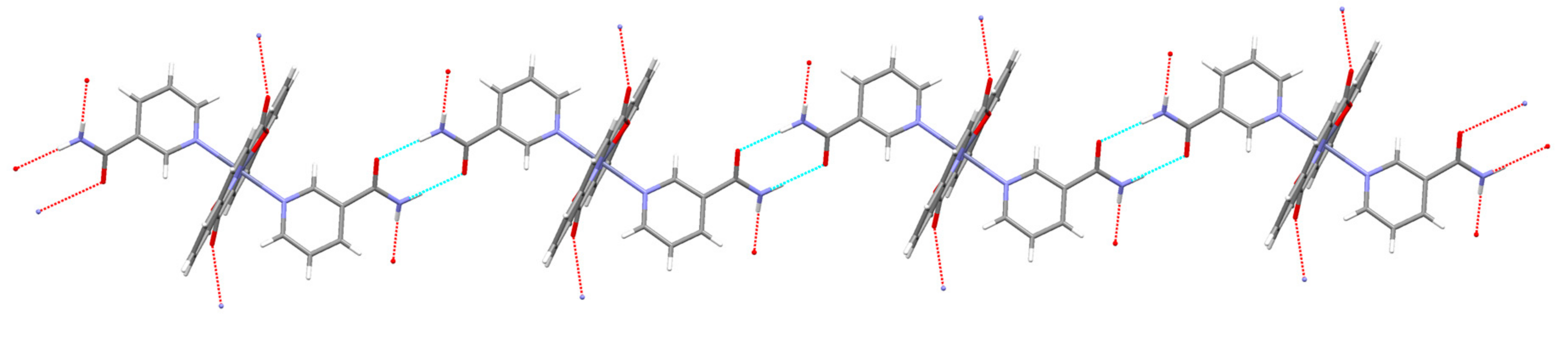
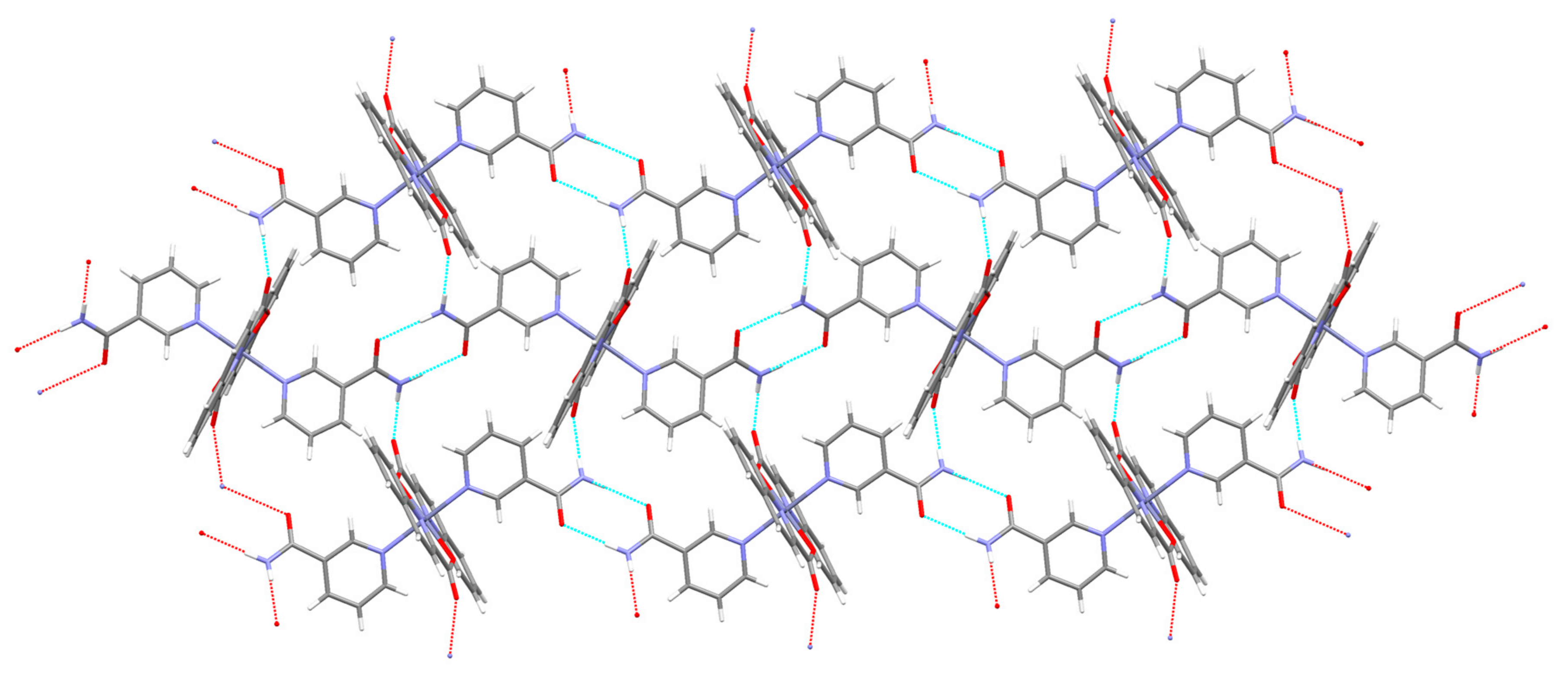
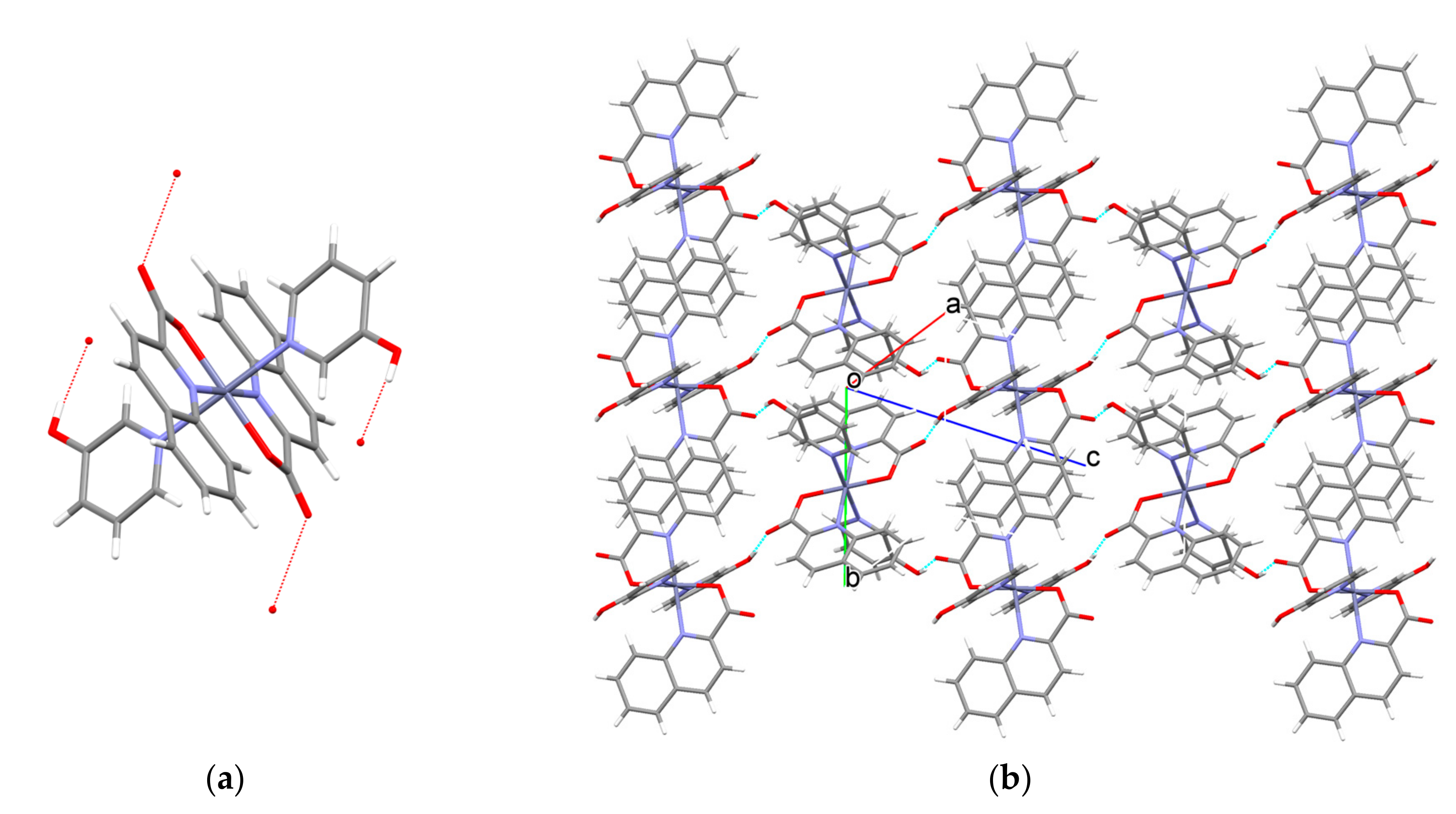
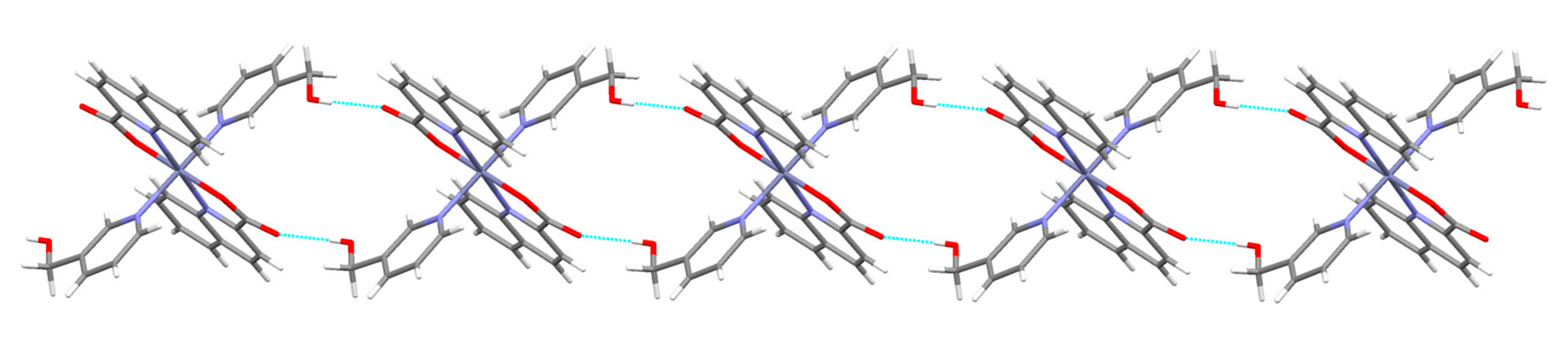
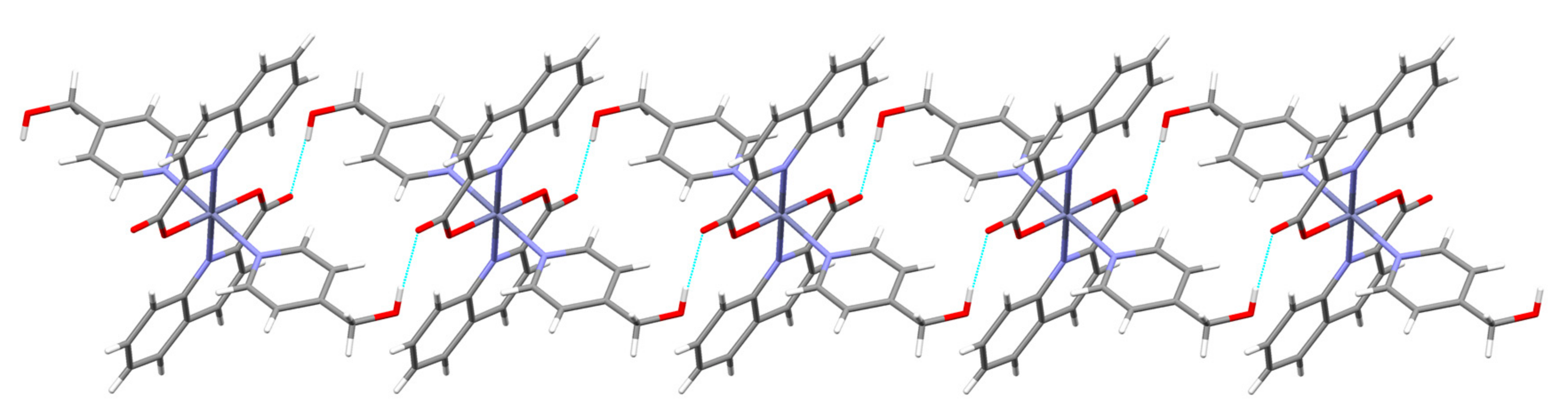
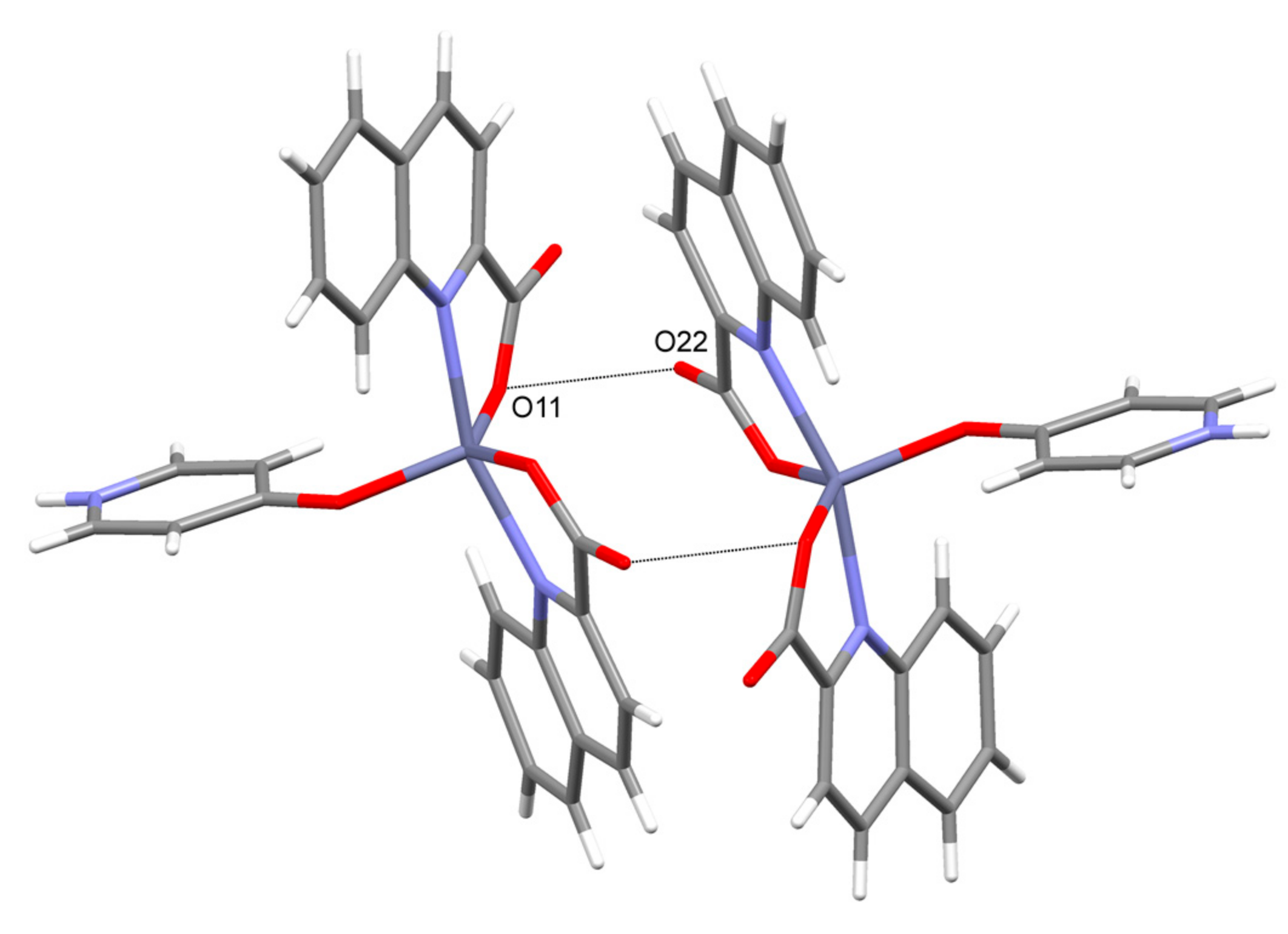
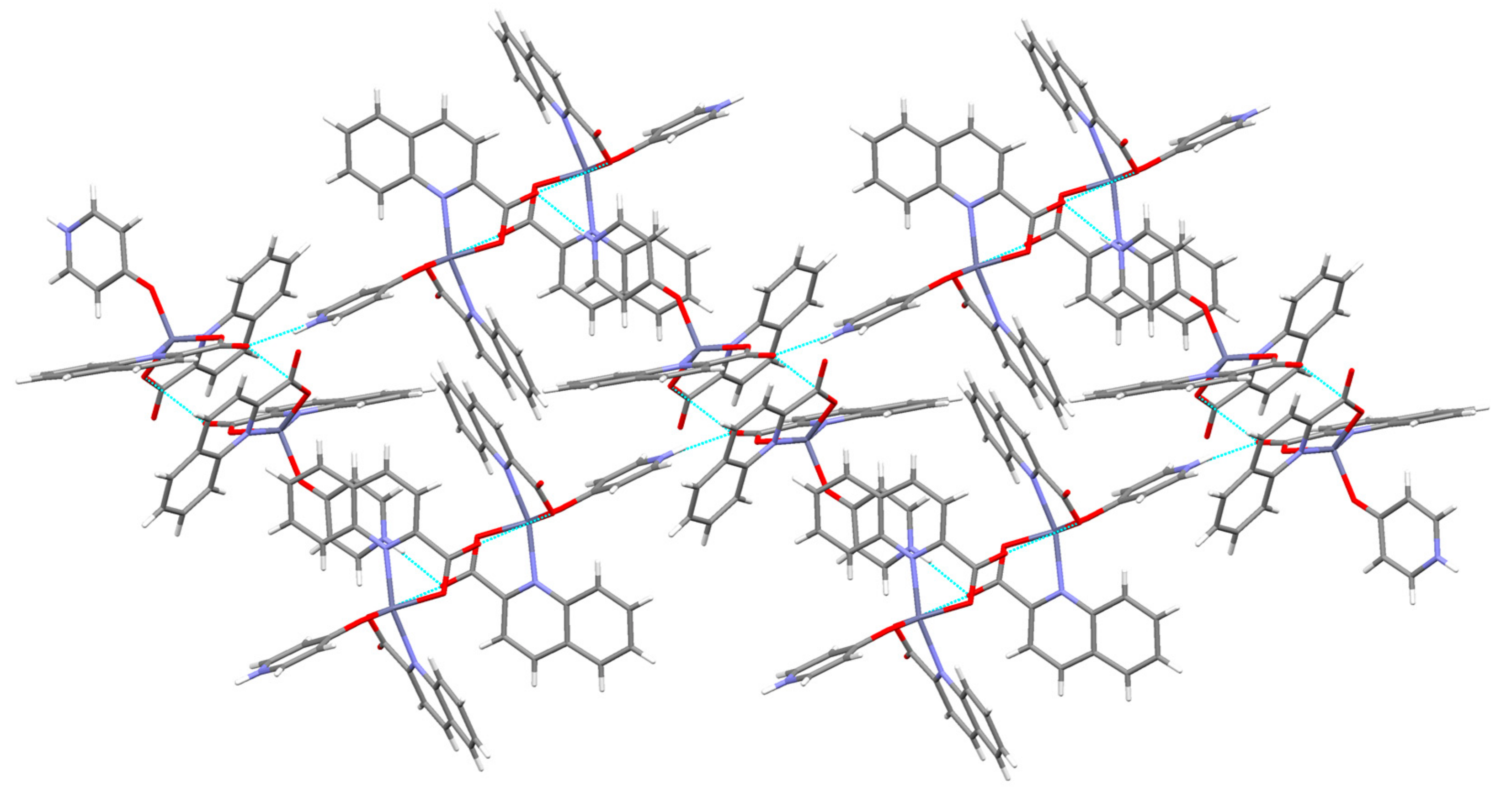
3.2. Description of Structures
3.3. Infrared Spectroscopy
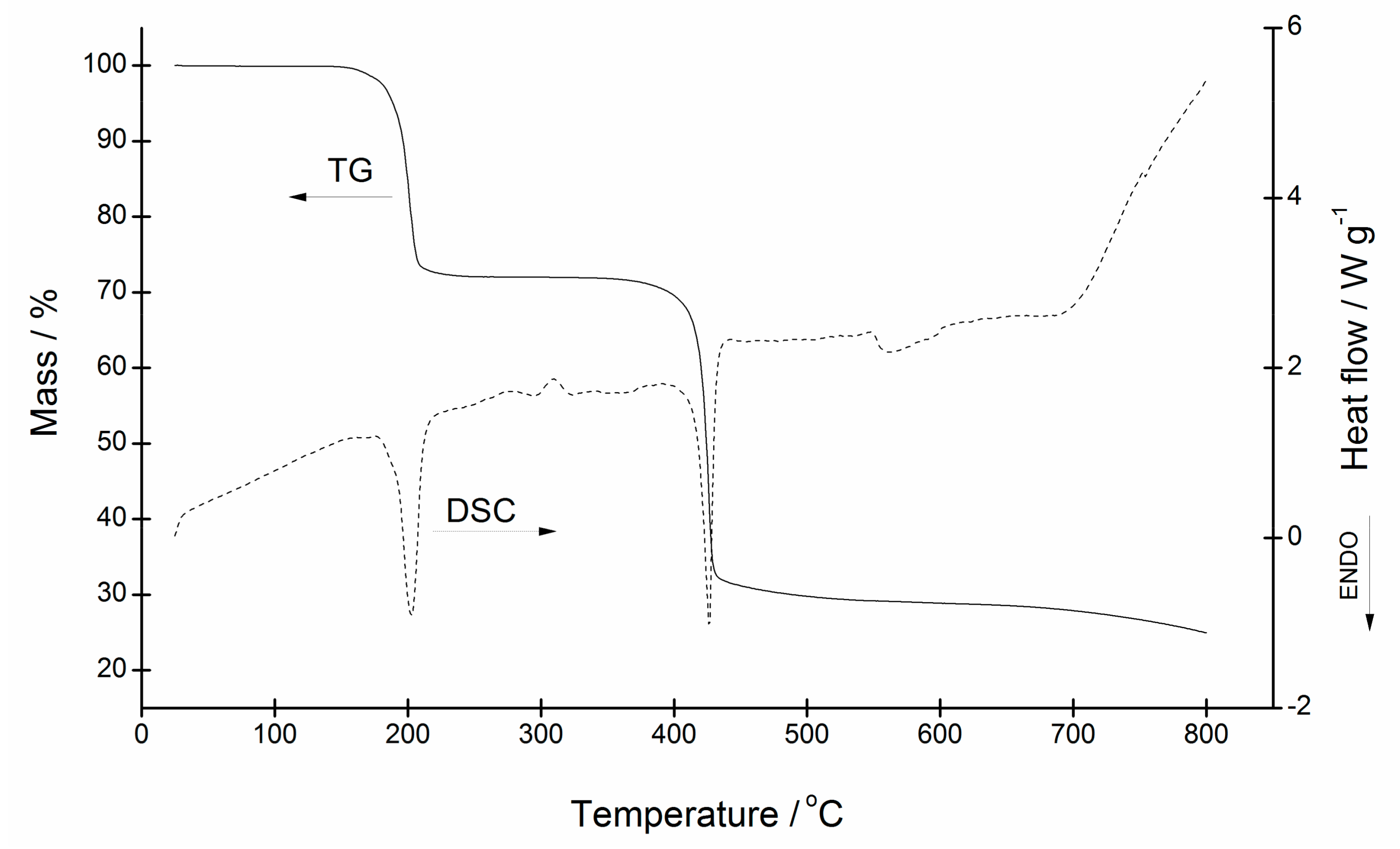
3.4. Thermal Analysis of [Zn(quin)2(Py)2] (1), [Zn(quin)2(3,5-Lut)2] (2), [Zn(quin)2(3-Hmpy)2] (5), [Zn(quin)2(4-Hmpy)2] (7), and [Zn(quin)2(4-Pyridone)] (6)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keilin, D.; Mann, T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem. J. 1940, 34, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. The structural biology of zinc. Adv. Prot. Chem. 1991, 42, 281–335. [Google Scholar]
- Aoki, S.; Kimura, E. Zinc hydrolases. In Comprehensive Coordination Chemistry; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, Nederland, 2004; pp. 601–640. [Google Scholar]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Vahrenkamp, H. Why does nature use zinc—A personal view. Dalton Trans. 2007, 4751–4759. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.F.; Lopes, A.B.; Fernandes, P.A.; Ramos, M.J. The zinc proteome: A tale of stability and functionality. Dalton Trans. 2009, 7946–7956. [Google Scholar] [CrossRef] [PubMed]
- Lipton, A.S.; Heck, R.W.; Ellis, P.D. Zinc solid-state NMR spectroscopy of human carbonic anhydrase: Implications for the enzymatic mechanism. J. Am. Chem. Soc. 2004, 126, 4735–4739. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S. The ins and outs of biological zinc sites. Biometals 2009, 22, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tarushi, A.; Kljun, J.; Turel, I.; Pantazaki, A.A.; Psomas, G.; Kessissoglou, D.P. Zinc(II) complexes with the quinolone antibacterial drug flumequine: Structure, DNA- and albumin-binding. New J. Chem. 2013, 37, 342–355. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, J.; Liu, C.; Fan, J.; Sun, Y.; Zhou, Z.; Diao, G. An asymmetric binuclear zinc(II) complex with mixed iminodiacetate and phenanthroline ligands: Synthesis, characterization, structural conversion and anticancer properties. Inorg. Chem. Front. 2016, 3, 959–968. [Google Scholar] [CrossRef]
- Indoria, S.; Lobana, T.S.; Sood, H.; Arora, D.S.; Hundal, G.; Jasinski, J.P. Synthesis, spectroscopy, structures and antimicrobial activity of mixed-ligand zinc(II) complexes of 5-nitro-salicylaldehyde thiosemicarbazones. New J. Chem. 2016, 40, 3642–3653. [Google Scholar] [CrossRef]
- Koleša-Dobravc, T.; Maejima, K.; Yoshikawa, Y.; Meden, A.; Yasui, H.; Perdih, F. Vanadium and zinc complexes of 5-cyanopicolinate and pyrazine derivatives: Synthesis, structural elucidation and in vitro insulin-mimetic activity study. New. J. Chem. 2017, 41, 735–746. [Google Scholar] [CrossRef]
- McCall, K.; Huang, C.-C.; Fierke, C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Huheey, J.E.; Keller, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed.; Harper Collins College Publishers: New York, NY, USA, 1993; pp. 394–413. ISBN 0-06-042995-X. [Google Scholar]
- Laitaoja, M.; Valjakka, J.; Janis, J. Zinc coordination spheres in protein structures. Inorg. Chem. 2013, 52, 10983–10991. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Flight, R.M.; Rouchka, E.C.; Moseley, H.N.B. A less-biased analysis of metalloproteins reveals novel zinc coordination geometries. Proteins Struct. Funct. Genet. 2015, 83, 1470–1487. [Google Scholar] [CrossRef] [PubMed]
- Parkin, G. The bioinorganic chemistry of zinc: Synthetic analogues of zinc enzymes that feature tripodal ligands. Chem. Commun. 2000, 1971–1985. [Google Scholar] [CrossRef]
- Bosch, S.; Comba, P.; Gahan, L.R.; Schenk, G. Dinuclear zinc(II) complexes with hydrogen bond donors as structural and functional phosphatase models. Inorg. Chem. 2014, 53, 9036–9051. [Google Scholar] [CrossRef] [PubMed]
- Linder, D.P.; Rodgers, K.R. Methanethiol binding strengths and deprotonation energies in Zn(II)-imidazole complexes from M05-2X and MP2 theories: Coordination number and geometry influences relevant to zinc enzymes. J. Phys. Chem. B 2015, 119, 12182–12192. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Ueda, E.; Kawabe, K.; Miyake, H.; Takino, T.; Sakurai, H.; Kojima, Y. Development of new insulinomimetic zinc(II) picolinate complexes with a Zn(N2O2) coordination mode: Structure characterization, in vitro, and in vivo studies. J. Biol. Inorg. Chem. 2002, 7, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Fan, H.-H.; Wang, H.-Z.; Chen, X.-M. A solvothermally in situ generated mixed-ligand approach for NLO-active metal-organic framework materials. Inorg. Chem. 2005, 44, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllopoulou, C.; Raptopoulou, C.P.; Terzis, A.; Janssens, J.F.; Manessi-Zoupa, E.; Perlepes, S.P.; Plakatouras, J.C. Assembly of a helical zinc(II) chain and a two-dimensional cadmium(II) coordination polymer using picolinate and sulfate anions as bridging ligands. Polyhedron 2007, 26, 4053–4064. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Polyzou, C.D.; Kostakis, G.E.; Tasiopoulos, A.J.; Roubeau, O.; Teat, S.J.; Manessi-Zoupa, E.; Powell, A.K.; Perlepes, S.P. Metal ion-assisted transformations of 2-pyridinealdoxime and hexafluorophosphate. Dalton Trans. 2012, 41, 2862–2865. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Wu, X.-F.; Weidauer, M.; Irran, E.; Döhlert, P. Exploring the coordination chemistry of 2-picolinic acid to zinc and application of the complexes in catalytic oxidation chemistry. Inorg. Chem. Commun. 2014, 46, 320–323. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Ghanbarpour, A.R.; Amini, M.M.; Ng, S.W. Bis(μ-quinoline-2-carboxylato)-κ3N,O:O;κ3O:N,O-bis[(acetato-κ2O,O′)(methanol-κO)lead(II)]. Acta Cryst. 2010, E66, m963. [Google Scholar] [CrossRef] [PubMed]
- Zurowska, B.; Mrozinski, J.; Ciunik, Z. Structure and magnetic properties of a copper(II) compound with syn-anti carboxylato- and linear Cu–Cl–Cu chloro-bridges. Polyhedron 2007, 26, 3085–3091. [Google Scholar] [CrossRef]
- Zurowska, B.; Slepokura, K. Structure and magnetic properties of polynuclear copper(II) compounds with syn-anti carboxylato- and bromo-bridges. Inorg. Chim. Acta 2008, 361, 1213–1221. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; De, P.; Mobin, S.M.; Lahiri, G.K. Influence of nitrosyl coordination on the binding mode of quinaldate in selective ruthenium frameworks. Electronic structure and reactivity aspects. RSC Adv. 2012, 2, 3437–3446. [Google Scholar] [CrossRef]
- Starynowicz, P. Structure of bis-μ-(2-quinolinecarboxylato-O,O,O′)bis[triaqua(2-quinolinecarboxylato-N,O)(2-quinolinecarboxylato-O)neodymium(III)] trihydrate. Acta Cryst. 1990, C46, 2068–2070. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Coxall, R.A.; Harris, S.G.; Henderson, D.K.; Parsons, S.; Tasker, P.A.; Winpenny, R.E.P. Inter-ligand reactions: In situ formation of new polydentate ligands. J. Chem. Soc. Dalton Trans. 2000, 2349–2356. [Google Scholar] [CrossRef]
- Yue, Z.-Y.; Cheng, C.; Gao, P.; Yan, P.-F. Trans-Bis(methanol-κO)bis(2-quinoline-carboxylato-κ2N,O)zinc(II). Acta Cryst. 2004, E60, m82–m84. [Google Scholar]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 751, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; Shuang, M.; Zhu, M.C.; Lei, L.; Gao, E.J. Synthesis, crystal structure, and photoluminescence of zinc complex [Zn(Qina)2(DMSO)2]·2DMSO. Russ. J. Coord. Chem. 2009, 35, 874–879. [Google Scholar] [CrossRef]
- CrysAlis PRO, Version 1.171.35.11; Agilent Technologies: Yarnton, Oxfordshire, UK, 2011.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, J.J. ORTEP-3 for Windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- CrystalMaker for Windows, Version 2.6.1; CrystalMaker Software, Ltd.: Oxfordshire, UK, 2007.
- Katritzky, A.R.; Lagowski, J.M. Prototropic tautomerism of heteroaromatic compounds: I. General discussion and methods of study. Adv. Heterocycl. Chem. 1963, 1, 311–338. [Google Scholar]
- Rawson, J.M.; Winpenny, R.E.P. The coordination chemistry of 2-pyridone and its derivatives. Coord. Chem. Rev. 1995, 139, 313–374. [Google Scholar] [CrossRef]
- Xu, H.; Ng, D.K.P. Construction of subphthalocyanine-porphyrin and subphthalocyanine heterodyads through axial coordination. Inorg. Chem. 2008, 47, 7921–7927. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.T.M.; Choi, C.-F.; Ng, D.K.P. Assembling tetrapyrole derivatives through axial coordination. Tetrahedron 2004, 60, 6889–6894. [Google Scholar] [CrossRef]
- Koval’chukova, O.V.; Strashnova, S.B.; Zaitsev, B.E.; Vovk, T.V. Synthesis and physicochemical properties of some transition metal complexes with 3-hydroxypyridine. Russ. J. Coord. Chem. 2002, 28, 767–770. [Google Scholar] [CrossRef]
- Zevaco, T.A.; Görls, H.; Dinjus, E. Synthesis, spectral and structural characterization of zinc carboxylate [Zn(2-quinolinecarboxylato)2(1-methylimidazole)2]. Inorg. Chim. Acta 1998, 269, 283–286. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E.; Biradha, K.; Desiraju, G.R. Hydrogen bonding in organometallic crystals. 6. X–H···M hydrogen bonds and M···(H–X) pseudo-agostic bonds. Organometallics 1997, 16, 1846–1856. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.H.; Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynska, D.; Lis, T.; Jerzykiewicz, L.B. 3D supramolecular network constructed by intermolecular interactions in mixed ligand complex of zinc. Inorg. Chem. Commun. 2005, 8, 1090–1093. [Google Scholar] [CrossRef]
- Brand, U.; Vahrenkamp, H. A new tridentate N,N,S ligand and its zinc complexes. Inorg. Chem. 1995, 34, 3285–3293. [Google Scholar] [CrossRef]
- Okabe, N.; Muranishi, Y. Aquabis(quinoline-2-carboxylato-κ2N,O)zinc(II). Acta Cryst. 2003, E59, m244–m246. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Redijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands: The crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate (τ is calculated using the equation (β–α)/60 where α and β stand for basal angles. Its value is 0 for a perfect square pyramid, and it becomes unity for a trigonal bipyramid). J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Jotani, M.M.; Arman, H.D.; Poplaukhin, P.; Tiekink, E.R.T. Bis(N,N-diethyldithiocarbamato-κ2S,S’)(3-hydroxypyridine-κN)zinc and bis[N-(2-hydroxyethyl)-N-methyldithiocarbamato-κ2S,S’](3-hydroxypyridine-κN)zinc: Crystal structures and Hirshfeld surface analysis. Acta Cryst. 2016, E72, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Goher, M.A.S.; Sodin, B.; Bitschnau, B.; Fuchs, E.C.; Mautner, F.A. Ladders, rings and cubes as structural motifs in three new zinc(II) azide complexes: Synthesis, spectral and structural characterization. Polyhedron 2008, 27, 1423–1431. [Google Scholar] [CrossRef]
- Masse, R.; Fur, Y.L. Crystal structure of bis(4-pyridone)dichlorozinc(II), ZnCl2(C5H5NO)2. Zeitschrift für Kristallographie New Cryst. Struct. 1998, 213, 114. [Google Scholar]
- Mautner, F.A.; Berger, C.; Gspan, C.; Sudy, B.; Fisher, R.C.; Massoud, S.S. Pyridyl and triazole ligands directing the assembling of zinc(II) into coordination polymers with different dimensionality through azides. Polyhedron 2017, 130, 136–144. [Google Scholar] [CrossRef]
- Feazell, R.P.; Carson, C.E.; Klausmeyer, K.K. Synthesis of a functionalized monomer of the rare M7O210+ double tetrahedron Zn cluster. Inorg. Chem. Commun. 2007, 10, 873–875. [Google Scholar] [CrossRef]
- Zelenak, V.; Cisarova, I.; Sabo, M.; Llewellyn, P.; Gyoryova, K. A Zn(II)-coordination polymer formed by benzoate and 3-pyridinemethanol ligands: Synthesis, spectroscopic properties, crystal structure and kinetics of thermal decomposition. J. Coord. Chem. 2004, 57, 87–96. [Google Scholar]
- Murugavel, R.; Kuppuswamy, S.; Boomishankar, R.; Steiner, A. Hierarchical structures built from a molecular zinc phosphate core. Angew. Chem. Int. Ed. 2006, 45, 5536–5540. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Scott, B.M.T.; Smith, M.M.; Urbina, J.F.; Desper, J. Establishing amide···amide reliability and synthon transferability in the supramolecular assembly of metal-containing one-dimensional architectures. Inorg. Chem. 2009, 48, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Douglas, B.B.; McDaniel, D.H.; Alexander, J.J. Concepts and Models of Inorganic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1994; ISBN 978-0-471-62978-8. [Google Scholar]
- Babu, N.J.; Reddy, L.S.; Nangia, A. Amide-N-oxide heterosynthon and amide dimer homosynthon in cocrystals of carboxamide drugs and pyridine N-oxides. Mol. Pharm. 2007, 4, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl-carbonyl interactions can be competitive with hydrogen bonds. Acta Cryst. 1998, B54, 320–329. [Google Scholar] [CrossRef]
- Prohens, R.; Portell, A.; Font-Bardia, M.; Bauza, A.; Frontera, A. Experimental and theoretical study of weak intermolecular interactions in crystalline tertiary squaramides. CrystEngComm 2016, 18, 6437–6443. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1997; pp. 23–30, 57–62. ISBN 0-471-16392-9. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon–oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared and Raman Spectroscopy; Academic Press International Edition: New York, NY, USA, 1964; pp. 263, 274. [Google Scholar]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Empirical formula | C30H22N4O4Zn | C34H30N4O4Zn | C36H30N8O6Zn | C30H22N4O6Zn | C32H26N4O6Zn | C25H17N3O5Zn | C32H26N4O6Zn |
| Formula weight | 567.89 | 623.99 | 736.05 | 599.89 | 627.94 | 504.79 | 627.94 |
| Crystal system | monoclinic | triclinic | monoclinic | monoclinic | triclinic | monoclinic | monoclinic |
| Space group | P 21/c | P −1 | P 21/n | P 21/n | P −1 | P 21/n | P 21/n |
| T [K] | 150(2) | 150(2) | 150(2) | 150(2) | 150(2) | 150(2) | 150(2) |
| a [Å] | 8.8768(11) | 8.0866(4) | 8.7982(4) | 9.8119(5) | 7.1034(4) | 11.2818(4) | 8.8653(6) |
| b [Å] | 9.5542(17) | 9.2741(3) | 14.0204(5) | 9.6039(4) | 9.6254(5) | 15.0187(6) | 17.9746(10) |
| c [Å] | 15.592(2) | 9.9121(4) | 14.3149(7) | 13.8912(9) | 10.7604(6) | 13.3256(6) | 9.6797(6) |
| α [°] | 90 | 96.315(3) | 90 | 90 | 97.779(5) | 90 | 90 |
| β [°] | 105.972(13) | 96.506(4) | 105.608(5) | 102.700(6) | 109.171(5) | 101.321(4) | 116.307(8) |
| γ [°] | 90 | 100.997(3) | 90 | 90 | 99.391(4) | 90 | 90 |
| V [Å3] | 1271.3(3) | 718.39(5) | 1700.69(13) | 1276.98(12) | 671.37(6) | 2213.93(15) | 1382.71(15) |
| Dcalcd [g/cm3] | 1.484 | 1.442 | 1.437 | 1.56 | 1.553 | 1.153 | 1.508 |
| Z | 2 | 1 | 2 | 2 | 1 | 4 | 2 |
| λ [Å] | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| μ [mm−1] | 1.011 | 0.902 | 0.781 | 1.017 | 0.971 | 1.153 | 0.943 |
| Collected reflections | 6568 | 6544 | 10541 | 7407 | 5739 | 12358 | 6568 |
| Unique reflections, Rint | 2899, 0.0839 | 3309, 0.0287 | 3907, 0.0490 | 2886, 0.0349 | 3077, 0.0304 | 5082, 0.0320 | 3179, 0.0267 |
| Observed reflections | 1931 | 3054 | 2849 | 2245 | 2788 | 4254 | 2621 |
| R1 1 (I >2σ(I)) | 0.052 | 0.0379 | 0.0409 | 0.034 | 0.0364 | 0.0325 | 0.0302 |
| wR2 2 (all data) | 0.1206 | 0.097 | 0.0915 | 0.075 | 0.0878 | 0.0801 | 0.0748 |
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Zn–quin− | |||||||
| Zn–N | 2.225(3) | 2.2277(16) | 2.2248(19) | 2.2298(15) | 2.2462(17) | 2.1425(15) and 2.1496(15) | 2.2214(14) |
| Zn–O | 2.0249(19) | 2.0147(14) | 2.0519(14) | 2.0391(13) | 2.0500(13) | 1.9952(13) and 2.0333(13) | 2.0208(11) |
| bite angle | 79.52(9) | 78.66(6) | 78.21(6) | 78.86(5) | 77.94(6) | 79.85(6) and 79.23(5) | 78.06(5) |
| torsion angle 1 | 6.9(4) | 4.1(3) | 11.4(3) | 13.6(3) | 7.6(3) | 6.1(2) and 6.0(2) | 2.9(2) |
| dihedral angle 2 | 8.5(5) | 5.5(3) | 11.6(4) | 13.7(3) | 7.0(3) | 7.1(3) and 7.4(3) | 2.7(3) |
| C–O | 1.230(3), 1.269(4) | 1.225(3), 1.273(2) | 1.238(3), 1.270(3) | 1.241(2), 1.263(2) | 1.235(2), 1.264(3) | 1.228(2), 1.282(2) | 1.235(2), 1.271(2) |
| Zn–pyridine ligand | |||||||
| L | Py | 3,5-Lut | Nia | 3-Py-OH | 3-Hmpy | 4-pyridone | 4-Hmpy |
| donor atom | N | N | N | N | N | O | N |
| Zn–L | 2.214(2) | 2.2395(17) | 2.1861(17) | 2.2008(17) | 2.1868(16) | 1.9602(13) | 2.2420(14) |
| Complex | Zn–N | Zn–O | Zn–L | Ref. |
|---|---|---|---|---|
| [Zn(quin)2(1-methylimidazole)2] | 2.244(2) | 2.057(2) | 2.144(2) | [49] |
| [Zn(quin)2(CH3OH)2] | 2.231(1) | 2.003(1) | 2.170(2) | [35] |
| cis-[Zn(quin)2(Him)2] | 2.293(4)–2.418(4) | 2.014(3)–2.052(3) | 2.088(4)–2.135(4) | [52] |
| [Zn(Mepa)(quin)] | 2.220(4) | 2.015(2) | / 2 | [53] |
| [Zn(quin)2(H2O)] 1 | 2.144(4), 2.147(4) | 2.017(4), 1.994(4) | 1.986(3) | [54] |
| Compound | Functions, Engaged in an Interaction 1 | Contact [Å] 2,3 |
|---|---|---|
| 3 | NH2···COO− | N(3)···O(2) [−x + 0.5, y + 0.5, −z + 0.5] = 2.885(3) |
| 4 | NH2···C=O(amide) OH···COO– | N(3)···O(3) [−x + 1, −y, −z + 1] = 2.975(3) O(3)···O(2) [−x + 1.5, y − 0.5, −z + 1.5] = 2.640(2) |
| 5 | OH···COO− | O(3)···O(2) [x + 1, y + 1, z] = 2.697(2) |
| 6 | NH(pyridone)···COO− | N(3)···O(22) [x + 1.5, −y + 0.5, z − 0.5] = 2.783(2) |
| COO−··· COO− 4 | O(11)···O(22) [−x, −y + 1, −z + 1] = 2.934(2) | |
| 7 | OH···COO− | O(3)···O(2) [−x + 1, −y, −z + 2] = 2.794(2) |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modec, B. Crystal Chemistry of Zinc Quinaldinate Complexes with Pyridine-Based Ligands. Crystals 2018, 8, 52. https://doi.org/10.3390/cryst8010052
Modec B. Crystal Chemistry of Zinc Quinaldinate Complexes with Pyridine-Based Ligands. Crystals. 2018; 8(1):52. https://doi.org/10.3390/cryst8010052
Chicago/Turabian StyleModec, Barbara. 2018. "Crystal Chemistry of Zinc Quinaldinate Complexes with Pyridine-Based Ligands" Crystals 8, no. 1: 52. https://doi.org/10.3390/cryst8010052
APA StyleModec, B. (2018). Crystal Chemistry of Zinc Quinaldinate Complexes with Pyridine-Based Ligands. Crystals, 8(1), 52. https://doi.org/10.3390/cryst8010052