Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure
Abstract
:1. Introduction
2. Results and Discussion
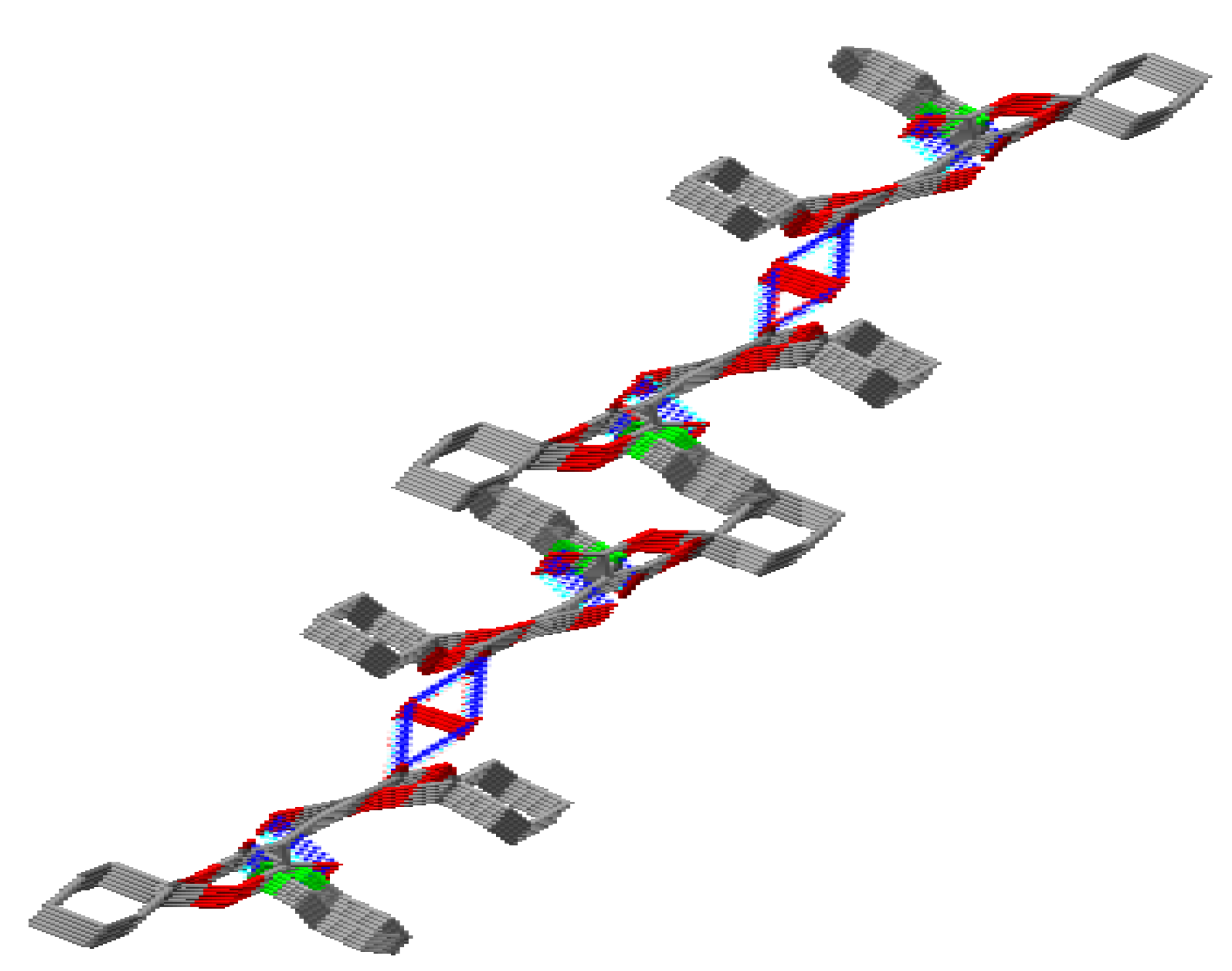
2.1. Crystal Structure
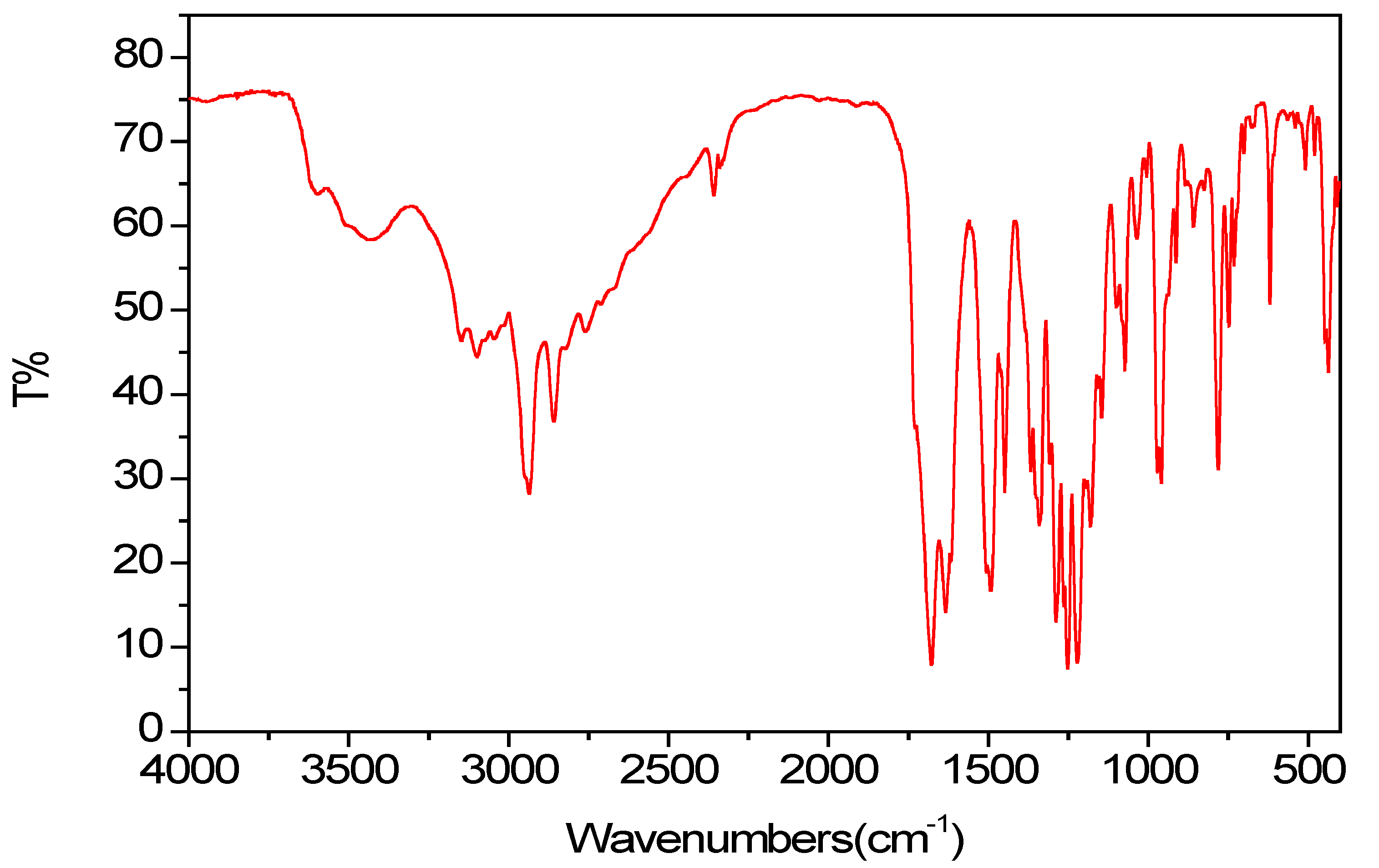
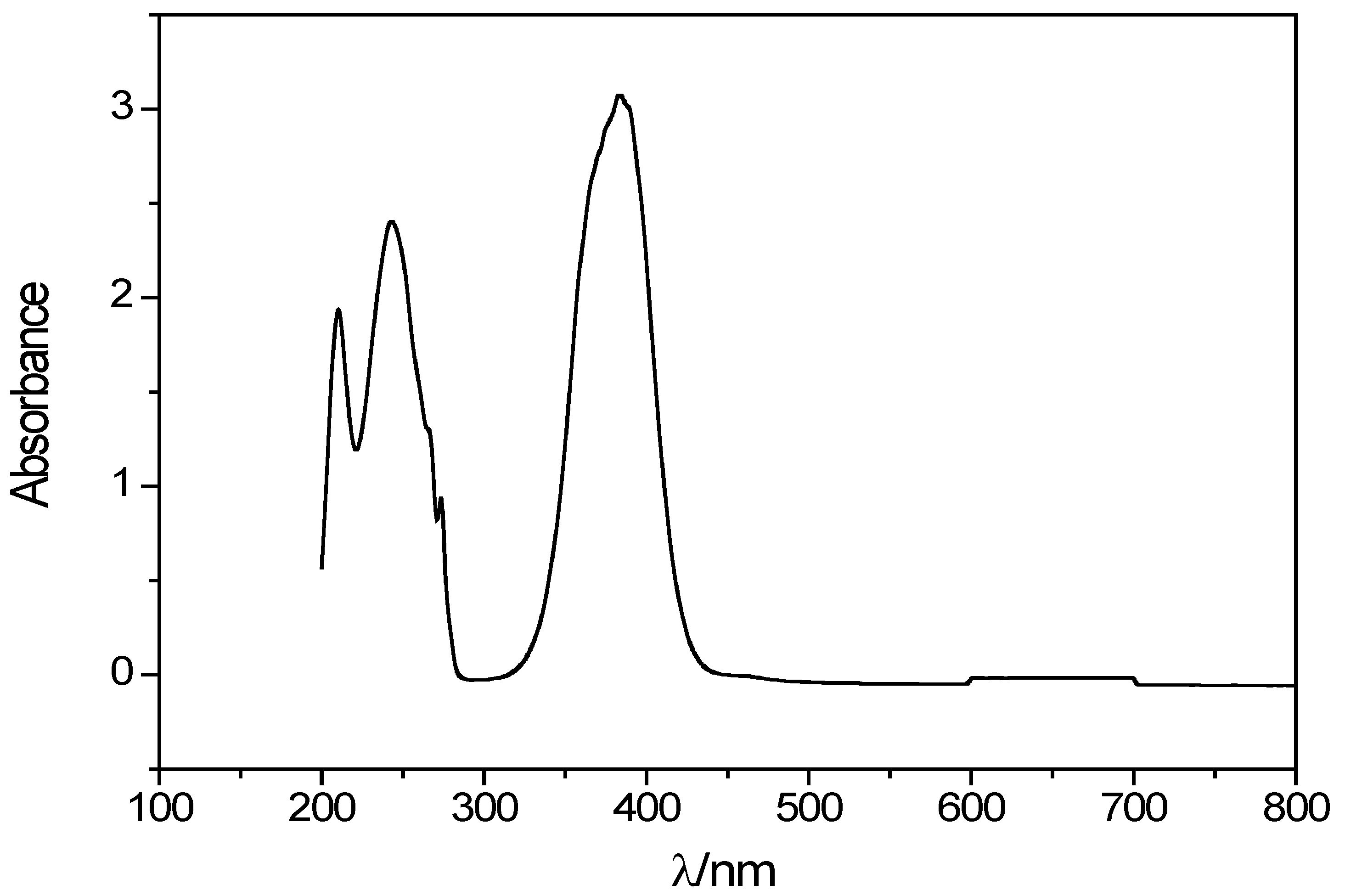
2.2. Spectroscopic Properties
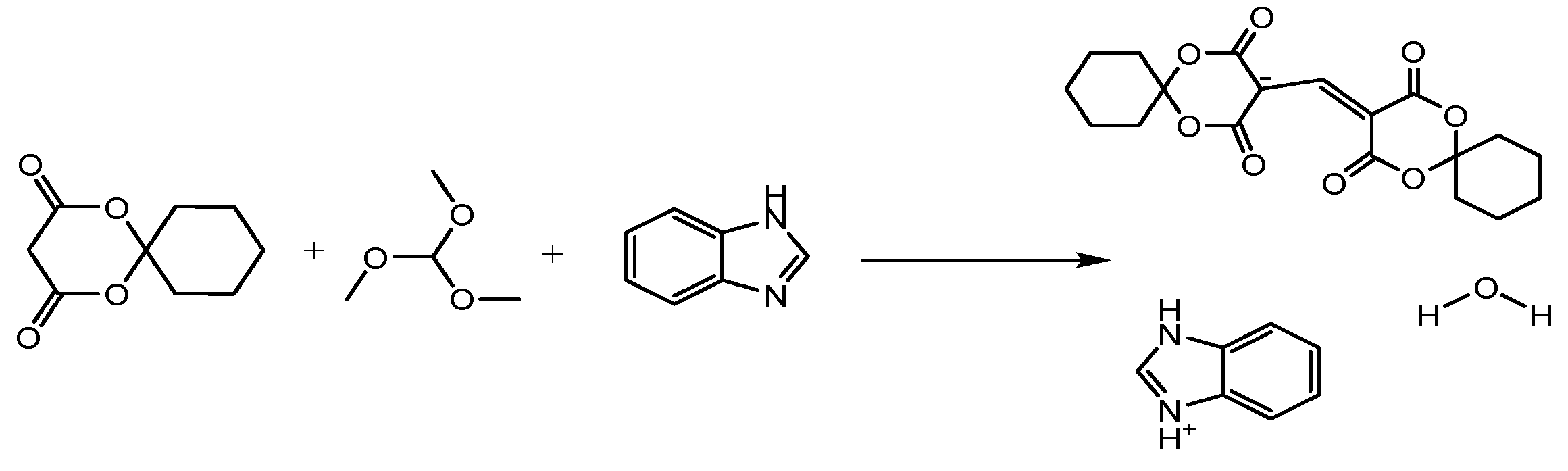
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of the Title Compound
3.3. Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lohar, T.; Jadhav, S.; Kumbhar, A.; Mane, A.; Salunkhe, R. Bis-amino methylation for the synthesis of spiro-fused piperidines using iron(III) trifluroacetate in aqueous micellar medium. Res. Chem. Intermediat. 2016, 42, 5329–5338. [Google Scholar] [CrossRef]
- Meena, K.; Kumari, S.; Khurana, J.M.; Malik, A.; Sharma, C.; Panwar, H. One pot three component synthesis of spiro[indolo-3,10′-indeno[1,2-b] quinolin]-2,4,11′-triones as a new class of antifungal and antimicrobial agents. Chin. Chem. Lett. 2017, 28, 136–142. [Google Scholar] [CrossRef]
- Shrestha, R.; Sharma, K.; Lee, Y.R.; Wee, Y.J. Cerium oxide-catalyzed multicomponent condensation. Mol. Divers. 2016, 20, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Khazir, J.; Singh, P.P.; Reddy, D.M.; Hyder, I.; Shafi, S.; Sawant, S.S.; Chashoo, G.; Mahajan, A.; Alam, M.S.; Saxena, A.K.; et al. Synthesis and anticancer activity of novel spiro-isoxazoline and spiro-isoxazolidine derivatives of asantonin. Eur. J. Med. Chem. 2013, 63, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Gandhi, D.M.; Kumar, A.V.; Srinivas, G.; Reddy, M.S.; Kanth, S.S.; Vijjulatha, M. Microwave assisted synthesis, biological evaluation, and molecular docking of novel chroman scaffolds incorporating spiro chromanone framework. Med. Chem. Res. 2016, 25, 2882–2894. [Google Scholar] [CrossRef]
- Borad, M.A.; Bhoi, M.N.; Rathwa, S.K.; Vasava, M.S.; Patel, H.D.; Patel, C.N.; Pandya, H.A.; Pithawala, E.A.; Georrge, J.J. Microwave assisted ZrSiO2 catalysed synthesis, characterization and computational study of novel spiro [indole-thiazolidines] derivatives as anti-tubercular agents. Interdiscip. Sci. Comput. Life Sci. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Baek, S.; Kim, S.; Song, J.H.; Lee, S.Y.; Kim, M. Trans-fused 5-(tert-butoxtycarbonyl) amino octahydroindenes as a protease activated receptor-1 (PAR1) antagonist. Arch. Pharm. Res. 2016, 39, 1275–1295. [Google Scholar] [CrossRef] [PubMed]
- Abdou, W.M.; Ganoub, N.A.; Sabry, E. Spiro- and substituted thiophenetriazaphospholes and phosphoramidates as potent antineoplastic agents: synthesis, biological evaluation, and SAR studies. Monatsh. Chem. 2016, 147, 619–626. [Google Scholar] [CrossRef]
- Iwamoto, K.; Kimura, H.; Oike, M.; Sato, M. Methylene-bridged bis(benzimidazolium) salt as a highly efficientcatalystfor thebenzoinreaction in aqueous media. Org. Biomol. Chem. 2008, 6, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Yıgıt, B.; Yetkin, Y.; Gök, Y.; Özdemır, I.; Gün, S. Synthesis and antimicrobial studies of 1-methyl-2-dimethylaminoethyl-substituted benzimidazolium salts and N-heterocyclic carbene–silver complexes. J. Coord. Chem. 2012, 65, 371–379. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Haque, R.A.; Ahamed, M.B.K.; Majid, A.M.S.; Al-Rawi, S.S. Synthesis and anticancer activity ofpara-xylyl linked bis-benzimidazolium salts and respective Ag(I)N-heterocyclic carbene complexes. Med. Chem. Res. 2013, 22, 2455–2466. [Google Scholar] [CrossRef]
- Lal, A.K.; Milton, M.D. Synthesis of new benzimidazolium salts with tunable emission intensities and their application as fluorescent probes for Fe3+ in pure aqueous media. Tetrahedron Lett. 2014, 55, 1810–1814. [Google Scholar] [CrossRef]
- Lee, J.P.; Yoo, B.; Suresh, T.; Kang, M.S.; Vital, R.; Kim, K.J. Novel silane-substituted benzimidazolium iodide as gel electrolyte for dye-sensitized solar cells. Electrochim. Acta 2009, 54, 4365–4370. [Google Scholar] [CrossRef]
- Zeng, W.L.; Cai, X.; Guo, H.M. Synthesis, experimental and theoretical characterization of 3-(4-(dimethylamino) benzylidene)-1,5-dioxaspiro [5.5]undecane-2,4-dione. Chin. J. Struct. Chem. 2013, 32, 1603–1610. [Google Scholar]
- Cremer, D.J.; Pople, A. A general definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Zeng, W.L. 5,5-[(2,4-Dichlorophenyl)methylene]bis(2,2-dimethyl-1,3dioxane-4,6-dione). Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Zeng, W.L. Synthesis and crystal structures of two new oxaspirocyclic compounds. Crystals 2016, 6, 134. [Google Scholar] [CrossRef]
- Jian, F.F.; Sian, H.L.; Sun, P.; Mukhopadhyay, U.; Bernal, I. Synthesis, structures and thermal properties of three crystalline polymorphs of the bis(n-phenylmethylbenzimidazole-n) dichloro cobalt(II) complex CoCl2(C7H5N2CH2Ph)2. J. Coord. Chem. 2004, 57, 923–934. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
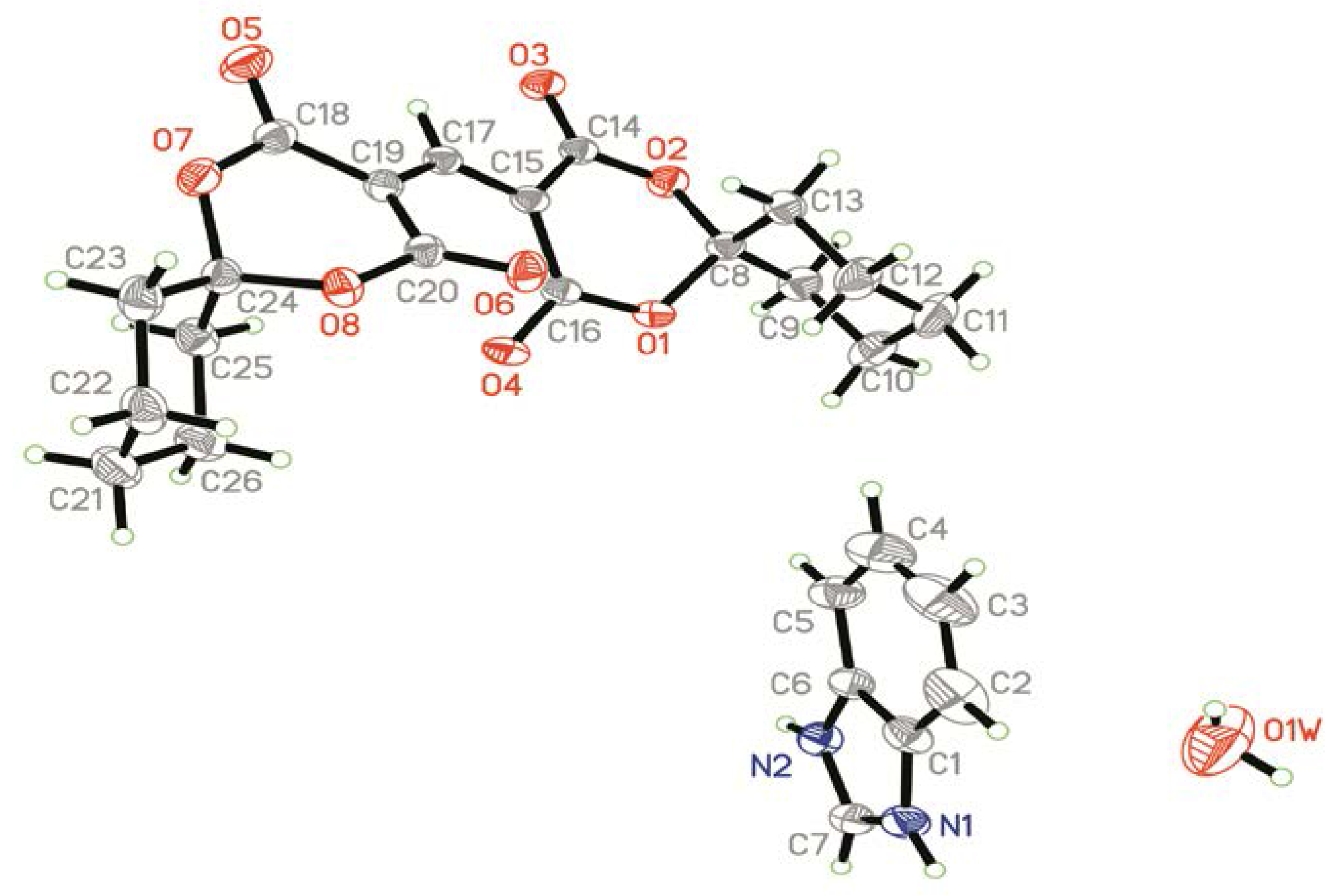
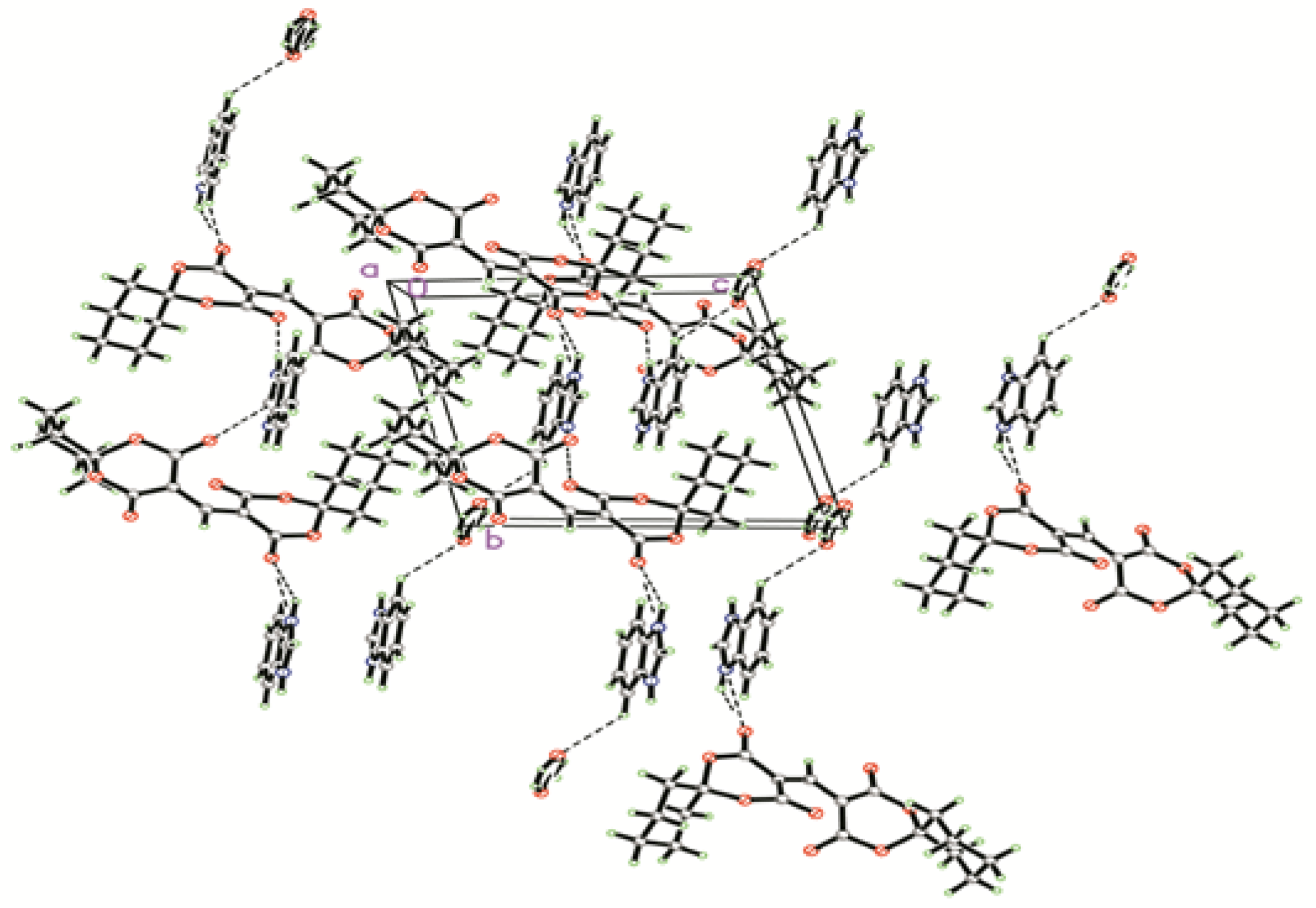
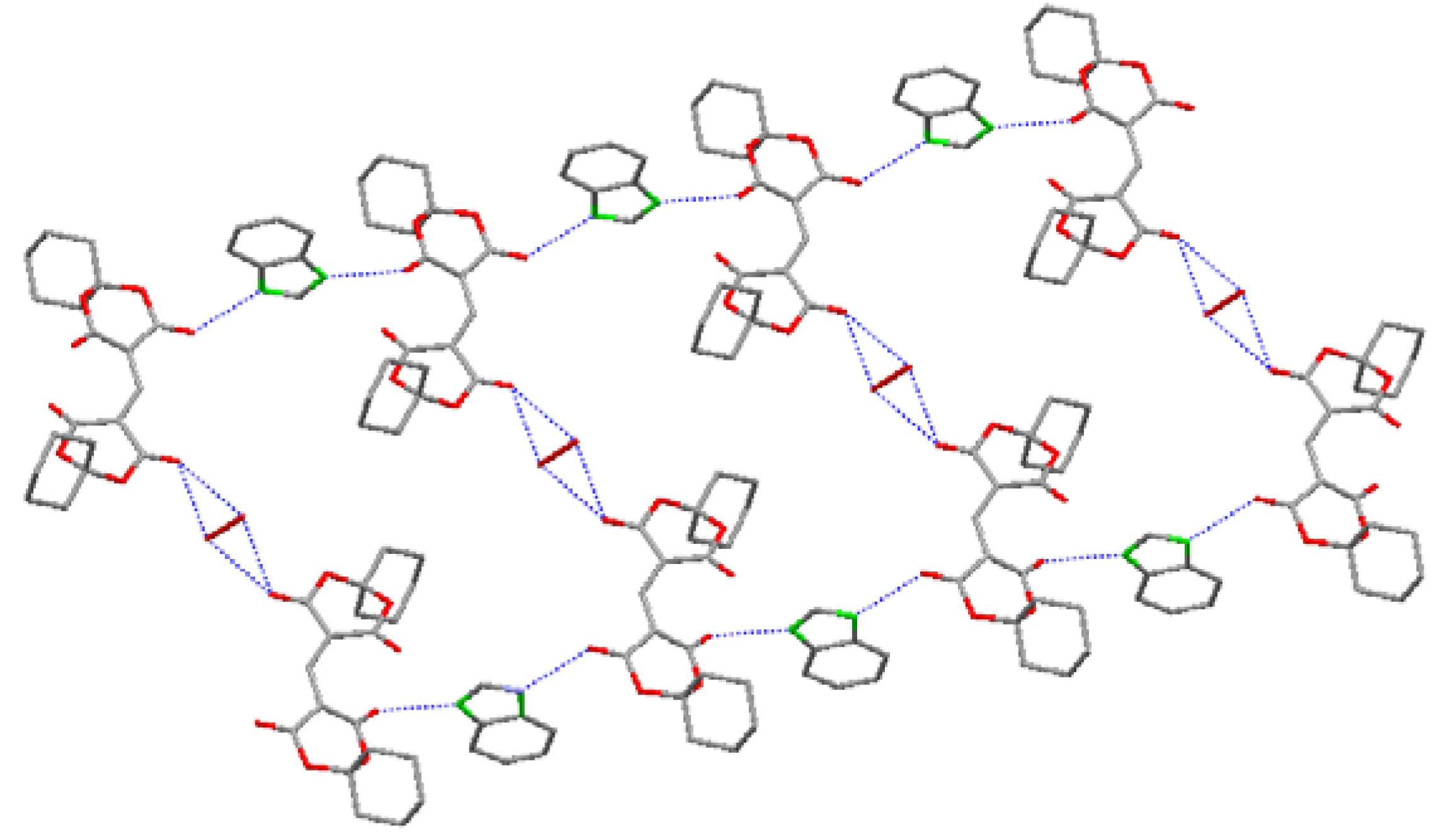
| Bond | Distances (Å) | Bond | Distances (Å) |
|---|---|---|---|
| N(1)−C(7) | 1.316(3) | N(2)−C(7) | 1.315(2) |
| N(1)−C(1) | 1.386(3) | N(2)−C(6) | 1.379(2) |
| C(14)−C(15) | 1.446(2) | C(18)−C(19) | 1.457(2) |
| C(15)−C(17) | 1.386(2) | C(19)−C(20) | 1.456(2) |
| C(15)−C(16) | 1.445(2) | O(2)−C(8) | 1.4422(2) |
| C(17)−C(19) | 1.380(2) | O(1)−C(8) | 1.4381(2) |
| O(7)−C(24) | 1.439(2) | O(8)−C(24) | 1.438(2) |
| Angle | (°) | Angle | (°) |
| C(19)−C(17)−C(15) | 131.18(1) | C(17)−C(15)−C(16) | 124.55(1) |
| C(17)−C(19)−C(18) | 116.84(1) | C(17)−C(15)−C(14) | 117.487(1) |
| C(20)−C(19)−C(18) | 117.55(2) | C(16)−C(15)−C(14) | 117.16(2) |
| O(8)−C(24)−O(7) | 108.89(1) | O(1)−C(8)−O(2) | 108.72(1) |
| Torsion | (°) | (°) | |
| O(3)−C(14)−C(15)−C(17) | −11.9(2) | C(17)−C(19)−C(20)−O(6) | 20.5(3) |
| O(2)−C(14)−C(15)−C(17) | 170.22(1) | O(5)−C(18)−C(19)−C(17) | −16.7(3) |
| O(3)−C(14)−C(15)−C(16) | 158.38(2) | C(17)−C(19)−C(20)−O(8) | −165.71(1) |
| C(15)−C(17)−C(19) −C(18) | −167.65(2) | C(14)−C(15)−C(17)−C(19) | −170.89(2) |
| D–H···A | Symmetry | D–H(Å) | H…A(Å) | D…A(Å) | ∠D–H···A (°) |
|---|---|---|---|---|---|
| N(1)–H(1A)···O(4) | −x + 1, −y + 1, −z + 1 | 0.899 | 1.832 | 2.728 | 173.53 |
| N(2)–H(2)···O(3) | −x + 1, −y, −z + 1 | 0.851 | 1.925 | 2.717 | 154.53 |
| O1W−H1W1…O(5) | x + 1, y + 1, z − 1 | 0.854 | 2.064 | 2.878 | 159.30 |
| O1W−H1W2…O(5) | −x, −y + 1, −z + 1 | 0.853 | 2.307 | 2.985 | 136.62 |
| Formula | (C19H21O8) (C7H7N2) (0.5H2O) |
|---|---|
| Formula Weight | 505.51 |
| Crystal System | Triclinic |
| Space group | P-1 |
| Crystal Size(mm3) | 0.40 × 0.38 × 0.37 |
| Wavelength (Å) | 0.71073 |
| a(Å) | 11.017(2) |
| b(Å) | 11.424(2) |
| c(Å) | 11.650(2) |
| α(°) | 70.60(3) |
| β(°) | 71.00(3) |
| γ(°) | 67.64(3) |
| V(Å3) | 1245.2(4) |
| Z | 2 |
| F(000) | 534 |
| D/g·cm−3 | 1.348 |
| −h, h/−k, k/−l, l | −13:14; −14:14; −15:15 |
| Reflections collected | 12207 |
| Independent reflections | 5629[R(int) = 0.0345] |
| Reflections observed(I > 2σ(I)) | 4279 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 5629/0/342 |
| Goodness-of-fit on F2 | 1.081 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0481, wR2 = 0.1265 |
| R indices (all data) | R1 = 0.0650, wR2 = 0.1490 |
| Largest diff. peak and hole (e Å−3) | 0.373, –0.218 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Jiang, J. Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure. Crystals 2017, 7, 303. https://doi.org/10.3390/cryst7100303
Zeng W, Jiang J. Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure. Crystals. 2017; 7(10):303. https://doi.org/10.3390/cryst7100303
Chicago/Turabian StyleZeng, Wulan, and Jinhe Jiang. 2017. "Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure" Crystals 7, no. 10: 303. https://doi.org/10.3390/cryst7100303
APA StyleZeng, W., & Jiang, J. (2017). Synthesis and Crystal Structure of a New Hydrated Benzimidazolium Salt Containing Spiro Structure. Crystals, 7(10), 303. https://doi.org/10.3390/cryst7100303






