Structure of a Novel Spinel Li0.5Zn5/3Sb2.5/3O4 by Neutron and Synchrotron Diffraction Analysis
Abstract
:1. Introduction
2. Experimental
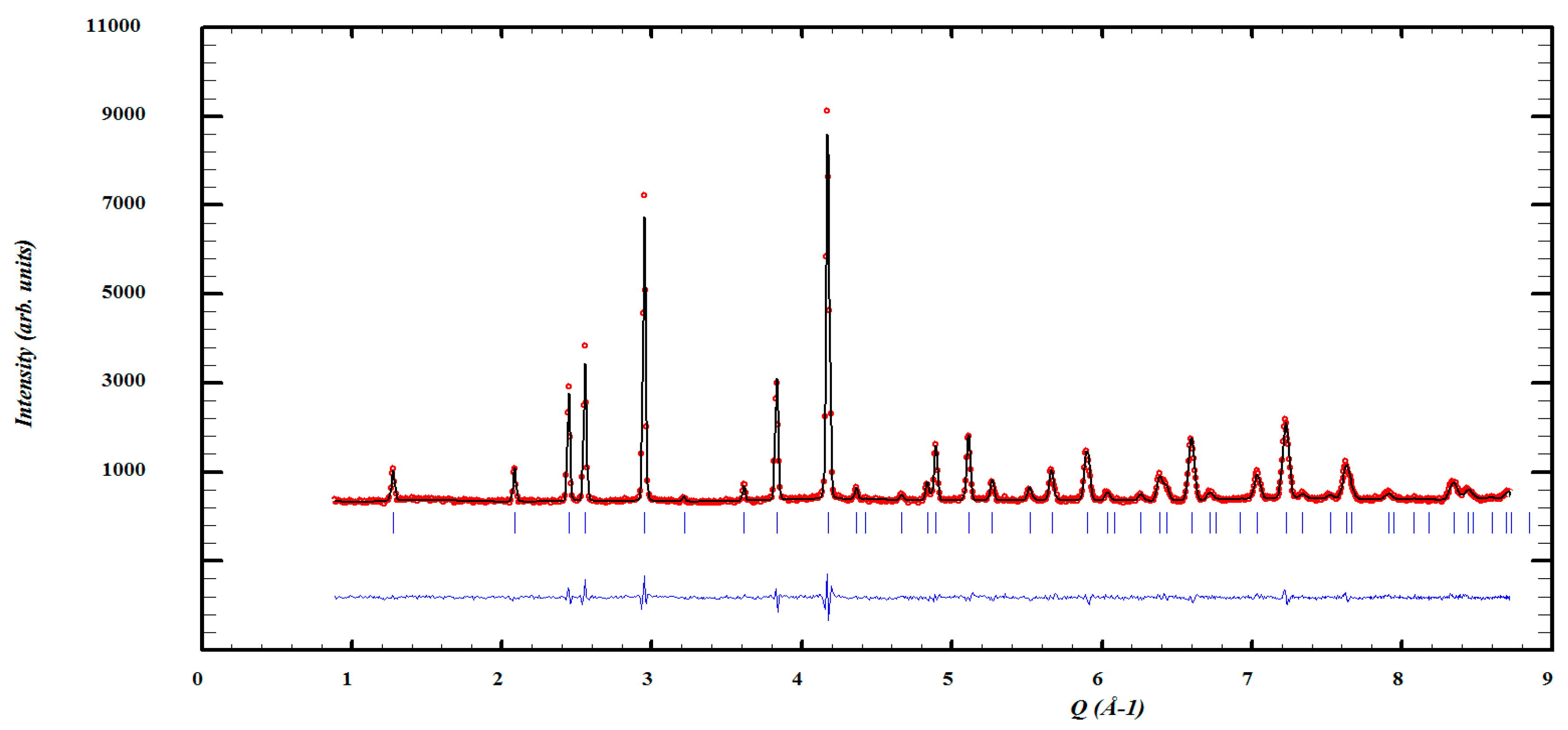
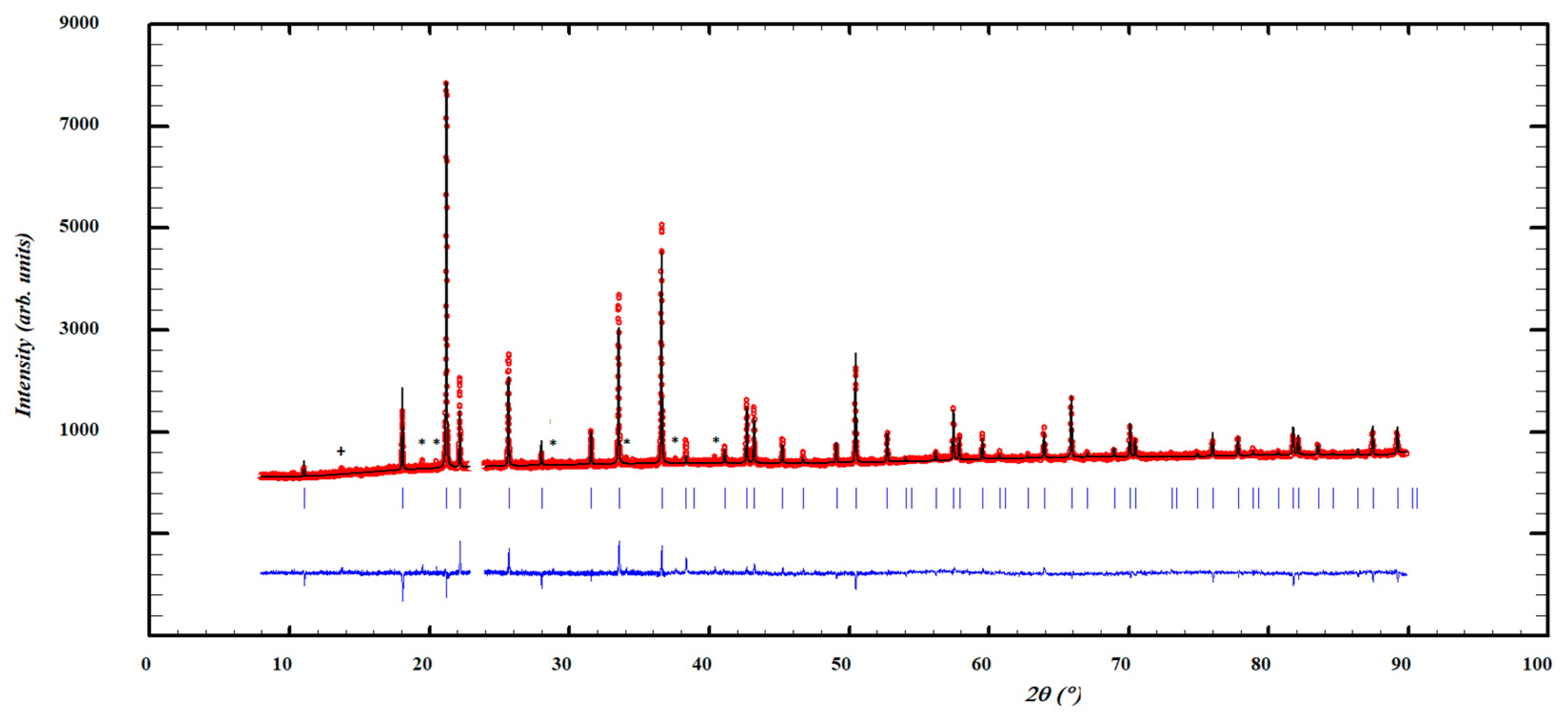
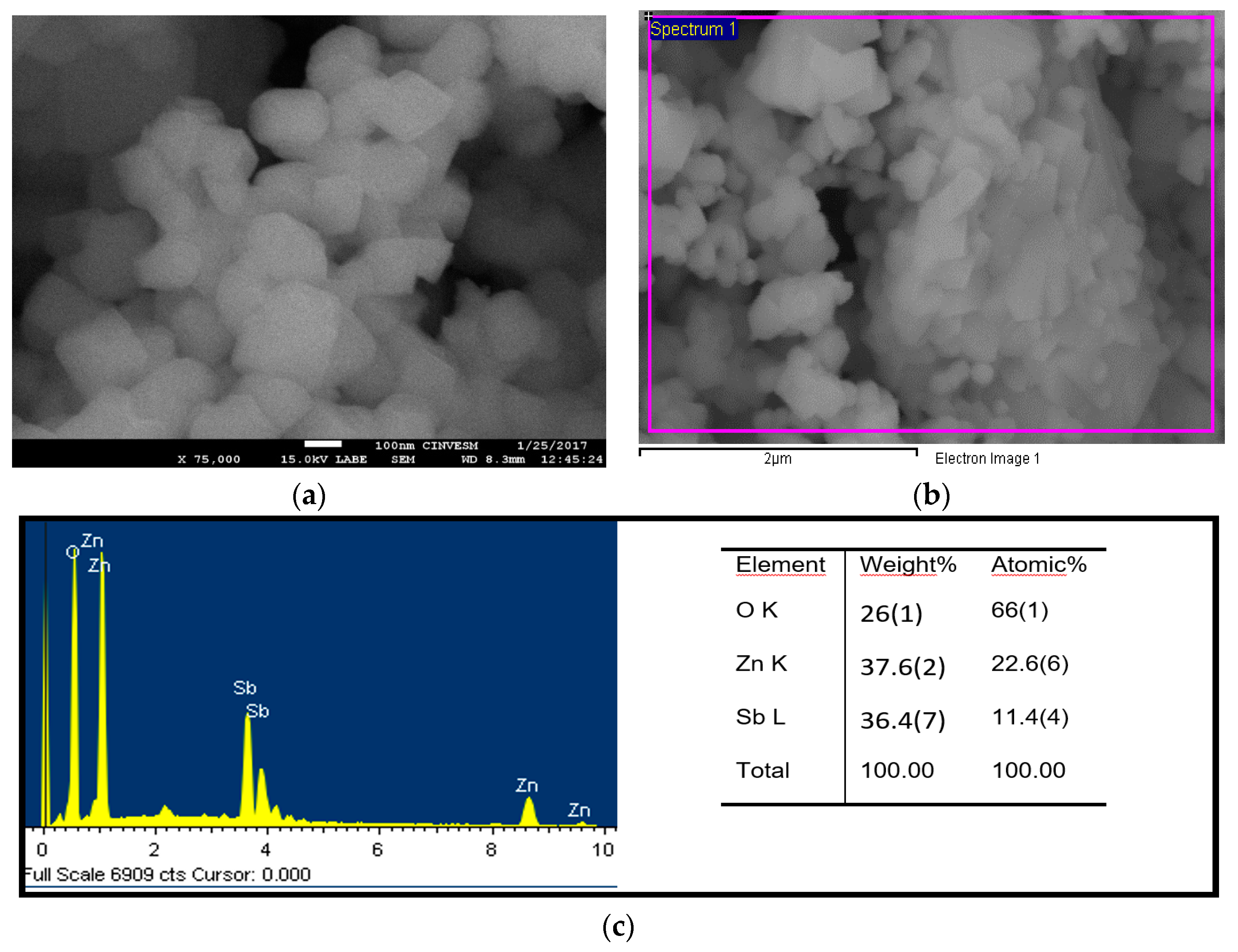
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talanov, V.M. Calculation of Structural Parmeters of Spinels. Phys. Status Solidi 1981, 106, 99–106. [Google Scholar] [CrossRef]
- Talanov, V.M. Structural Modelling of Low-Symmetry Phases of Spinels. I. Phases with 1:1 Octahedral Order. Phys. Status Solidi 1990, 162, 61–73. [Google Scholar] [CrossRef]
- Biagioni, C.; Pasero, M. The systematics of the spinel-type minerals: An overview. Am. Mineral. 2014, 99, 1254–1264. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of Spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Lisboa-Filho, P.N.; Vila, C.; Góes, M.S.; Morilla-Santos, C.; Gama, L.; Longo, E.; Schreiner, W.H.; Paiva-Santos, C.O. Composition and electronic structure of Zn7-xMxSb2O12 (M=Ni and Co) spinel compounds. Mater. Chem. Phys. 2004, 85, 377–382. [Google Scholar] [CrossRef]
- Bernik, S.; Branković, G.; Rustja, S.; Žunić, M.; Podlogar, M.; Branković, Z. Microstructural and compositional aspects of ZnO-based varistor ceramics prepared by direct mixing of the constituent phases and high-energy milling. Ceram. Int. 2008, 34, 1495–1502. [Google Scholar] [CrossRef]
- Branković, Z.; Branković, G.; Poleti, D.; Varela, J. Structural and electrical properties of ZnO varistors containing different spinel phases. Ceram. Int. 2001, 27, 115–122. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Zeng, Y.; Li, C. The effect of secondary phases on electrical properties of ZnO-based ceramic films prepared by a sol-gel method. J. Mater. Sci. Mater. Electron. 2004, 5, 549–553. [Google Scholar] [CrossRef]
- Harrington, R.; Miles, G.C.; West, A.R. Phase equilibria, crystal chemistry and polymorphism of Zn7Sb2O12 doped with Cr and Ni. Mater. Res. Bull. 2008, 43, 1949–1956. [Google Scholar] [CrossRef]
- Harrington, R.; Miles, G.C.; West, A.R. Crystallography of Ni-doped Zn7Sb2O12 and phase equilibria in the system ZnO-Sb2O5-NiO. J. Eur. Ceram. Soc. 2006, 26, 2307–2311. [Google Scholar] [CrossRef]
- Miles, G.C.; West, A.R. Polymorphism and Thermodynamic Stability of Zn7Sb2O12. J. Am. Ceram. Soc. 2005, 88, 396–398. [Google Scholar] [CrossRef]
- Filipek, E.; Dąbrowska, G. Phase relations up to the solidus line in the part of the Sb-Zn-O system. Open Chem. 2009, 7, 192–196. [Google Scholar] [CrossRef]
- Kuramochi, K.; Suzuki, K.; Yamazaki, T.; Mitsuishi, K.; Furuya, K.; Hashimoto, I.; Watanabe, K. Quantitative structural analysis of twin boundary in using {HAADF} {STEM} method. Ultramicroscopy 2008, 109, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Talanov, V.M.; Shirokov, V.B. Tilting structures in spinels. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Miles, G.C.; West, A.R. Crystal chemistry of Co-doped Zn7Sb2O12. J. Solid State Chem. 2008, 181, 334–339. [Google Scholar] [CrossRef]
- Braun, P.B. A Superstructure in Spinels. Nature 1952, 170, 1123. [Google Scholar] [CrossRef]
- Houabes, M.; Metz, R. The effect of lithium oxide on the threshold field of ZnO varistors. Ceram. Int. 2009, 35, 1385–1389. [Google Scholar] [CrossRef]
- Clarke, D.R. Varistor Ceramics. J. Am. Ceram. Soc. 2004, 82, 485–502. [Google Scholar] [CrossRef]
- Meyer, B.K.; Stehr, J.; Hofstaetter, A.; Volbers, N.; Zeuner, A.; Sann, J. On the role of group I elements in ZnO. Appl. Phys. A. 2007, 88, 119–123. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Fuentes-Cobas, L.E. Synchrotron Radiation Diffraction and Scattering in Ferroelectrics. In Multifunctional Polycrystalline Ferroelectric Materials; Springer: Dordrecht, The Netherlands, 2011; pp. 217–280. [Google Scholar]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Lonardelli, I.; Wenk, H.R. Rietveld texture analysis from diffraction images. Z. Krist. Suppl. 2007, 1, 125–130. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method. International Union of Crystallography Monographs on Crystallography; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Delacourt, C.; Rodríguez-Carvajal, J.; Schmitt, B.; Tarascon, J.M.; Masquelier, C. Crystal chemistry of the olivine-type LixFePO4 system (0 ≤ x ≤ 1) between 25 and 370°C. Solid State Sci. 2005, 7, 1506–1516. [Google Scholar] [CrossRef]
- Wang, J.; Peng, G.; Guo, Y.; Yang, X. XPS investigation of segregation of Sb in SnO2 powders. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 95–99. [Google Scholar] [CrossRef]
- Gurgul, J.; Rinke, M.T.; Schellenberg, I.; Pöttgen, R. The antimonide oxides REZnSbO and REMnSbO (RE = Ce, Pr)-An XPS study. Solid State Sci. 2013, 17, 122–127. [Google Scholar] [CrossRef]
- Wagner, C.D.; Davis, L.E.; Zeller, M.V.; Taylor, J.A.; Raymond, R.H.; Gale, L.H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf. Interface Anal. 1981, 3, 211–225. [Google Scholar] [CrossRef]
- Redhammer, G.J.; Tippelt, G. Crystal structure of spinel-type Li0.64Fe2.15Ge0.21O4. Acta Crystallogr. Sect. E Crystallogr. Commun. 2016, 72, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Ezhilvalavan, S.; Kutty, T.R.N. Low-voltage varistors based on zinc antimony spinel Zn7Sb2O12. Appl. Phys. Lett. 1996, 68, 2693–2695. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels; crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]


| Crystal Data | |||||
|---|---|---|---|---|---|
| Radiation | |||||
| Cubic, (227) | Synchrotron X-rays, λ = 0.9500 Å | ||||
| Constant wavelength Neutron, λ = 1.28 Å | |||||
| a = 8.5567(1) Å | Particle morphology: equiaxial powder | ||||
| V = 626.5078(2) Å3 | Color: White | ||||
| Z = 8 | |||||
| Rietveld reliability factors | |||||
| ND Data | XRD Data | ||||
| Rp = 0.05 | Rp = 0.06 | ||||
| Rwp = 0.06 | Rwp = 0.08 | ||||
| Rexp = 0.05 | Rexp = 0.05 | ||||
| RBragg = 3.928 | RBragg = 5.377 | ||||
| χ2 = 1.631 | χ2 = 4.0342 | ||||
| 1150 data points | 9794 data points | ||||
| Excluded region(s): none | Excluded region(s): 1 (23° ≤ 2θ ≤ 24°) | ||||
| x | y | z | Uiso | Occupancy | |
| Zn1 | 0.12500 | 0.12500 | 0.12500 | 0.0107(10) | 0.82(4) |
| Li1 | 0.12500 | 0.12500 | 0.12500 | 0.0107(10) | 0.18(4) |
| Li2 | 0.50000 | 0.50000 | 0.50000 | 0.0121(6) | 0.16(2) |
| Zn2 | 0.50000 | 0.50000 | 0.50000 | 0.0121(6) | 0.42(2) |
| Sb1 | 0.50000 | 0.50000 | 0.50000 | 0.0121(6) | 0.42(2) |
| O1 | 0.2596(1) | 0.2596(1) | 0.2596(1) | 0.0131(3) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Romero, J.A.; Fuentes-Cobas, L.E.; Rodríguez-Carvajal, J.; Tabasco-Novelo, C.; Quintana, P. Structure of a Novel Spinel Li0.5Zn5/3Sb2.5/3O4 by Neutron and Synchrotron Diffraction Analysis. Crystals 2017, 7, 280. https://doi.org/10.3390/cryst7090280
Marín-Romero JA, Fuentes-Cobas LE, Rodríguez-Carvajal J, Tabasco-Novelo C, Quintana P. Structure of a Novel Spinel Li0.5Zn5/3Sb2.5/3O4 by Neutron and Synchrotron Diffraction Analysis. Crystals. 2017; 7(9):280. https://doi.org/10.3390/cryst7090280
Chicago/Turabian StyleMarín-Romero, José Alfredo, Luis Edmundo Fuentes-Cobas, Juan Rodríguez-Carvajal, Carolina Tabasco-Novelo, and Patricia Quintana. 2017. "Structure of a Novel Spinel Li0.5Zn5/3Sb2.5/3O4 by Neutron and Synchrotron Diffraction Analysis" Crystals 7, no. 9: 280. https://doi.org/10.3390/cryst7090280
APA StyleMarín-Romero, J. A., Fuentes-Cobas, L. E., Rodríguez-Carvajal, J., Tabasco-Novelo, C., & Quintana, P. (2017). Structure of a Novel Spinel Li0.5Zn5/3Sb2.5/3O4 by Neutron and Synchrotron Diffraction Analysis. Crystals, 7(9), 280. https://doi.org/10.3390/cryst7090280






