Determination of the Correct Composition of “Hydrous Lead(II) Oxotellurate(IV)” as PbTeO3, Crystallizing as a New Polymorph
Abstract
1. Introduction
2. Experimental
2.1. Synthesis and Crystallization
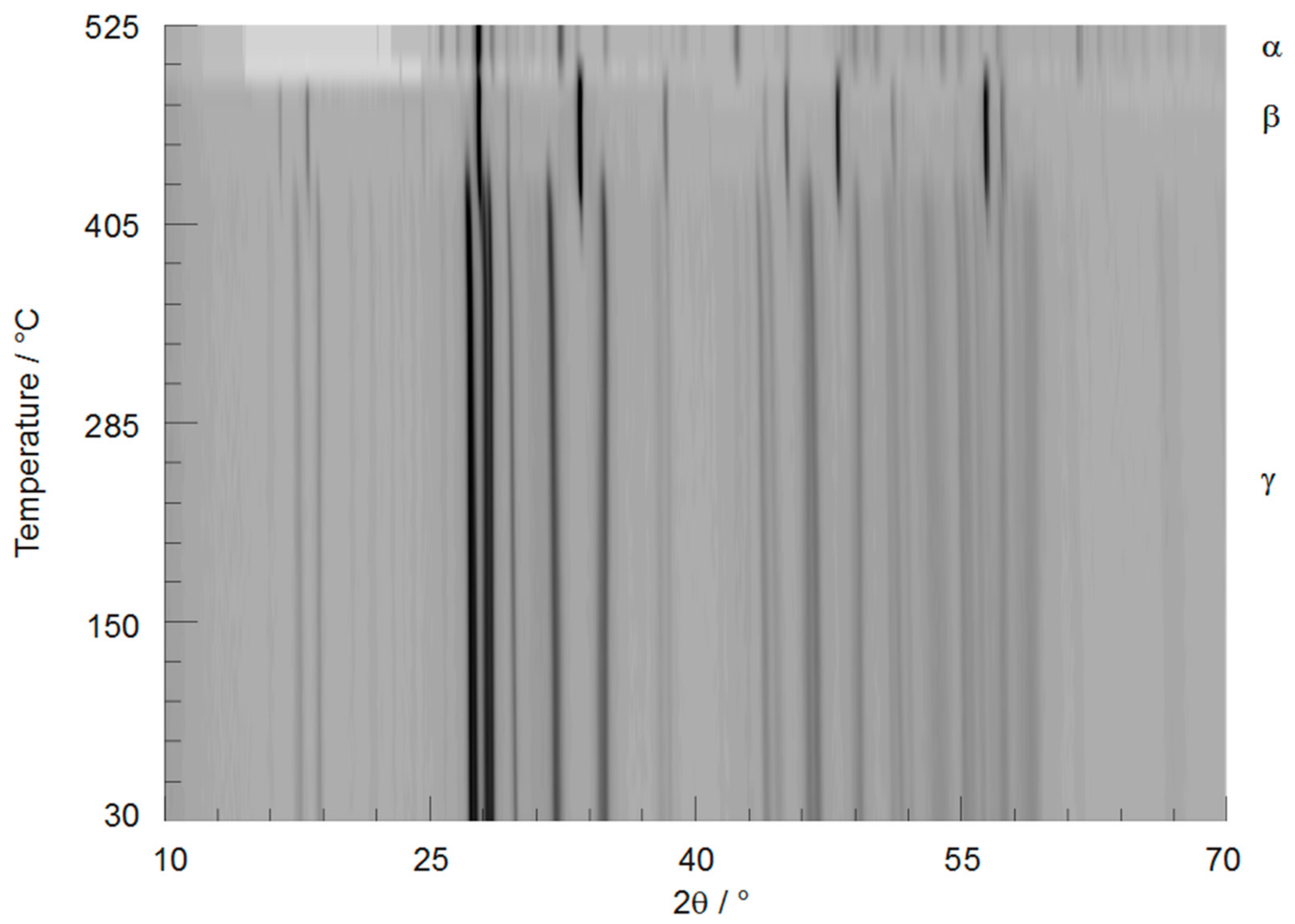
2.2. X-ray Powder Diffraction (XRPD)
2.3. Single Crystal Diffraction
2.4. Vibrational Spectroscopy
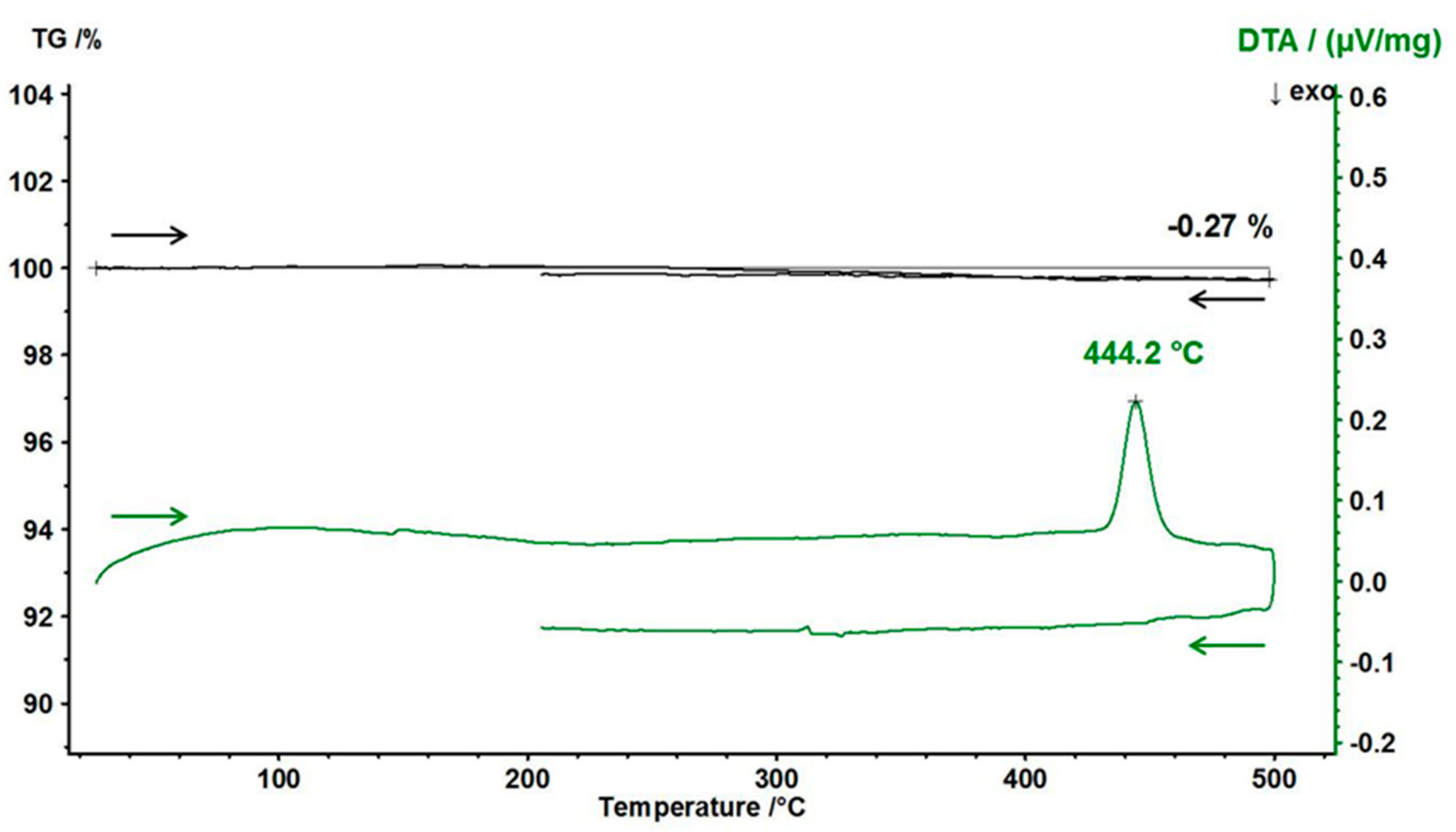
2.5. Thermal Analysis
3. Results and Discussion
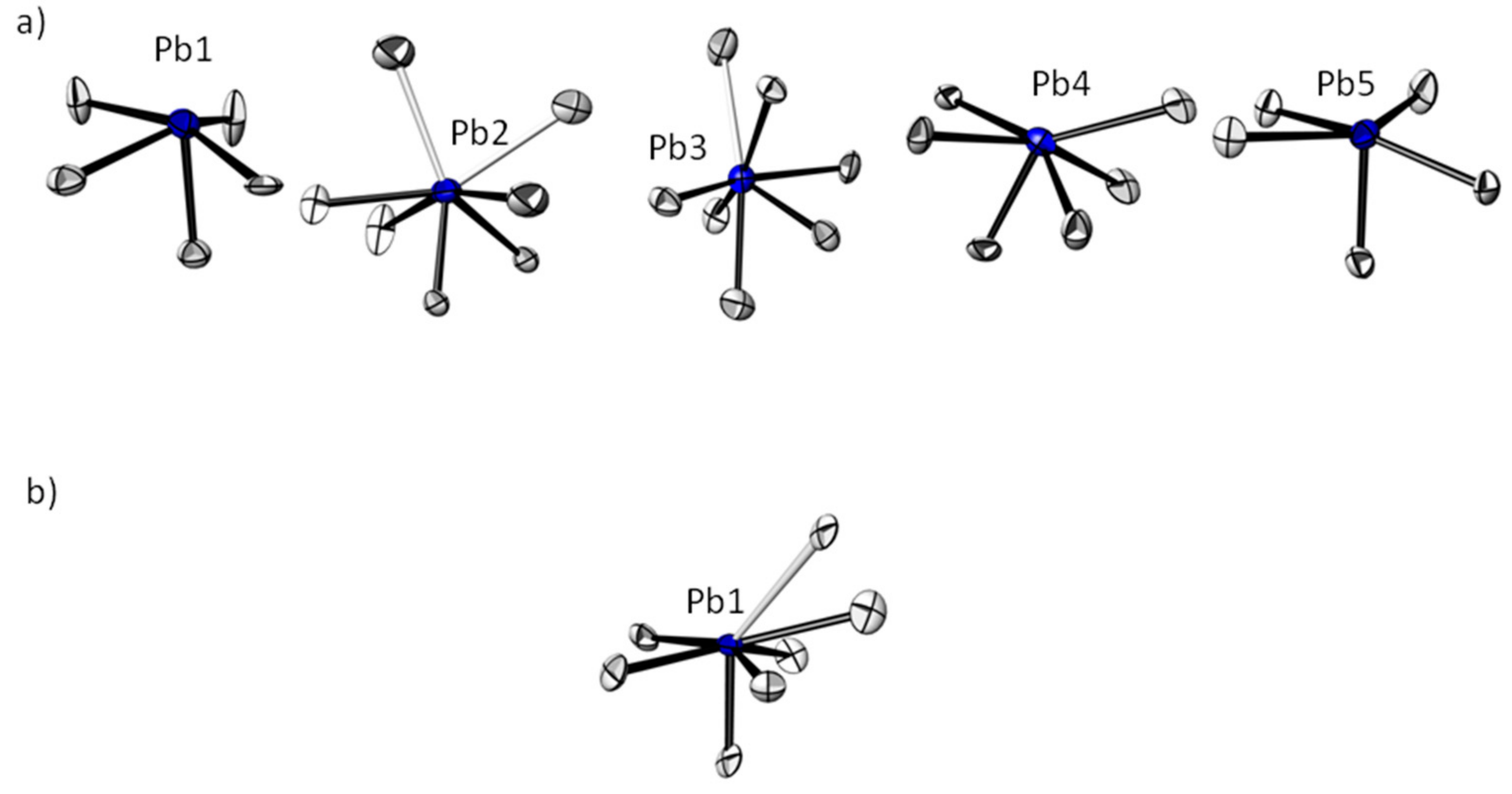
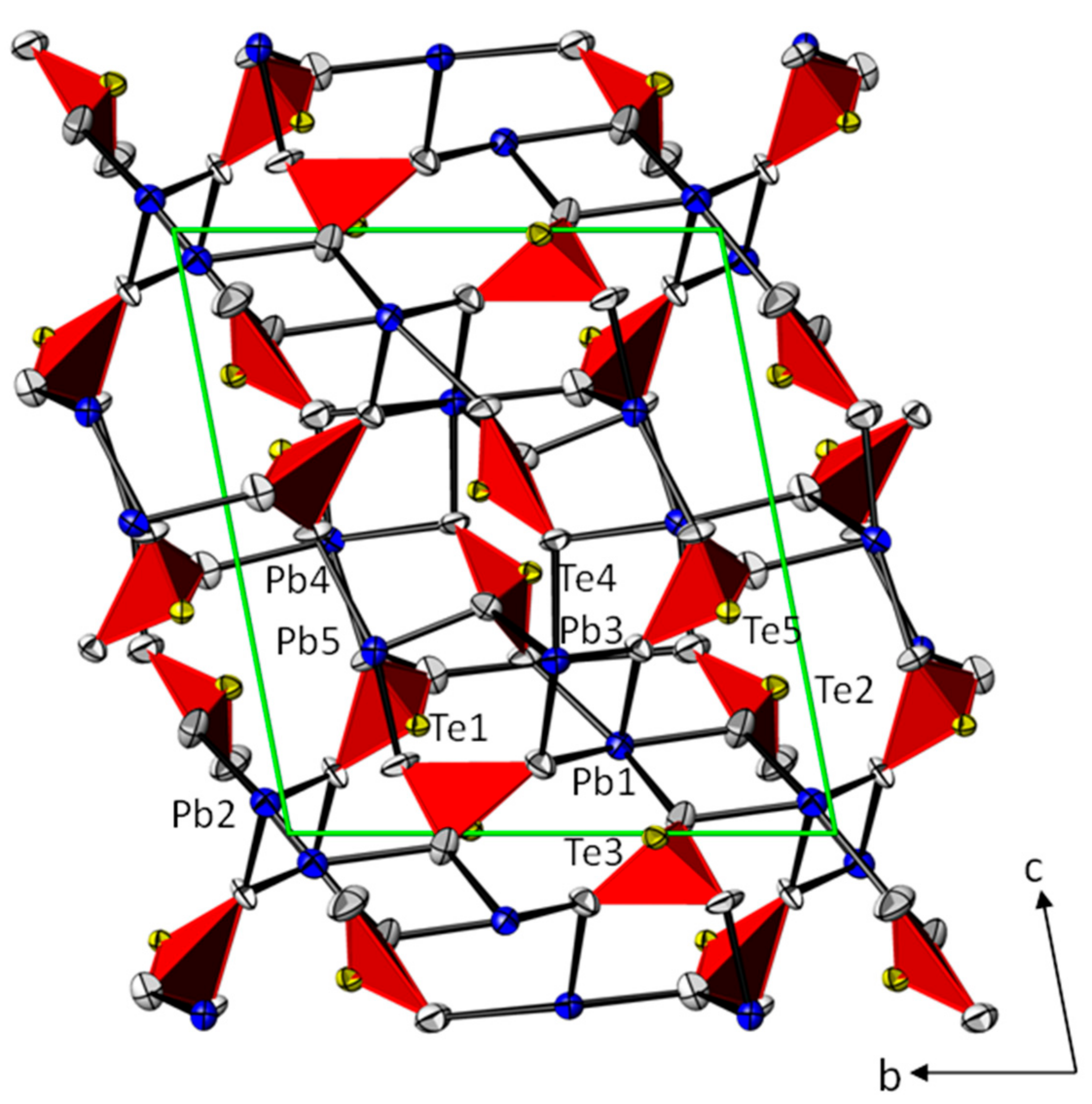
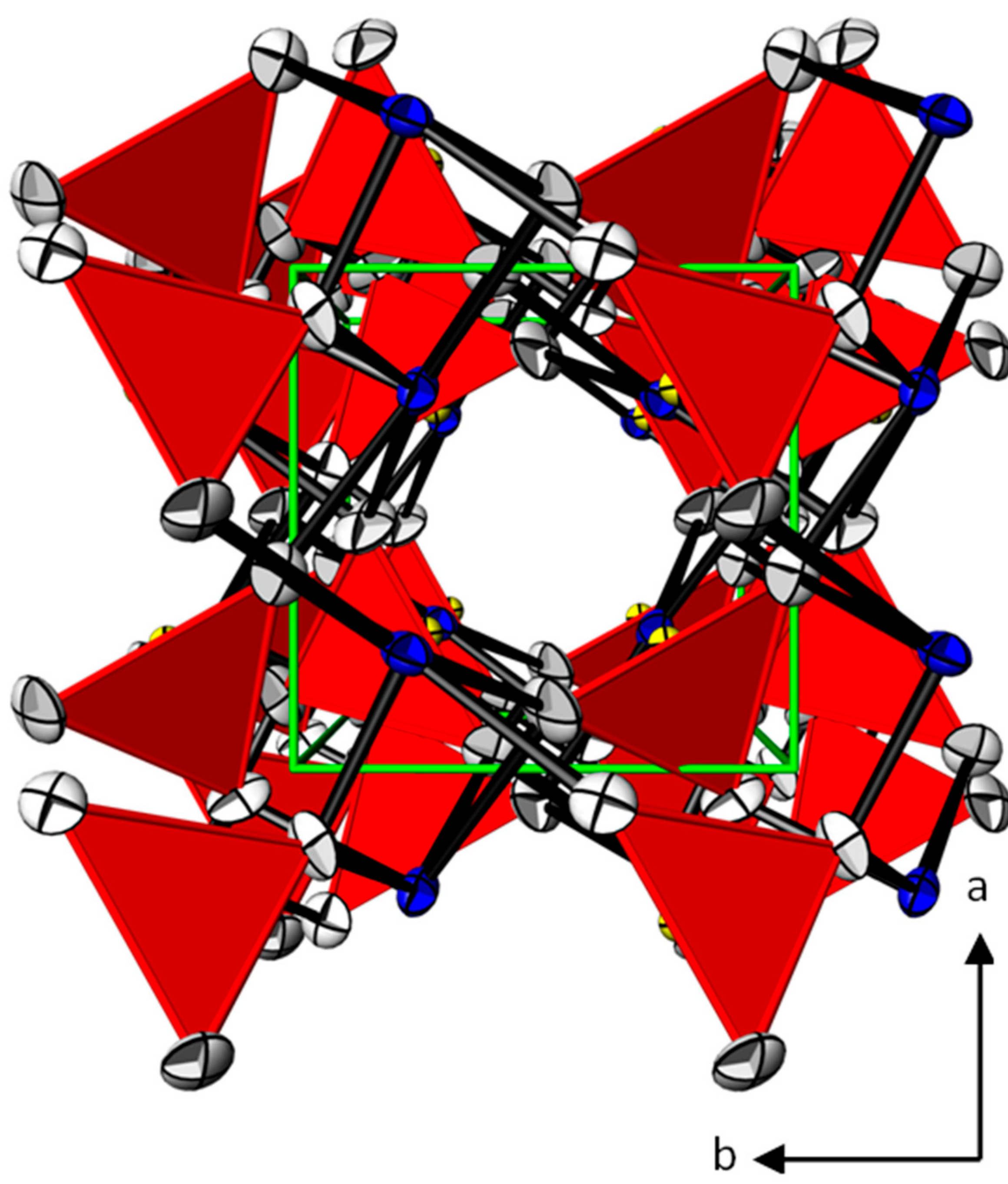
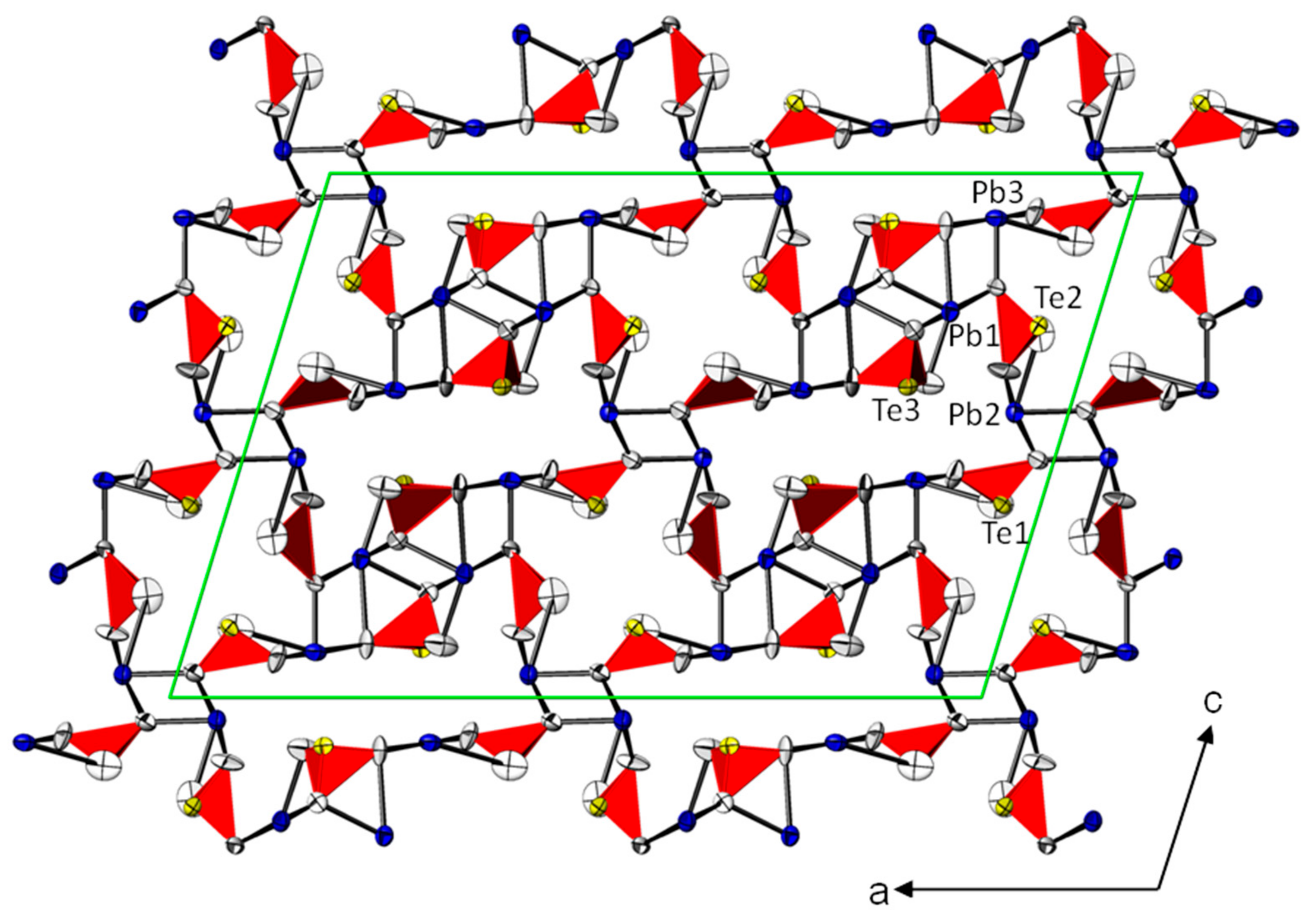
3.1. Crystal Structures of the PbTeO3 Polymorphs—General Features
3.2. Crystal Structures of the PbTeO3 Polymorphs
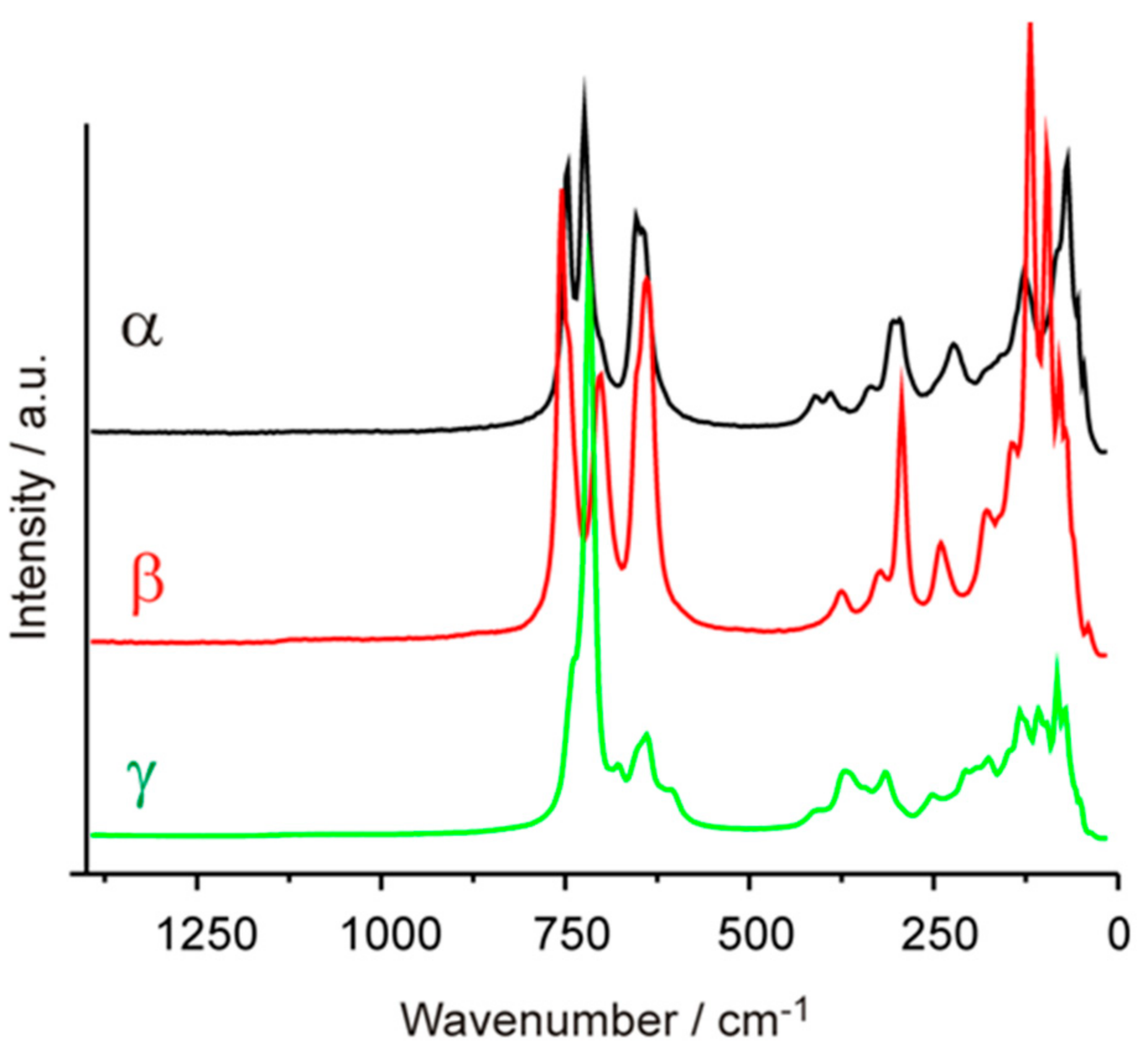
3.3. Raman Spectroscopy
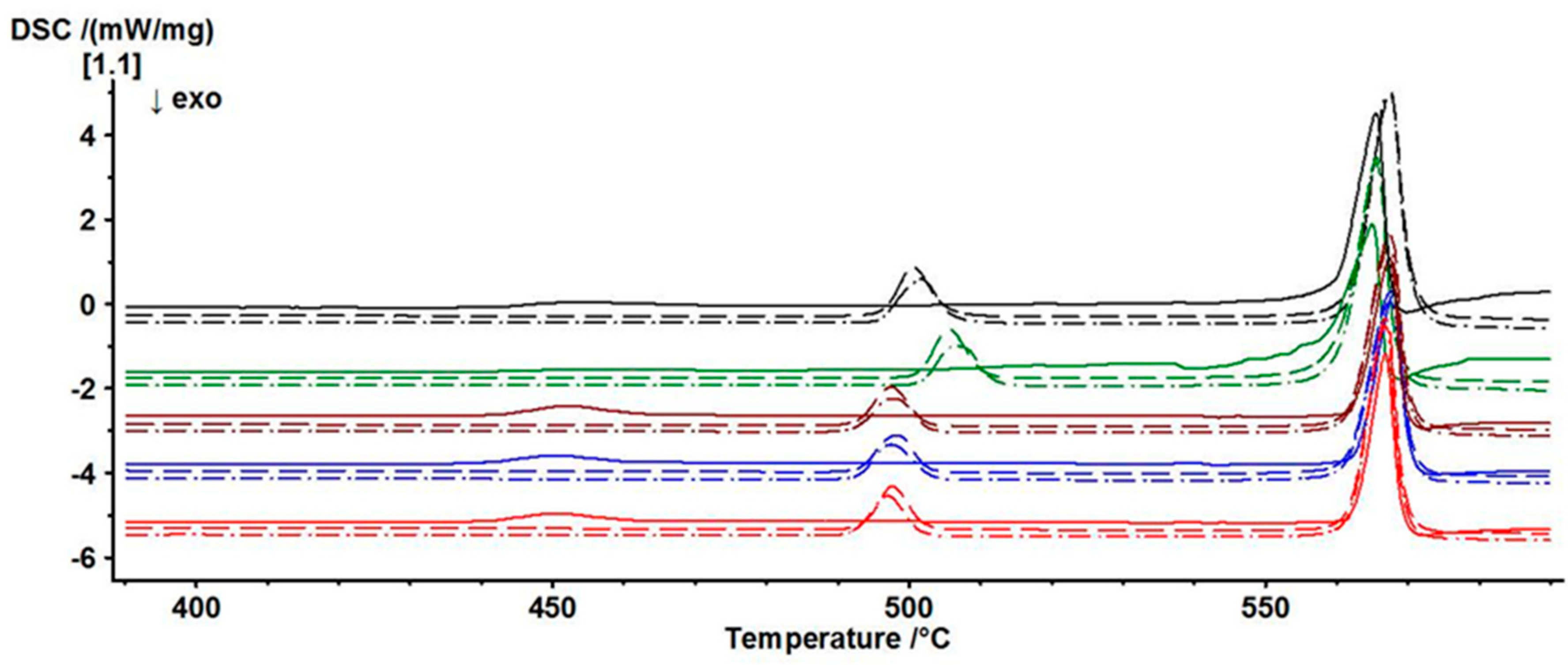
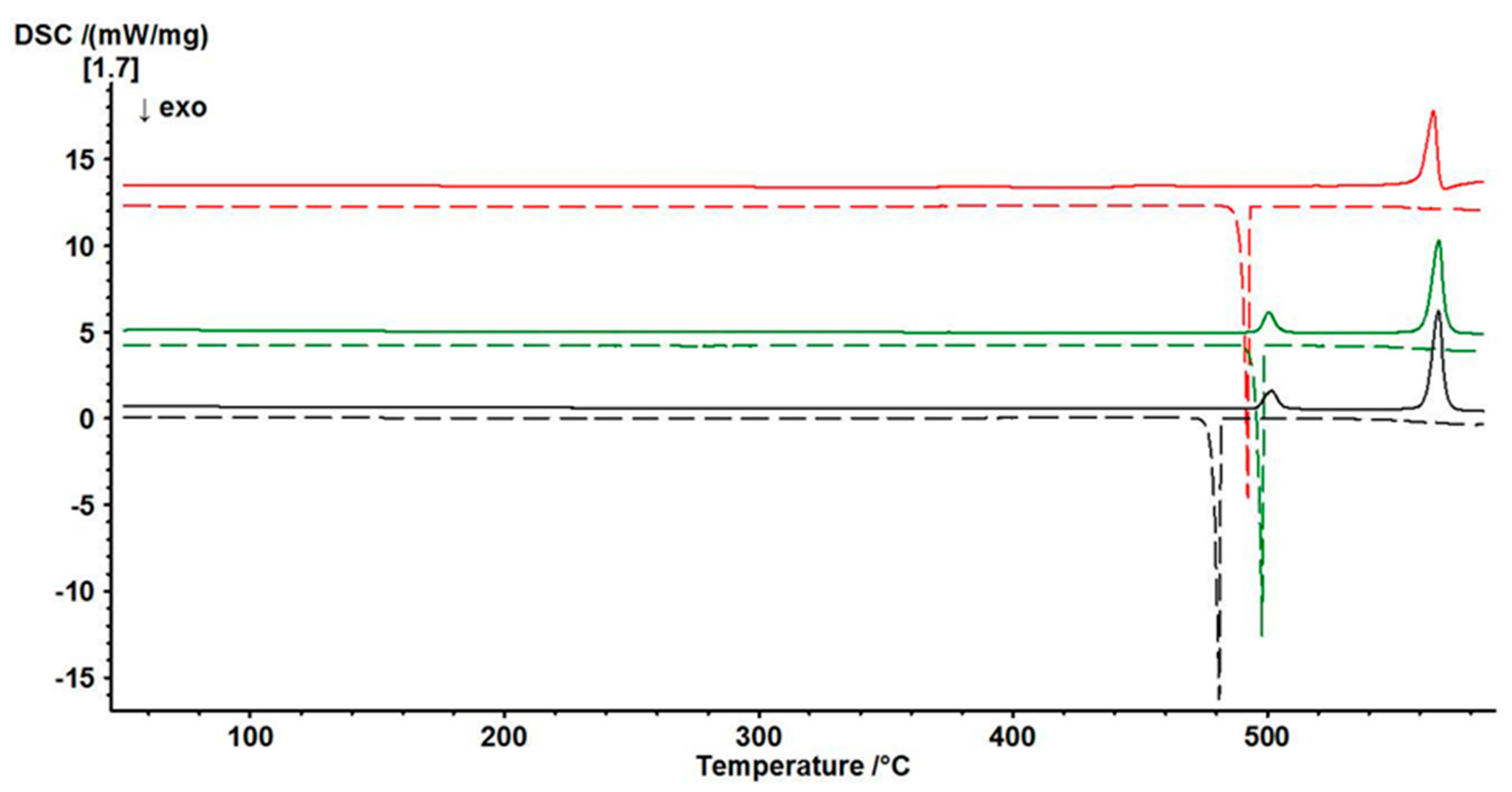
3.4. Thermal Behaviour of γ-PbTeO3
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Galy, J.; Meunier, G.; Andersson, S.; Åström, A. Stéréochimie des eléments comportant des paires non liées: Ge(II), As(III), Se(IV), Br(V), Sn(II), Sb(III), Te(IV), I(V), Xe(VI), Tl(I), Pb(II), et Bi(III) (oxydes, fluorures et oxyfluorures). J. Solid State Chem. 1975, 13, 142–159. [Google Scholar] [CrossRef]
- Shimoni-Livny, L.; Glusker, J.P.; Bock, C.W. Lone pair functionality in divalent lead compounds. Inorg. Chem. 1998, 37, 1853–1867. [Google Scholar] [CrossRef]
- Kosse, L.I.; Politova, E.D.; Bush, A.A.; Astaf’ev, A.V.; Stefanovich, S.Y.; Myzgin, E.A.; Venevtsev, Y.N. Growing and some properties of β-PbTeO3 monocrystals. Kristallografiya 1983, 28, 510–513. [Google Scholar]
- Sciau, P.; Lapasset, J.; Moret, J. Structure de la phase quadratique de PbTeO3. Acta Crystallogr. 1986, 42, 1688–1690. [Google Scholar] [CrossRef]
- Mariolacos, K. Die Kristallstruktur von PbTeO3. Anzeiger der Österreichische Akademie der Wissenschaften Mathematisch-Naturwissenschatliche Klasse 1969, 106, 129–130. [Google Scholar]
- Zavodnik, V.E.; Ivanov, S.A.; Stash, A.I. α-Lead tellurite from single-crystal data. Acta Crystallogr. 2008, 64, 16. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A. Girdite, Oboyerite, Fairbankite, and Winstanleyite, Four New Tellurium Minerals from Tombstone, Arizona. Mineral. Mag. 1979, 43, 453–457. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Tananeyva, O.I. Plumbotellurite, α-PbTeO3, a new mineral. Doklady Akademii Nauk SSSR 1982, 262, 1231–1235. [Google Scholar]
- Gaitan, M.; Jerez, A.; Noguerales, P.; Pico, C.; Veiga, M.L. New Methods of synthesis of mixed oxides of Te and Pb: characterization of the new phases PbTeO3 (cubic) and PbTeO4 (orthorhombic). Synth. React. Inorg. Met–Org. Chem. 1987, 17, 479–490. [Google Scholar] [CrossRef]
- Tananaeva, O.I.; Latypova, Z.K.; Novoselova, A.V. Study of PbTe-PbTeO3 and PbTeO4-PbO cross-sections of Pb-Te-O system. Izvestiya Akademii Nauk SSSR Neorganicheskie Materialy 1977, 13, 386–387. [Google Scholar]
- Young, I.M. The central region of the PbO/TeO2 phase diagram. J. Mater. Sci. 1979, 14, 1579–1585. [Google Scholar] [CrossRef]
- Stavrakieva, D.; Ivanova, Y.; Pyrov, J. On the composition of the crystal phases in the PbO-TeO2 system. J. Mater. Sci. 1988, 23, 1871–1876. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Berchenko, N.; Fadeev, S.; Savchyn, V.; Kurbanov, K.; Trzyna, M.; Cebulski, J. Pb–Te–O phase equilibrium diagram and the lead telluride thermal oxidation. Thermochim. Acta 2014, 579, 64–69. [Google Scholar] [CrossRef]
- El-Rabaie, S.; Taha, T.A.; Higazy, A.A. PbTe quantum dots formation in a novel germanate glass. J. Alloys Compd. 2014, 594, 102–106. [Google Scholar] [CrossRef]
- Scheele, M.; Oeschler, N.; Veremchuk, I.; Peters, S.O.; Littig, A.; Kornowski, A.; Klinke, C.; Weller, H. Thermoelectric properties of lead chalcogenide core–shell nanostructures. ACS Nano 2011, 5, 8541–8551. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, J. Chimie douce approaches to the synthesis of metastable oxide materials. Chem. Mater. 1995, 7, 1265–1275. [Google Scholar] [CrossRef]
- Stöger, B.; Weil, M.; Baran, E.J.; González-Baró, A.C.; Malo, S.; Rueff, J.M.; Petit, S.; Lepetit, M.B.; Raveau, B.; Barrier, N. The dehydration of SrTeO3(H2O)—A topotactic reaction for preparation of the new metastable strontium oxotellurate(IV) phase ε-SrTeO3. Dalton Trans. 2011, 40, 5538–5548. [Google Scholar] [CrossRef] [PubMed]
- Tananaeva, O.I.; Novoselova, A.V. Thermal stability of lead tellurite(IV) and tellurate(VI). Izvestiya Akademii Nauk SSSR Neorganicheskie Materialy 1967, 3, 114–118. [Google Scholar]
- Berchenko, M.A.; Belyaev, A.I. Preparation of lead, barium, and copper tellurites by precipitation from solution. Zhurnal Neorganicheskoi Khimii 1967, 12, 1774–1781. [Google Scholar]
- Champarnaud-Mesjard, J.C.; Thomas, P.; Colas-Dutreilh, M.; Oufkir, A. Crystal structure of dilead tritellurate (IV), Pb2Te3O8. Z. Kristallogr. New Cryst. Struct. 2001, 216, 185–186. [Google Scholar]
- Artner, C.; Weil, M. Re-examination of “Pb3TeO6”: Determination of its correct composition as Pb5TeO8. J. Solid State Chem. 2013, 199, 240–247. [Google Scholar] [CrossRef]
- APEX-2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2013.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Herrendorf, W. HABITUS; Universities of Karlsruhe and Gießen: Karlsruhe, Germany, 1997. [Google Scholar]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Krivovichev, S.V.; Brown, I.D. Are the compressive effects of encapsulation an artifact of the bond valence parameters? Z. Kristallogr. 2001, 216, 245–247. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Dowty, E. ATOMS for Windows, Version 6.3; Shape Software: Kingsport, TN, USA, 2006.
- Christy, A.G.; Mills, S.J.; Kampf, A.R. A review of the structural architecture of tellurium oxycompounds. Mineral. Mag. 2016, 80, 415–545. [Google Scholar] [CrossRef]
- Zemann, J. Zur Stereochemie des Te(IV) gegenüber Sauerstoff. Monatsh. Chem. 1971, 102, 1209–1216. [Google Scholar] [CrossRef]
- Bondi, A. Van der waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Weidlein, J.; Müller, U.; Dehnicke, K. Schwingungsspektroskopie; Thieme Verlag Press: Stuttgart, Baden-Württemberg, Germany, 1988. [Google Scholar]
- Farmer, V.C. (Ed.) Mineralogical Society Monograph 4: The Infrared Spectra of Minerals; The Mineralogical Society Press: London, UK, 1974. [Google Scholar]
- Rieger, F.; Mudring, A.V. Phase Transition in Tl2TeO3: Influence and origin of the thallium lone pair distortion. Inorg. Chem. 2007, 46, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Keeffe, E.C. Raman spectroscopic study of the tellurite mineral: Sonoraite Fe3+Te4+O3(OH)·H2O. J. Raman Spectrosc. 2009, 40, 133–136. [Google Scholar] [CrossRef]
| Formula | PbTeO3 | |
|---|---|---|
| Modification | γ- | β- |
| Diffractometer | Bruker APEX-II CCD | |
| Radiation; wavelength/Å | Mo K; 0.71073 | |
| Temperature/°C | 22(1) | |
| Formula weight | 382.79 | |
| Space group (No.) | P (2) | P41 (76) |
| Crystal description | colourless block | colourless fragment |
| Crystal dimensions/mm3 | 0.022 × 0.034 × 0.045 | 0.060 × 0.080 × 0.080 |
| Formula units Z | 10 | 4 |
| a/Å | 7.0185(3) | 5.3193(6) |
| b/Å | 10.6166(4) | |
| c/Å | 11.9616(5) | 11.9326(15) |
| α/° | 78.548(3) | |
| β/° | 82.992(2) | |
| γ/° | 84.048(2) | |
| Volume/Å3 | 864.12(6) | 337.63(9) |
| μ/mm−1 | 56.887 | 58.238 |
| X-ray density/g·cm−3 | 7.356 | 7.531 |
| Rangeθmin–θmax/° | 1.75 → 41.40 | 3.83 → 29.98 |
| Range h; k; l | −11 → 13; −19 → 19; −22 → 22 | −7 → 7; −5 → 7; −16 → 16 |
| Measured reflections | 61288 | 3495 |
| Independent reflections | 11367 | 976 |
| Observed reflections [I > 2σ(I)] | 6799 | 622 |
| Ri | 0.075 | 0.034 |
| Absorption correction | numerical, HABITUS [25] | numerical, SADABS [23] |
| Coef. Of Transmission Tmin; Tmax | 0.177; 0.409 | 0.383; 0.750 |
| Structure solution and refinement | SHELXTL [24] | |
| Number of parameters | 227 | 46 |
| Extinction coefficient (SHELXTL) | 0.000504(5) | - |
| Absolute structure parameter | Flack x = −0.017(6) | |
| Difference electron density/e·Å−3, with distance to atom/Å | Δρmax = 2.41 [0.67; Pb3]; Δρmin = −2.53 [0.48; Pb2] | Δρmax = 1.03 [1.14; O3]; Δρmin = −2.52 [0.68; Pb1] |
| R[F2 > 2σ(F2)]; wR(F2 all) | 0.0289; 0.0360 | 0.0301; 0.0722 |
| Goof | 0.653 | 1.037 |
| CSD number | 432744 | 432745 |
| γ-PbTeO3 | |||||||
|---|---|---|---|---|---|---|---|
| Pb1 | – | O1 | 2.293(3) | Te1 | – | O6 | 1.868(3) |
| Pb1 | – | O12 | 2.366(3) | Te1 | – | O3 | 1.872(3) |
| Pb1 | – | O14 | 2.387(3) | Te1 | – | O2 | 1.903(3) |
| Pb1 | – | O7 | 2.485(3) | Te2 | – | O15 | 1.851(3) |
| Pb1 | – | O13 | 2.673(3) | Te2 | – | O10 | 1.882(3) |
| Pb2 | – | O2 | 2.309(3) | Te2 | – | O12 | 1.885(3) |
| Pb2 | – | O12 | 2.527(3) | Te3 | – | O14 | 1.863(3) |
| Pb2 | – | O2 | 2.540(3) | Te3 | – | O7 | 1.872(3) |
| Pb2 | – | O14 | 2.571(3) | Te3 | – | O11 | 1.875(3) |
| Pb2 | – | O15 | 2.624(4) | Te4 | – | O13 | 1.866(3) |
| Pb2 | – | O11 | 2.916(3) | Te4 | – | O8 | 1.871(3) |
| Pb2 | – | O15 | 2.948(4) | Te4 | – | O9 | 1.919(3) |
| Pb3 | – | O8 | 2.405(3) | Te5 | – | O5 | 1.868(3) |
| Pb3 | – | O3 | 2.470(3) | Te5 | – | O4 | 1.872(3) |
| Pb3 | – | O9 | 2.484(3) | Te5 | – | O1 | 1.900(3) |
| Pb3 | – | O7 | 2.549(3) | ||||
| Pb3 | – | O13 | 2.729(3) | O6–Te1–O3 | 98.43(14) | ||
| Pb3 | – | O1 | 2.748(3) | O6–Te1–O2 | 90.87(13) | ||
| Pb3 | – | O10 | 2.837(3) | O3–Te1–O2 | 99.32(13) | ||
| Pb4 | – | O4 | 2.366(3) | O15–Te2–O10 | 97.23(14) | ||
| Pb4 | – | O10 | 2.457(3) | O15–Te2–O12 | 101.14(16) | ||
| Pb4 | – | O5 | 2.505(3) | O10–Te2–O12 | 98.19(15) | ||
| Pb4 | – | O8 | 2.558(3) | O14–Te3–O7 | 100.31(14) | ||
| Pb4 | – | O6 | 2.561(3) | O14–Te3–O11 | 97.59(14) | ||
| Pb4 | – | O5 | 2.736(3) | O7–Te3–O11 | 96.61(14) | ||
| Pb5 | – | O11 | 2.320(3) | O13–Te4–O8 | 102.54(13) | ||
| Pb5 | – | O9 | 2.334(3) | O13–Te4–O9 | 96.33(13) | ||
| Pb5 | – | O6 | 2.363(3) | O8–Te4–O9 | 97.58(14) | ||
| Pb5 | – | O3 | 2.446(3) | O5–Te5–O4 | 97.42(14) | ||
| Pb5 | – | O4 | 2.755(3) | O5–Te5–O1 | 101.38(13) | ||
| O4–Te5–O1 | 95.96(13) | ||||||
| BVS. Pb1 1.95, Pb2 1.94, Pb3 1.99, Pb4 1.93, Pb5 1.96, Te1 3.89, Te2 3.98, Te3 4.02, Te4 3.85, Te5 3.90, O1 (1 Te, 2 Pb) 1.95, O2 (1 Te, 2 Pb) 2.02, O3 (1 Te, 2 Pb) 2.06, O4 (1 Te, 2 Pb) 1.96, O5 (1 Te, 2 Pb) 1.88, O6 (1 Te, 2 Pb) 2.08, O7 (1 Te, 2 Pb) 1.98, O8 (1 Te, 2 Pb) 2.03, O9 (1Te, 2Pb) 1.98, O10 (1 Te, 2 Pb) 1.83, O11 (1 Te, 2 Pb) 1.95, O12 (1 Te, 2 Pb) 2.03, O13 (1 Te, 2 Pb) 1.98, O14 (1 Te, 2 Pb) 2.08, O15 (1 Te, 2 Pb) 1.80. | |||||||
| β-PbTeO3 | |||||||
| Pb1 | – | O3 | 2.245(8) | Te1 | – | O1 | 1.877(8) |
| Pb1 | – | O1 | 2.427(9) | Te1 | – | O3 | 1.877(8) |
| Pb1 | – | O2 | 2.510(9) | Te1 | – | O2 | 1.883(8) |
| Pb1 | – | O2 | 2.604(8) | ||||
| Pb1 | – | O1 | 2.698(9) | O1–Te1–O3 | 95.3(4) | ||
| Pb1 | – | O3 | 2.703(10) | O1–Te1–O2 | 98.8(4) | ||
| Pb1 | – | O3 | 3.545(8) | O3–Te1–O2 | 103.8(4) | ||
| BVS. Pb1 1.99, Te1 3.91, O1 1.92 (1 Te, 2 Pb) O2 1.89 (1 Te, 2 Pb) O3 2.09 (1 Te, 2 Pb). | |||||||
| Measurement | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|
| 1–1 | 453.8 | --- | 565.6 |
| 1–2 | --- | 500.5 | 567.5 |
| 1–3 | --- | 501.8 | 567.4 |
| 2–1 | 455.8 | --- | 565.0 |
| 2–2 | --- | 505.7 | 565.5 |
| 2–3 | --- | 506.5 | 565.6 |
| 3–1 | 452.3 | --- | 567.6 |
| 3–2 | --- | 497.4 | 567.5 |
| 3–3 | --- | 497.6 | 567.3 |
| 4–1 | 450.6 | --- | 568.0 |
| 4–2 | --- | 498.3 | 567.6 |
| 4–3 | --- | 497.4 | 567.5 |
| 5–1 | 450.6 | --- | 566.9 |
| 5–2 | --- | 497.7 | 566.9 |
| 5–3 | --- | 496.9 | 566.8 |
| Measurement | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|
| 1–1 (heating) | 453.7 | --- | 565.6 |
| 1–2 (cooling) | 492.3 | ||
| 2–1 (heating) | --- | 500.5 | 567.5 |
| 2–2 (cooling) | 497.9 | ||
| 3–1 (heating) | --- | 501.8 | 567.4 |
| 3–2 (cooling) | 481.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, M.; Shirkhanlou, M.; Füglein, E.; Libowitzky, E. Determination of the Correct Composition of “Hydrous Lead(II) Oxotellurate(IV)” as PbTeO3, Crystallizing as a New Polymorph. Crystals 2018, 8, 51. https://doi.org/10.3390/cryst8010051
Weil M, Shirkhanlou M, Füglein E, Libowitzky E. Determination of the Correct Composition of “Hydrous Lead(II) Oxotellurate(IV)” as PbTeO3, Crystallizing as a New Polymorph. Crystals. 2018; 8(1):51. https://doi.org/10.3390/cryst8010051
Chicago/Turabian StyleWeil, Matthias, Mahdi Shirkhanlou, Ekkehard Füglein, and Eugen Libowitzky. 2018. "Determination of the Correct Composition of “Hydrous Lead(II) Oxotellurate(IV)” as PbTeO3, Crystallizing as a New Polymorph" Crystals 8, no. 1: 51. https://doi.org/10.3390/cryst8010051
APA StyleWeil, M., Shirkhanlou, M., Füglein, E., & Libowitzky, E. (2018). Determination of the Correct Composition of “Hydrous Lead(II) Oxotellurate(IV)” as PbTeO3, Crystallizing as a New Polymorph. Crystals, 8(1), 51. https://doi.org/10.3390/cryst8010051






