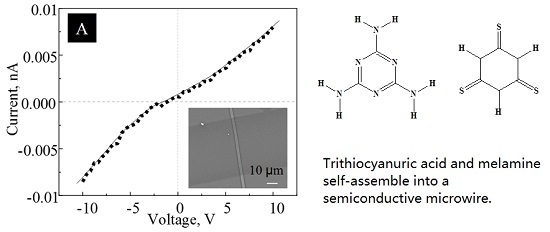
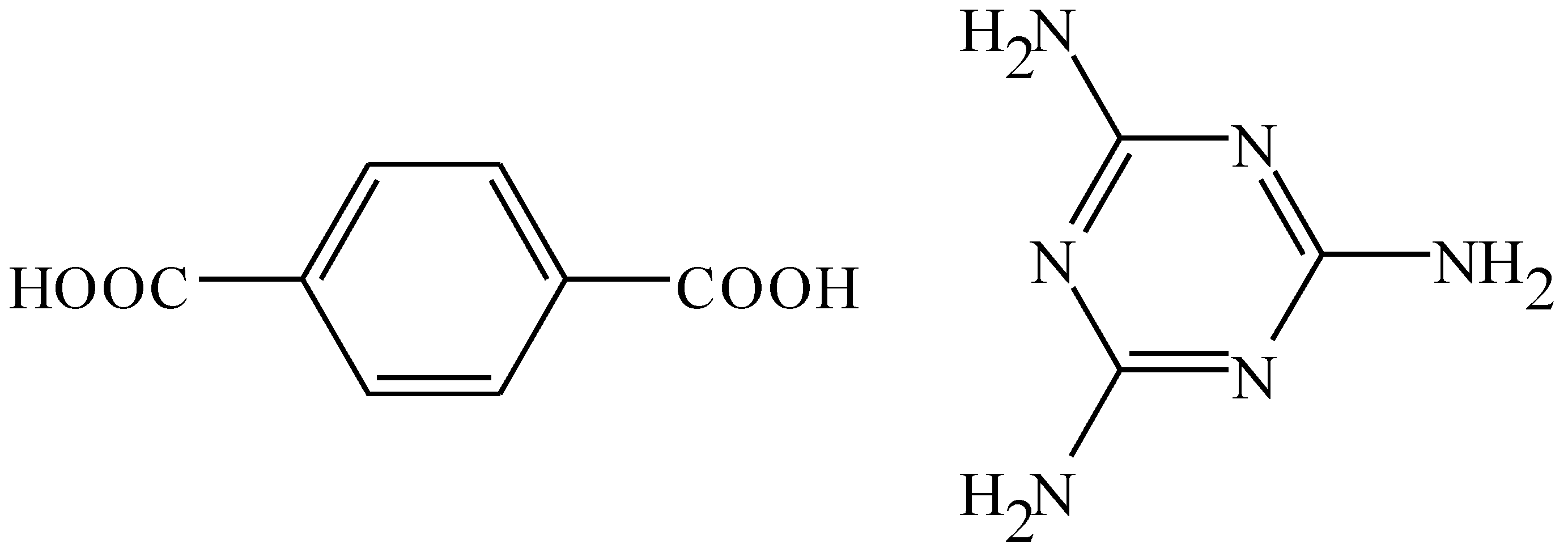
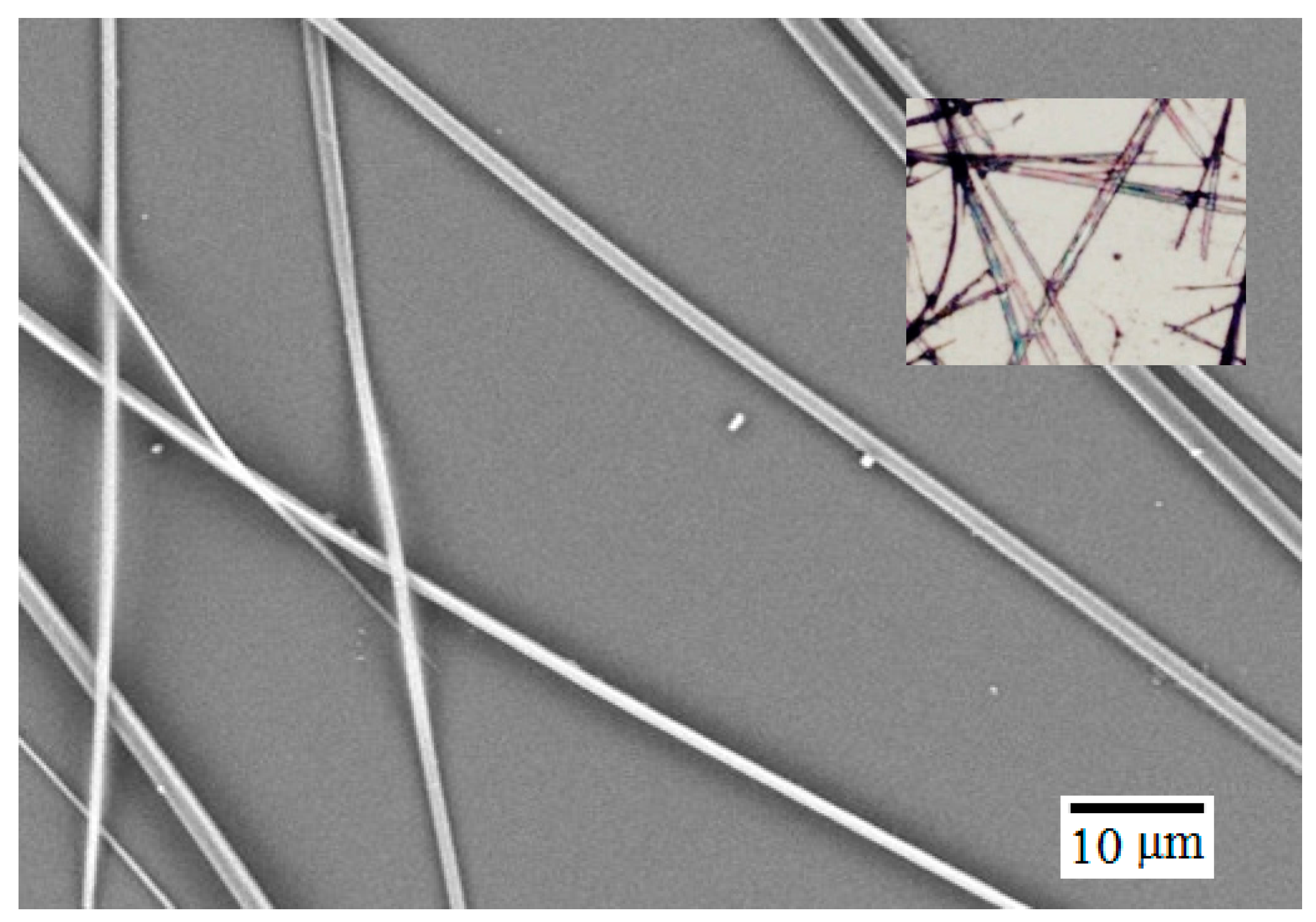
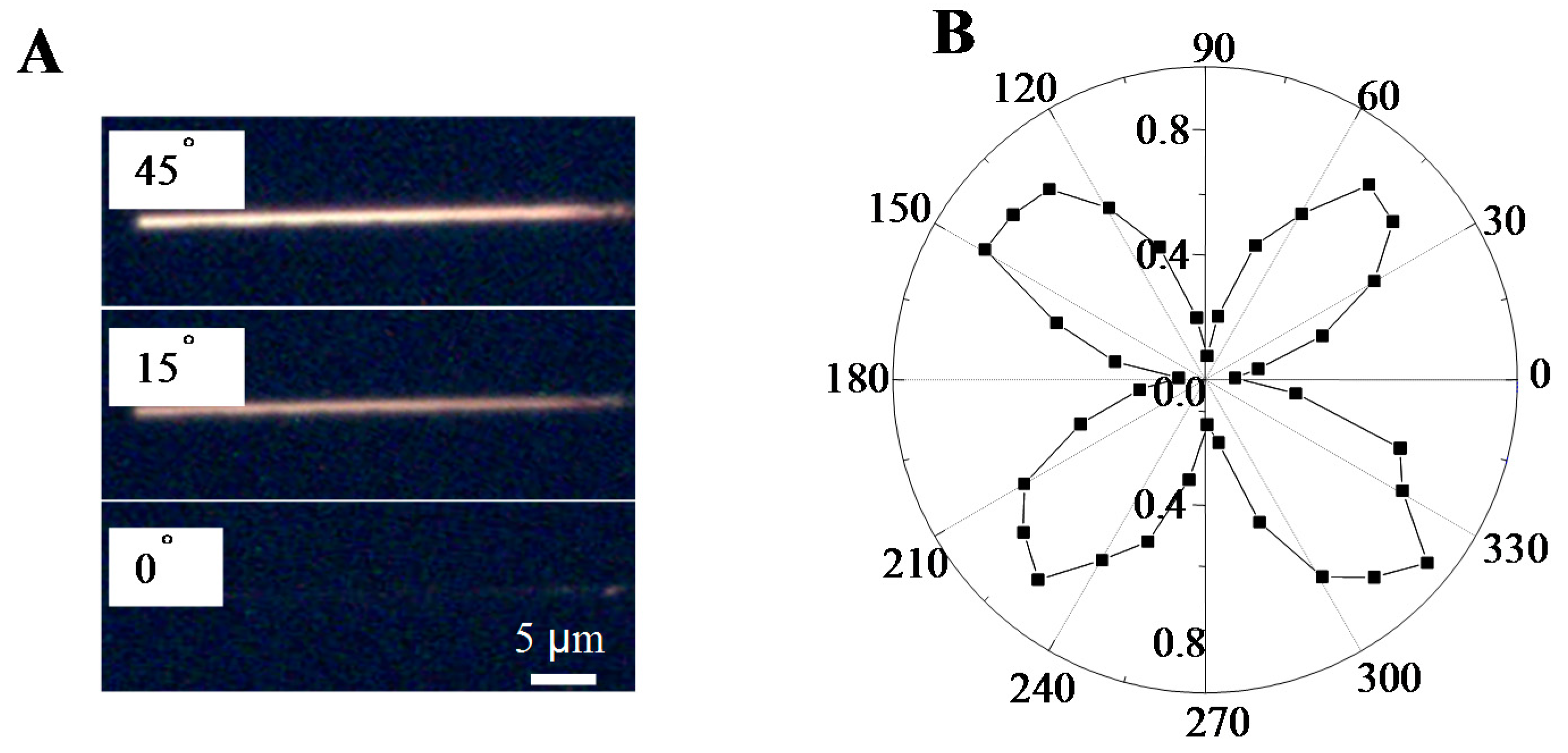
Self-Assembled Microwires of Terephthalic Acid and Melamine
Abstract
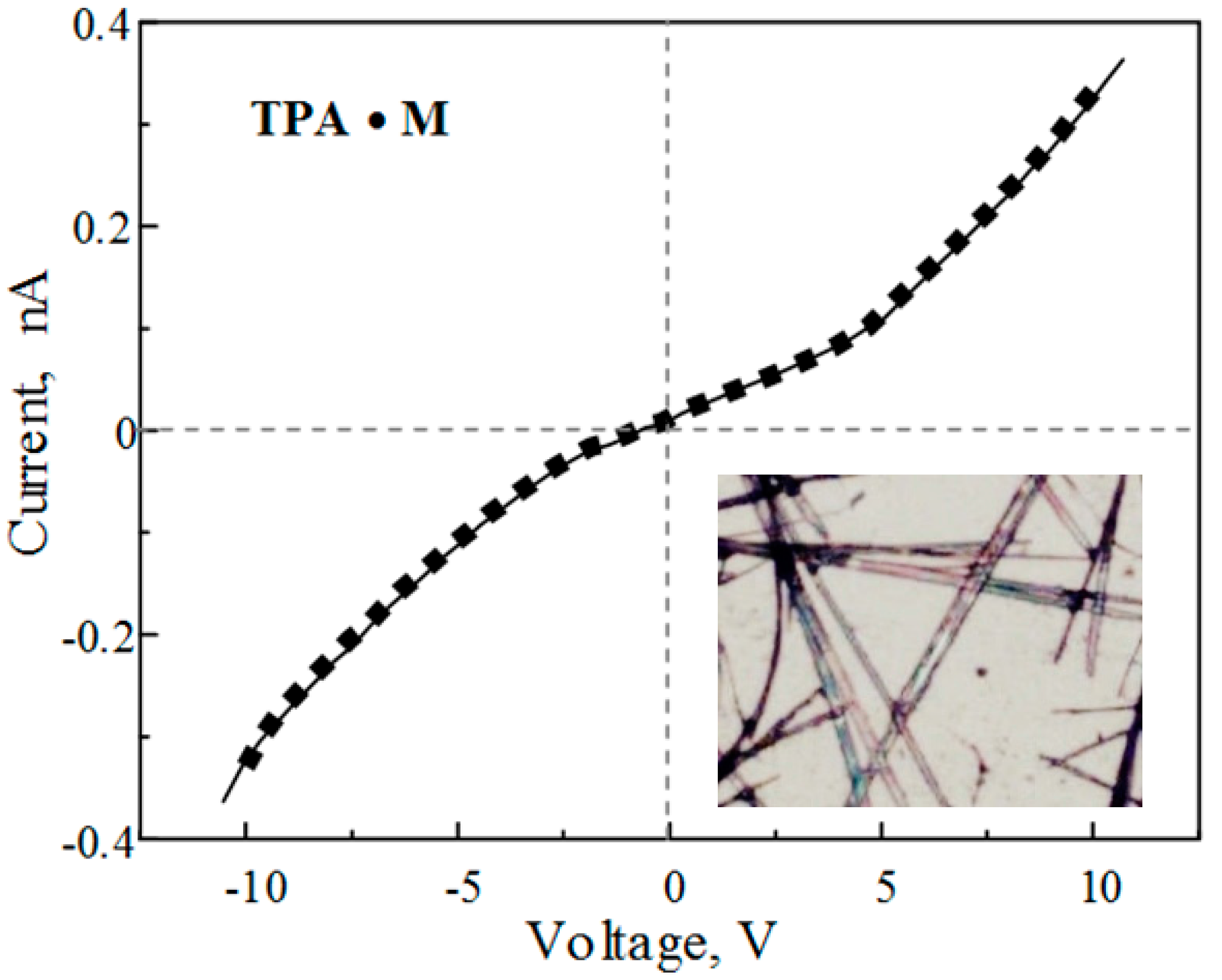
:Introduction
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zimmerman, S.C.; Zeng, F.; Reichert, D.F.C.; Kolotuchin, C.V. Self-assembling dendrimers. Science 1996, 271, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Schenning, A.P.H.J.; Meijer, E.W. Supramolecular electronics; nanowires from self-assembled π-conjugated systems. Chem. Commun. 2005, 41, 3245–3258. [Google Scholar] [CrossRef] [PubMed]
- Oshovsky, G.V.; Reinhoudt, D.N.; Verboom, W. Supramolecular chemistry in water. Angew. Chem. Int. Ed. 2007, 46, 2366–2393. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O.; Kodama, T.; Ogino, J.; Migake, Y. High-conductivity solid proton conductors: Dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals. Chem. Lett. 1979, 1, 17–18. [Google Scholar] [CrossRef]
- Zerkowski, J.A.; Seto, C.T.; Wierda, D.A.; Whitesides, G.M. The design of organic structures in the solid state: Hydrogen-bonded molecular “tapes”. J. Am. Chem. Soc. 1990, 112, 9025–9026. [Google Scholar] [CrossRef]
- Seto, C.T.; Whitesides, G.M. Molecular self-assembly through hydrogen bonding: Supramolecular aggregates based on the cyanuric acid-melamine lattice. J. Am. Chem. Soc. 1993, 115, 905–916. [Google Scholar] [CrossRef]
- Seto, C.T.; Whitesides, G.M. Self-assembly based on the cyanuric acid-melamine lattice. J. Am. Chem. Soc. 1990, 112, 6409–6411. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Xu, X. Hexagonal organic nanopillar array from the melamine−cyanuric acid complex. Langmuir 2010, 26, 4620–4622. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, M.S.; Kim, K. Infrared and raman spectroscopic study of terephthalic acid adsorbed on silver surfaces. J. Mol. Struct. 1997, 415, 93–100. [Google Scholar] [CrossRef]
- Christian, P.; Isabel, H.; Karl, K.; Eyal, N.; Mattanjah, S.V. Pairing of isolated nucleobases: Double resonance laser spectroscopy of adenine–thymine. ChemPhysChem 2003, 4, 838–842. [Google Scholar]
- Habeeb, M.M.; Al-Wakil, H.A.; El-Dissouky, A.; Refat, N.M. Vibrational spectroscopic studies of hydrogen-bonded complexes between 2,5-dihydroxy-P-benzoquinone and amines. Spctroscopy 2001, 15, 33–44. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, J.T.; Park, Y.D.; Jang, Y.; Cho, J.H.; Hwang, M.; Cho, K. Single-crystal polythiophene microwires grown by self-assembly. Adv. Mater. 2006, 18, 719. [Google Scholar] [CrossRef]
- Pisula, W.; Kastler, M.; Wasserfallen, D.; Pakula, T.; Mullen, K. Exceptionally long-range self-assembly of hexa-peri-hexabenzocoronene with dove-tailed alkyl substituents. J. Am. Chem. Soc. 2004, 126, 8074. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Kojtari, A.; Xu, X.; Ji, H.-F. Self-Assembled Microwires of Terephthalic Acid and Melamine. Crystals 2017, 7, 236. https://doi.org/10.3390/cryst7080236
Wang H, Kojtari A, Xu X, Ji H-F. Self-Assembled Microwires of Terephthalic Acid and Melamine. Crystals. 2017; 7(8):236. https://doi.org/10.3390/cryst7080236
Chicago/Turabian StyleWang, Hong, Arben Kojtari, Xiaohe Xu, and Hai-Feng Ji. 2017. "Self-Assembled Microwires of Terephthalic Acid and Melamine" Crystals 7, no. 8: 236. https://doi.org/10.3390/cryst7080236
APA StyleWang, H., Kojtari, A., Xu, X., & Ji, H.-F. (2017). Self-Assembled Microwires of Terephthalic Acid and Melamine. Crystals, 7(8), 236. https://doi.org/10.3390/cryst7080236