Removal of SO42− from Li2CO3 by Recrystallization in Na2CO3 Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
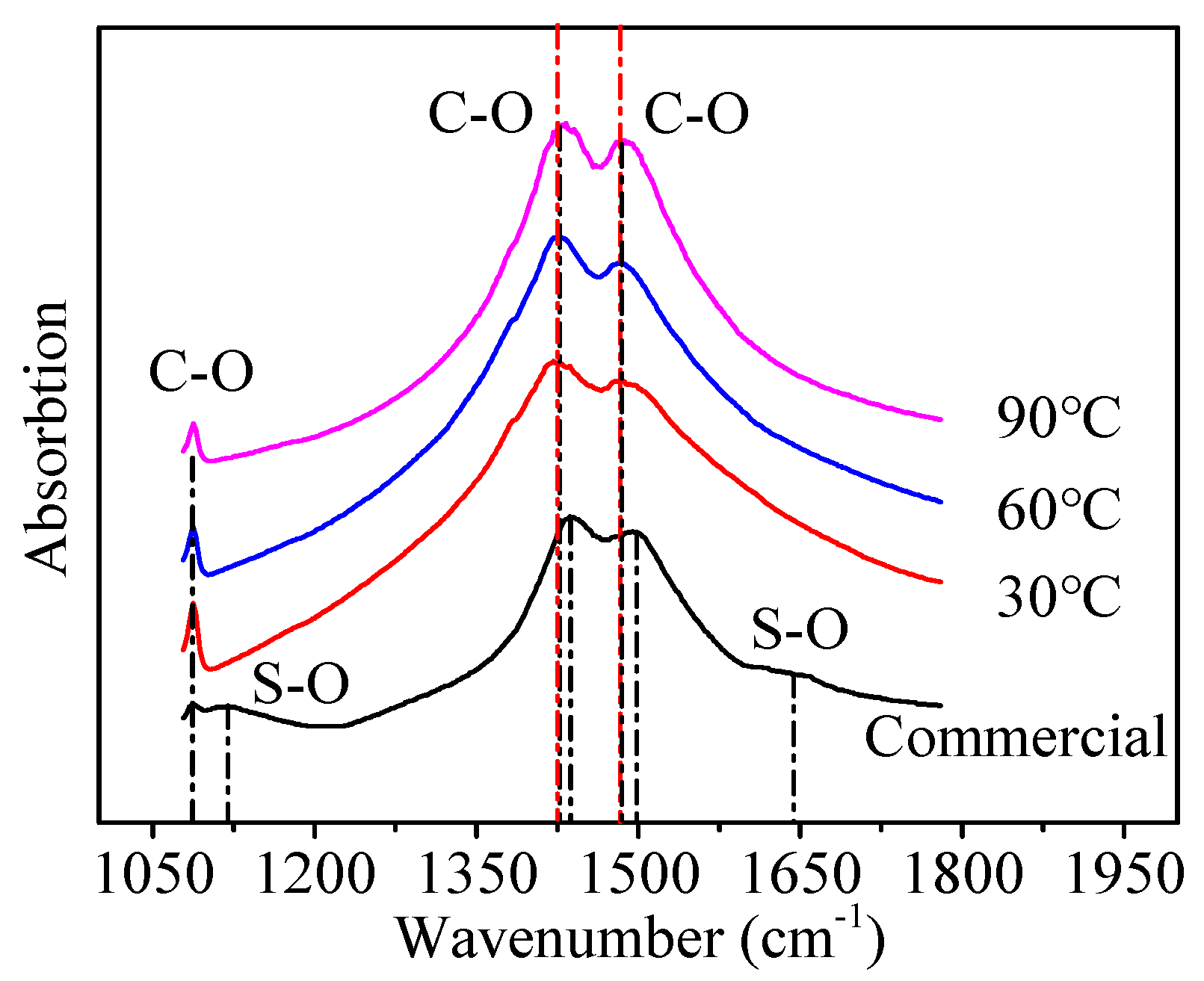
3.1. Characterization of Commercial Li2CO3
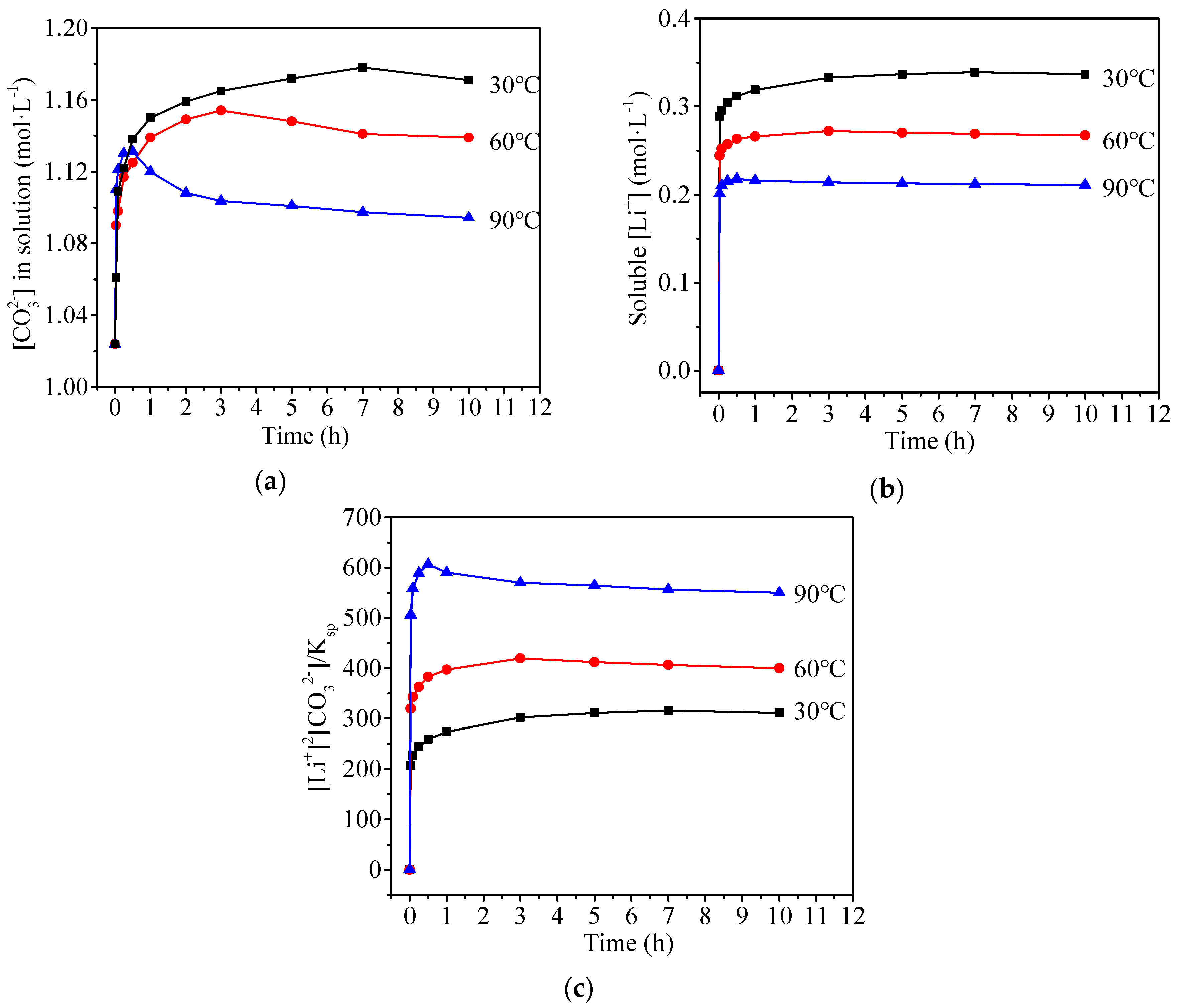
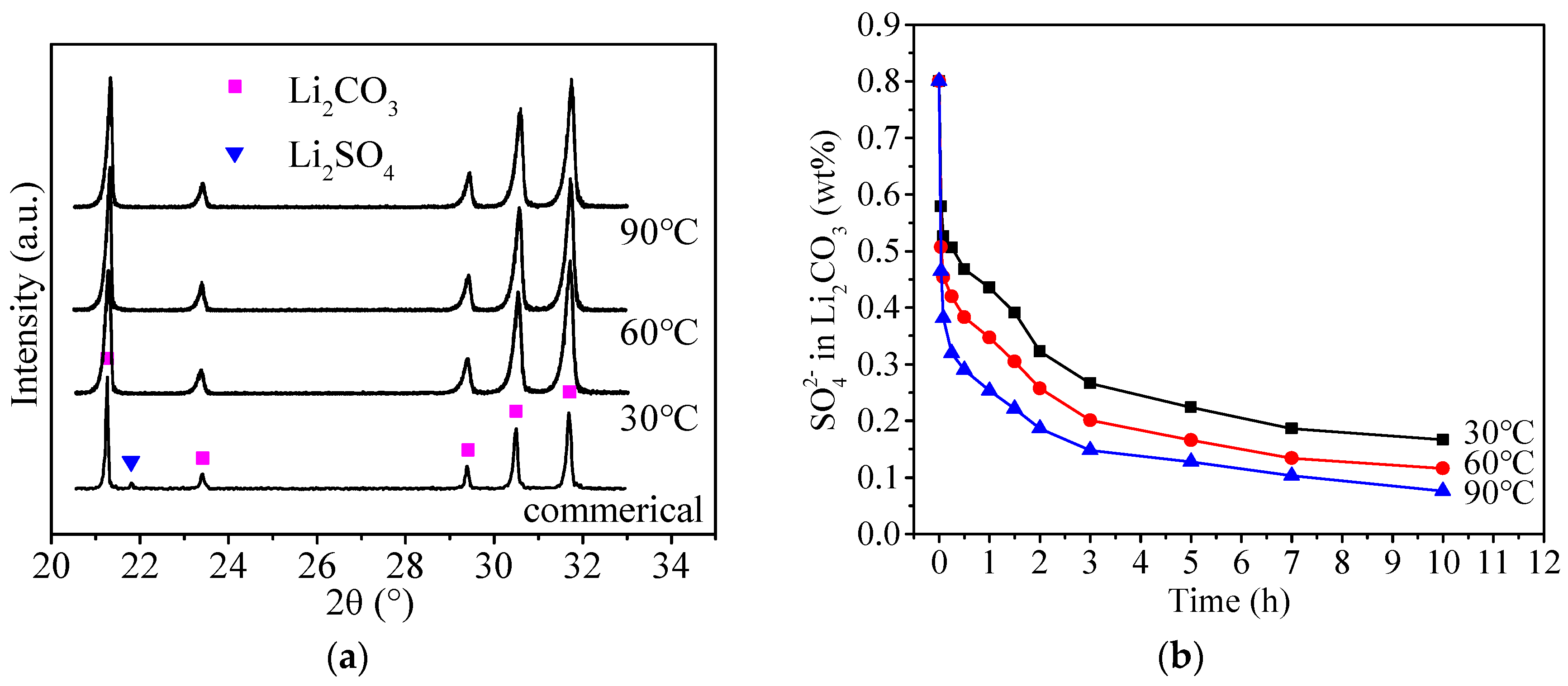
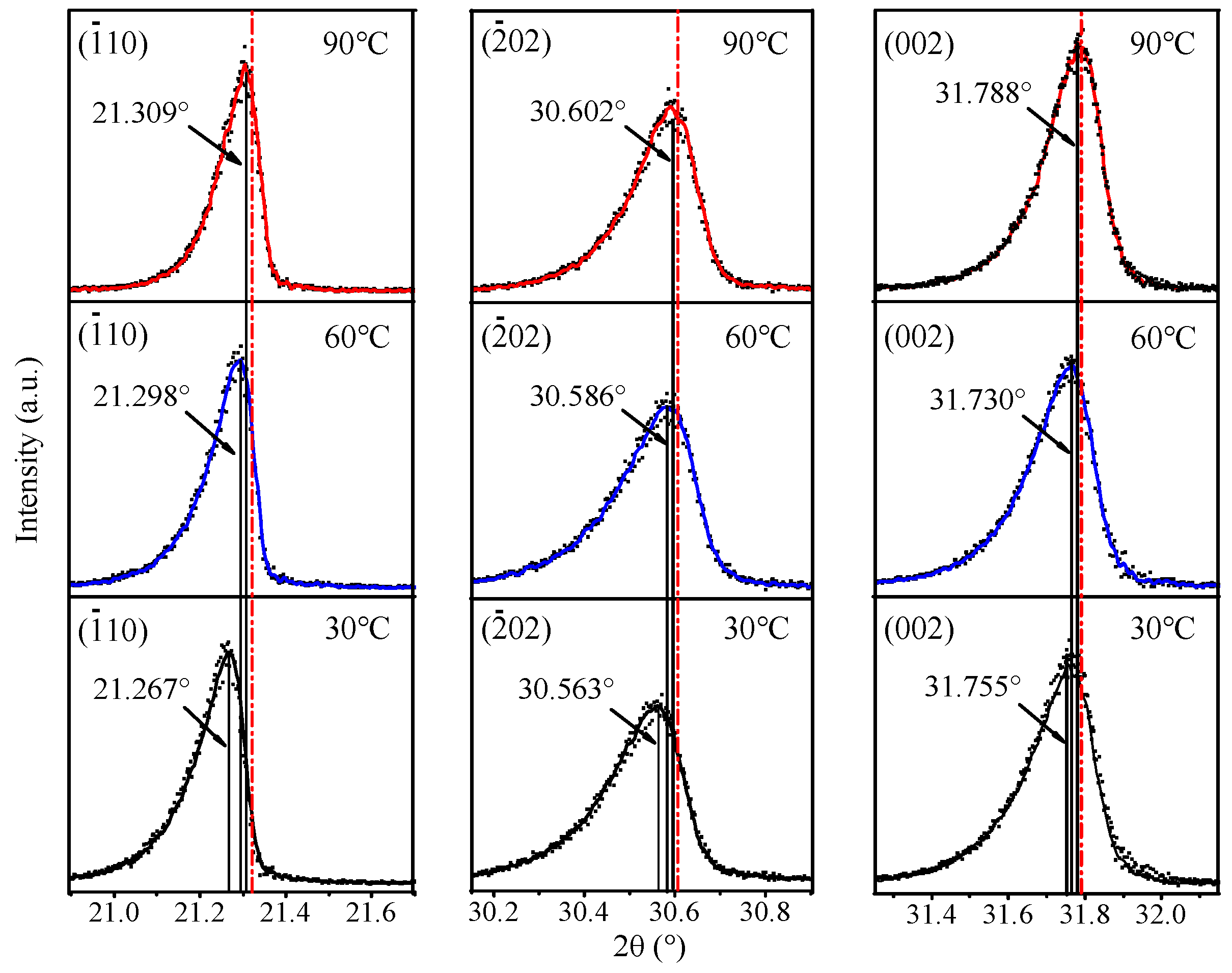
3.2. Recrystallization of Commercial Li2CO3
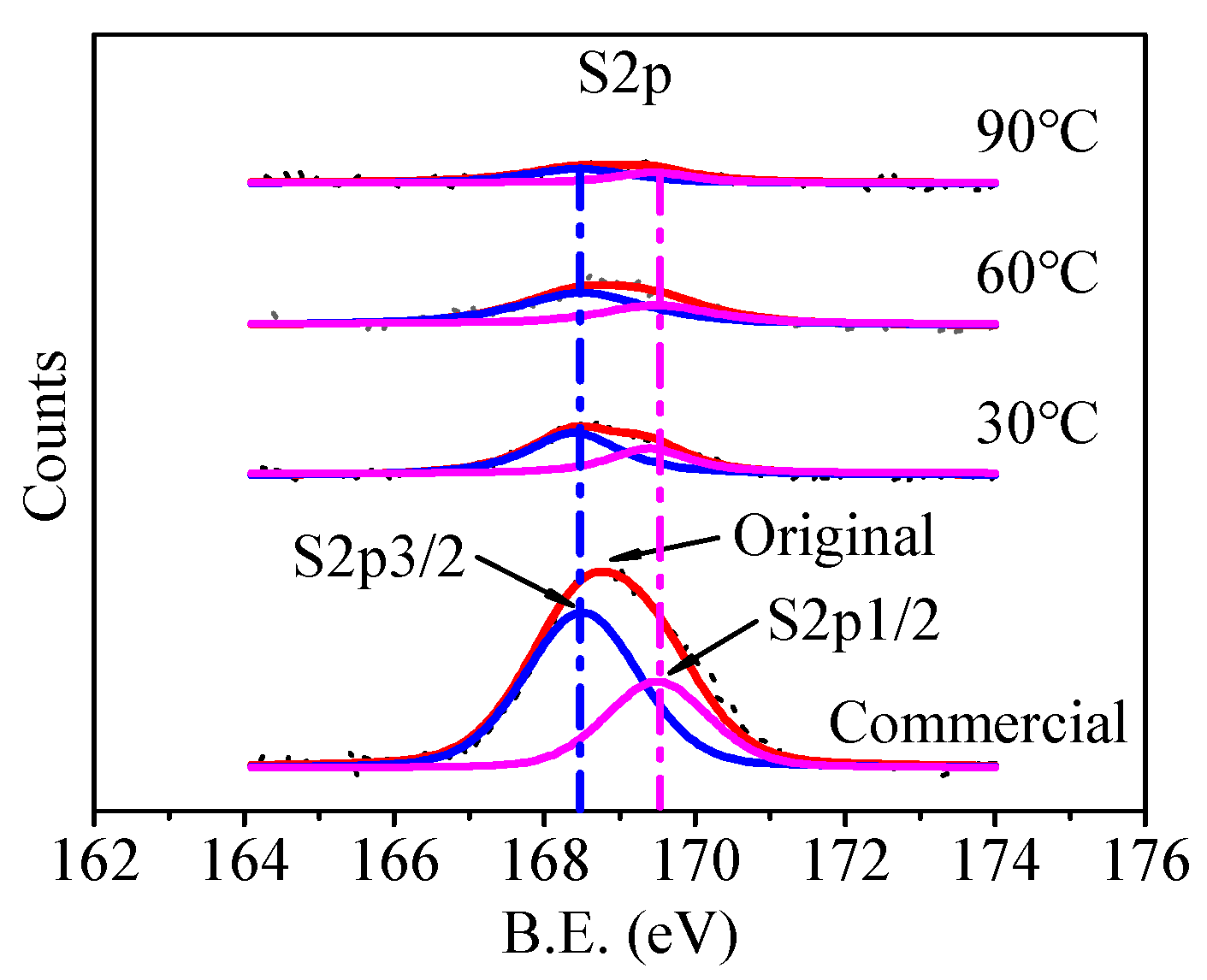
3.3. Removal of SO42− from Li2CO3
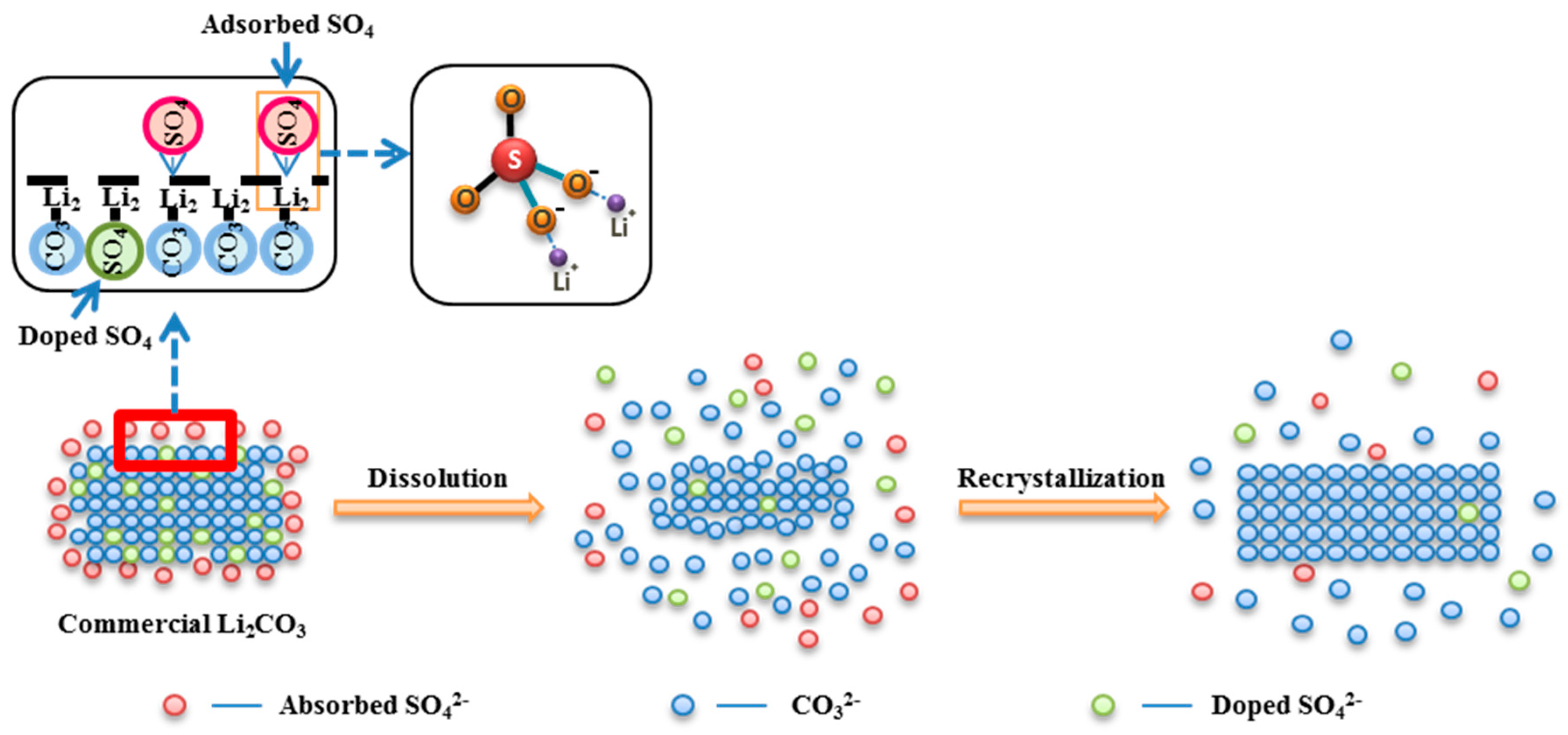
3.3.1. Behavior of Adsorbed SO42−
3.3.2. Behavior of Doped SO42−
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yan, Q. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110, 1–5. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Kim, M.J.; Tran, T. Factors affecting the extraction of lithium from lepidolite. Hydrometallurgy 2013, 134, 54–61. [Google Scholar] [CrossRef]
- Luong, V.T.; Kang, D.J.; An, J.W.; Dao, D.A.; Kim, M.J.; Tran, T. Iron sulphate roasting for extraction of lithium from lepidolite. Hydrometallurgy 2014, 141, 8–16. [Google Scholar] [CrossRef]
- Averill, W.A.; Olson, D.L. A review of extractive processes for lithium from ores and brines. Energy 1978, 3, 305–313. [Google Scholar] [CrossRef]
- Amouzegar, K.; Amant, G.S.; Harrison, S. Process for the Purification of Lithium Carbonate. U.S. Patent No. 6,048,507 A, 11 April 2000. [Google Scholar]
- Yi, W.; Yan, C.-Y.; Ma, P.-H.; Li, F.Q.; Wen, X.M. Refining of crude Li2CO3 via slurry phase dissolution using CO2. Sep. Purif. Technol. 2007, 56, 241–248. [Google Scholar] [CrossRef]
- Yi, W.-T.; Yan, C.-Y.; Ma, P.-H. Crystallization kinetics of Li2CO3 from LiHCO3 solutions. J. Cryst. Growth 2010, 312, 2345–2350. [Google Scholar] [CrossRef]
- Friedrich, H.; Pfeffinger, J.; Leutner, B. Method Producing Highly Pure Lithium Salts. U.S. Patent No. 6,592,832, 15 July 2003. [Google Scholar]
- Inorganic Chemistry, 4th ed.; Higher Education Press: Beijing, China, 2003; p. 151.
- Abraham, K.M.; Chaudhri, S.M. The lithium surface film in the Li/SO2 cell. J. Electrochem. Soc. 1986, 133, 1307–1311. [Google Scholar] [CrossRef]
- Sun, Y.; Song, X.-F.; Wang, J.; Luo, Y.; Yu, J.-G. Unseeded supersolubility of lithium carbonate: Experimental measurement and simulation with mathematical models. J. Cryst. Growth 2009, 311, 4714–4719. [Google Scholar] [CrossRef]
- Wan, S.Q.; Yi, Z.; Hao, Z.; Chia-Chung, S. Ab initio Investigation on Unhydrated Ion-associated Species between Na+, Li+, Mg2+ and SO42− Ions. Chem. J. Chin. Univ. 2012, 33, 1765–1770. [Google Scholar]
- Xu, C.Y.; Zhang, P.X.; Yan, L. Blue shift of Raman peak from coated TiO2 nanoparticles. J. Raman Spectrosc. 2001, 32, 862–865. [Google Scholar] [CrossRef]
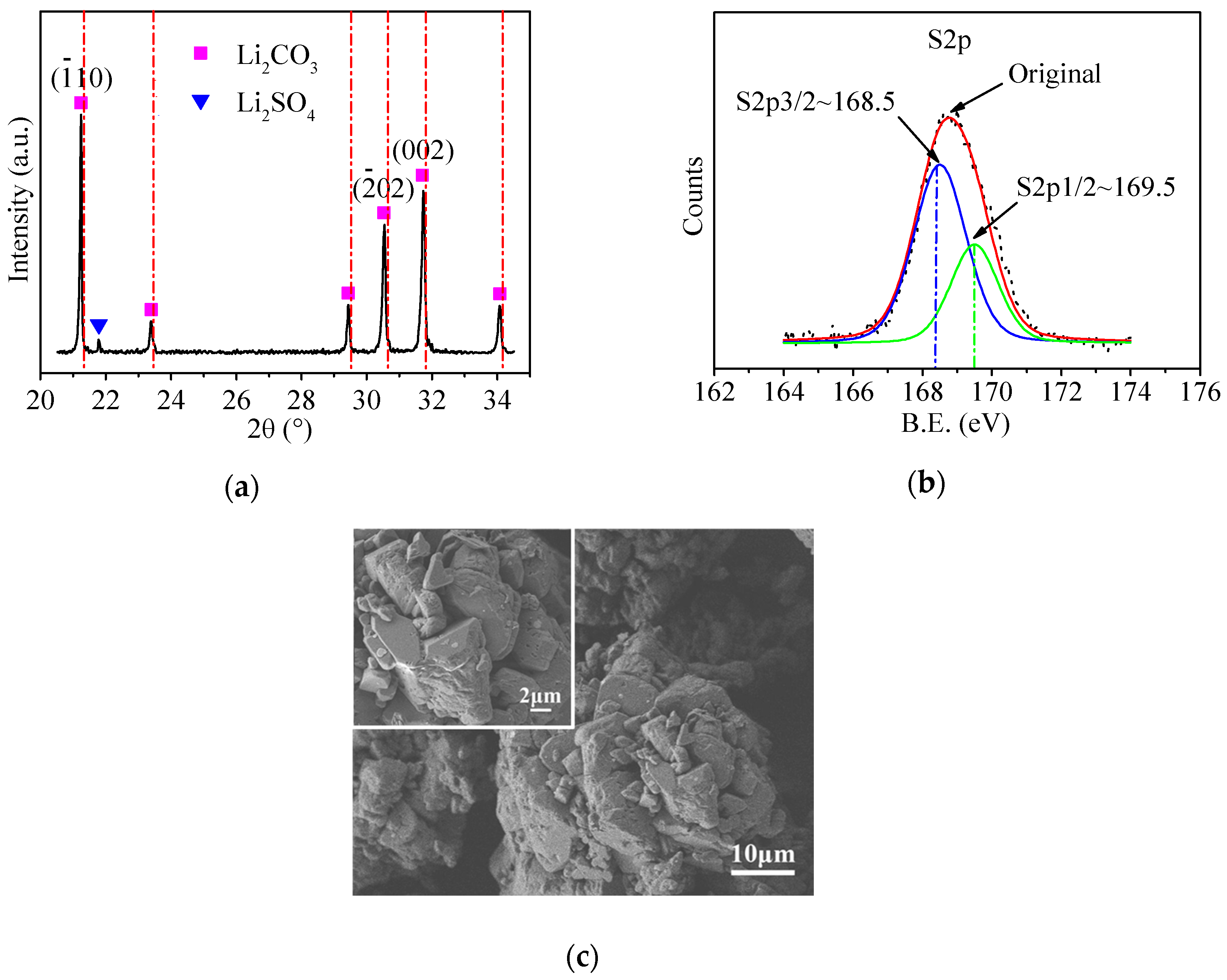
| Li2CO3 Samples | Surface Composition | SO42− on Surface (wt %) | SO42− in Bulk (wt %) | ||||
|---|---|---|---|---|---|---|---|
| Li (%) | S (%) | C (%) | O (%) | C:S | |||
| Commercial | 34.13 | 0.94 | 16.13 | 48.80 | 17.16 | 7.1 | 0.8 |
| Recrystallized at 30 °C | 34.09 | 0.34 | 16.71 | 48.86 | 49.15 | 2.62 | 0.17 |
| Recrystallized at 60 °C | 34.87 | 0.28 | 17.16 | 47.69 | 61.29 | 2.11 | 0.12 |
| Recrystallized at 90 °C | 33.90 | 0.19 | 16.76 | 49.15 | 88.21 | 1.49 | 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Chen, R.; Yang, Y.; Yi, M.; Xiang, L. Removal of SO42− from Li2CO3 by Recrystallization in Na2CO3 Solution. Crystals 2018, 8, 19. https://doi.org/10.3390/cryst8010019
Cai W, Chen R, Yang Y, Yi M, Xiang L. Removal of SO42− from Li2CO3 by Recrystallization in Na2CO3 Solution. Crystals. 2018; 8(1):19. https://doi.org/10.3390/cryst8010019
Chicago/Turabian StyleCai, Wei, Ruosong Chen, Yurong Yang, Meigui Yi, and Lan Xiang. 2018. "Removal of SO42− from Li2CO3 by Recrystallization in Na2CO3 Solution" Crystals 8, no. 1: 19. https://doi.org/10.3390/cryst8010019
APA StyleCai, W., Chen, R., Yang, Y., Yi, M., & Xiang, L. (2018). Removal of SO42− from Li2CO3 by Recrystallization in Na2CO3 Solution. Crystals, 8(1), 19. https://doi.org/10.3390/cryst8010019




