Synthesis and X-ray Crystal Structure of N’-Cyano-N,N’-dimethyl-4-nitrobenzohydrazide
Abstract
:1. Introduction
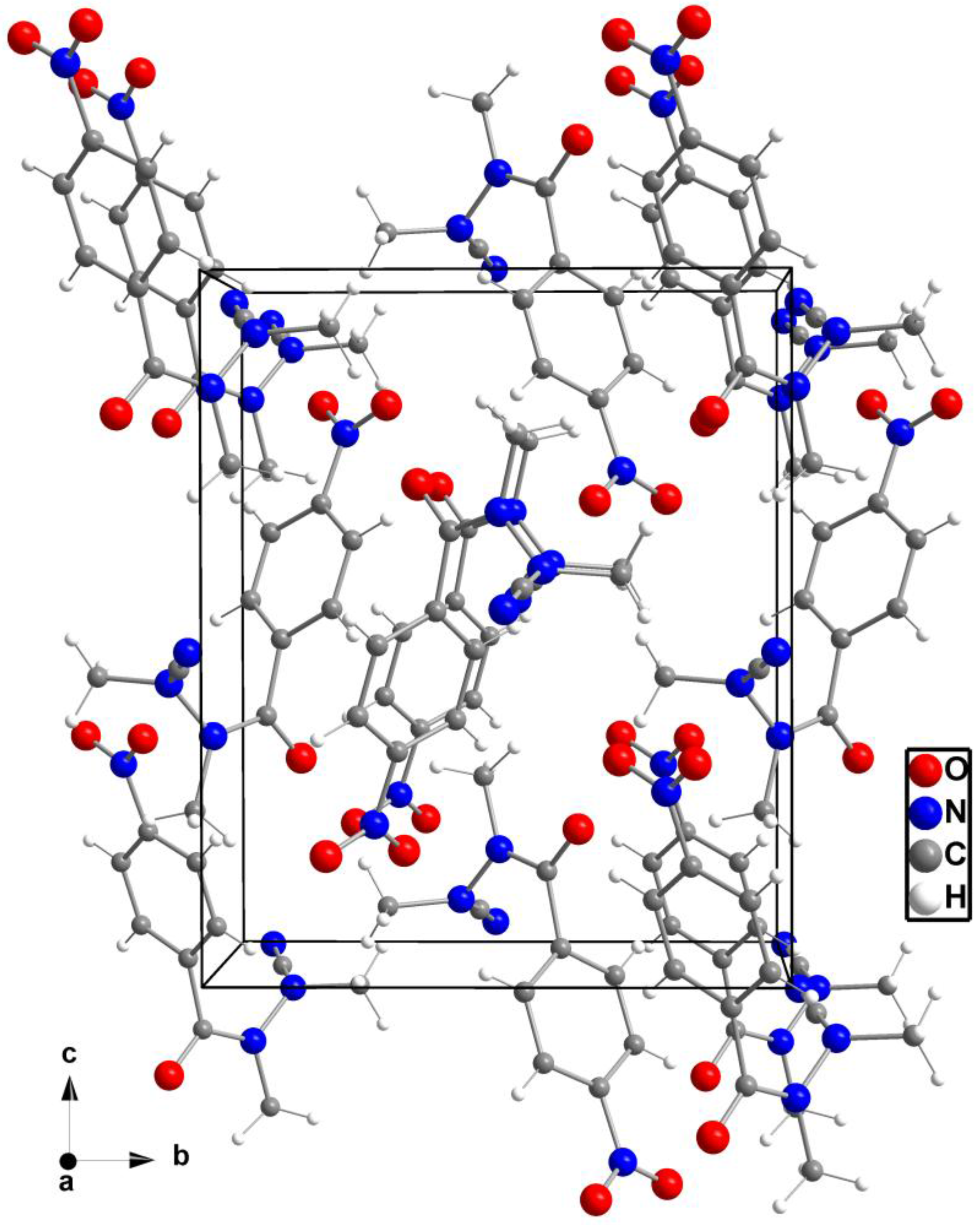
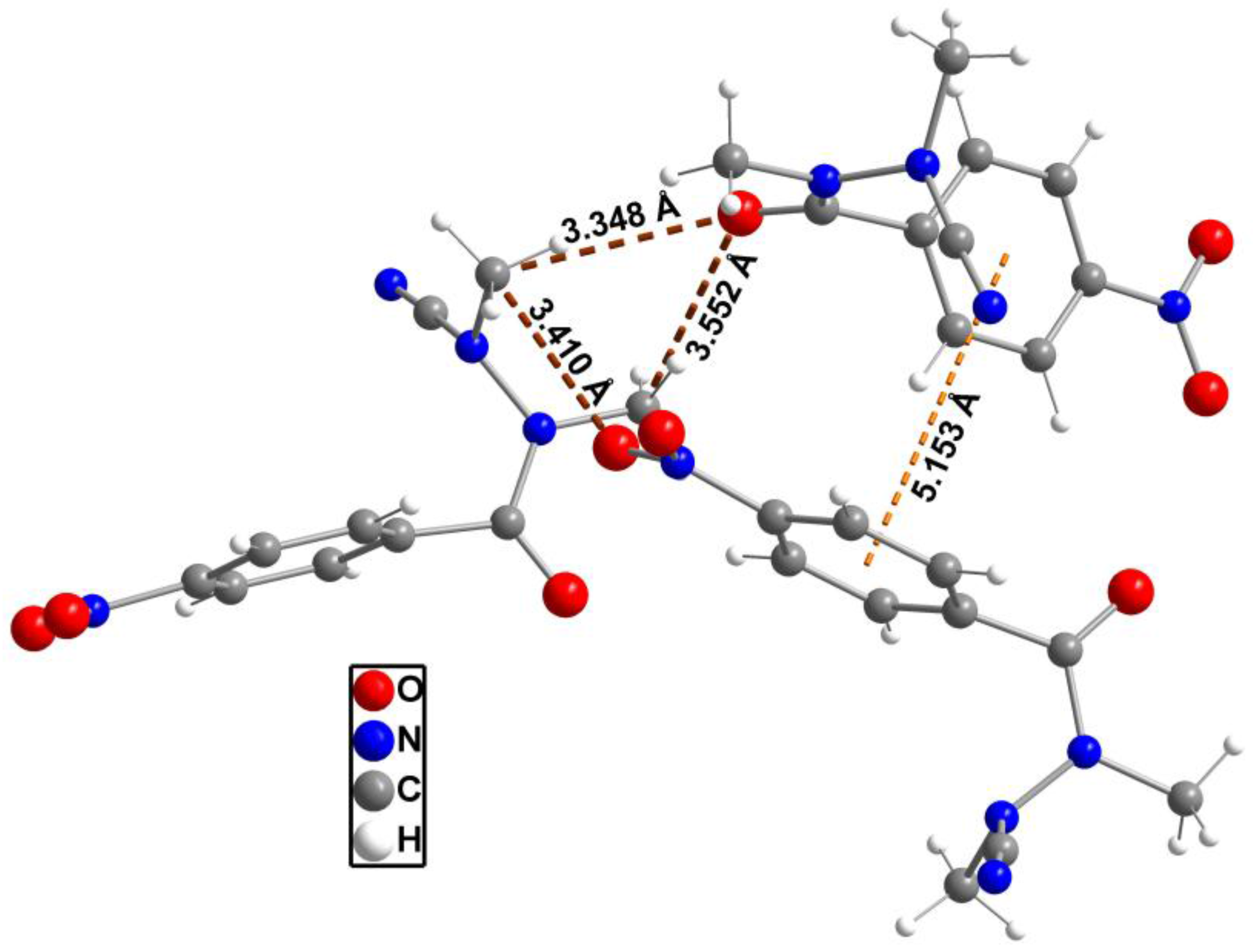
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Synthesis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Löser, R.; Frizler, M.; Schilling, K.; Gütschow, M. Azadipeptide nitriles: Highly potent and proteolytically stable inhibitors of papain-like cysteine proteases. Angew. Chem. Int. Ed. 2008, 47, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Löser, R.; Nieger, M.; Gütschow, M. Synthesis and crystal structure of benzyl [(1S)-1-(5-amino-1,3,4-oxadiazol-2-yl)-2-phenylethyl]carbamate. Crystals 2012, 2, 1201–1209. [Google Scholar] [CrossRef]
- Loh, Y.; Shi, H.; Hu, M.; Yao, S.Q. “Click” synthesis of small molecule-peptide conjugates for organelle-specific delivery and inhibition of lysosomal cysteine proteases. Chem. Commun. 2010, 46, 8407–8409. [Google Scholar] [CrossRef] [PubMed]
- Frizler, M.; Lohr, F.; Furtmann, N.; Kläs, J.; Gütschow, M. Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J. Med. Chem. 2011, 54, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.F.; Li, H.W.; Fang, X.; Wu, Y.; Wang, L.; Zou, S. Highly selective azadipeptide nitrile inhibitors for cathepsin K: Design, synthesis and activity assays. Org. Biomol. Chem. 2013, 11, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Löser, R.; Bergmann, R.; Frizler, M.; Mosch, B.; Dombrowski, L.; Kuchar, M.; Steinbach, J.; Gütschow, M.; Pietzsch, J. Synthesis and radiopharmacological characterisation of a fluorine-18-labelled azadipeptide nitrile as a potential PET tracer for in vivo imaging of cysteine cathepsins. ChemMedChem 2013, 8, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xiao, F.; Zhan, K.; Kim, Y.P.; Xie, H.; Xia, Z.; Rao, J. A biocompatible condensation reaction for the labeling of terminal cysteine residues on proteins. Angew. Chem. Int. Ed. 2009, 48, 9658–9662. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jeon, J.; Gambhir, S.S.; Rao, J.; Chin, F.T. 18F-Cyanobenzolthiol ([18F]CBT): A novel 18F-prosthetic group for labeling peptide or protein. J. Label. Compd. Radiopharm. 2011, 54, S503. [Google Scholar] [CrossRef]
- Inkster, J.A.; Colin, D.J.; Seimbille, Y. A novel 2-cyanobenzothiazole-based 18F prosthetic group for conjugation to 1,2-aminothiol-bearing targeting vectors. Org. Biomol. Chem. 2015, 13, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.W.; Cobb, J.; Nyburg, S.C.; Parkins, A.W. Some stereochemical aspects of the Pellizzari rearrangement. Tetrahedron 1995, 51, 13161–13166. [Google Scholar] [CrossRef]
- Bilewicz, E.; Malecka, M.; Grabowski, S.J.; Mloston, G. 2′-(4-Chlorophenyl)-2,3,4,5,6,7-hexahydro-4′,7′-methanospiro[9H-fluorene-9,3′-1H-indazole]-1′-carbo-nitrile and methyl 4′-chloro-2′-(4-chlorophenyl)-1′-cyanospiro[9H-fluorene-9,3′-pyrazolidine]-4′-carboxylate. Acta Cryst. 2007, C63, o739–o742. [Google Scholar] [CrossRef]
- Yang, P.Y.; Wang, M.; Li, L.; Wu, H.; He, C.Y.; Yao, S.Q. Design, synthesis and biological evaluation of potent azadipeptide nitrile inhibitors and activity-based probes as promising anti-Trypanosoma brucei agents. Chem. Eur. J. 2012, 18, 6528–6541. [Google Scholar] [CrossRef] [PubMed]
- McNab, H.; Hulme, A.; Benstead, D.; Wight, P. An efficient synthesis of substituted hydrazides. Synlett 2005, 1571–1574. [Google Scholar] [CrossRef]
- Ottersbach, P.A.; Schnakenburg, G.; Gütschow, M. Induction of chirality: Experimental evidence of atropisomerism in azapeptides. Chem. Commun. 2012, 48, 5772–5774. [Google Scholar] [CrossRef] [PubMed]
- Ottersbach, P.A.; Schnakenburg, G.; Gütschow, M. Atropisomerism in azadipeptides: Evaluation of N1-methylation and thioamide introduction. Tetrahedron Lett. 2015, 56, 4889–4891. [Google Scholar] [CrossRef]
- Ottersbach, P.A.; Schmitz, J.; Schnakenburg, G.; Gütschow, M. An access to aza-Freidinger lactams and E-locked analogs. Org. Lett. 2013, 15, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Chingle, R.; Lubell, W.D. Azopeptides: Synthesis and pericyclic chemistry. Org. Lett. 2015, 17, 5400–5403. [Google Scholar] [CrossRef] [PubMed]
- Licandro, E.; Perdicchia, D. N-Acylhydrazines: Future perspectives offered by new syntheses and chemistry. Eur. J. Org. Chem. 2004, 665–675. [Google Scholar] [CrossRef]
- Panaka, S.; Trivedi, R.; Sony, T.; Prabhakar, S.; Raju Chowhan, L. Silver(I) catalyzed intramolecular cyclization of N-(2-(alk-1-yn-1-yl))-1H-tetrazoles leading to the formation of N-cyano-2-substituted indoles under ambient conditions. Org. Chem. Front. 2017, 4, 1574–1579. [Google Scholar] [CrossRef]
- Paciaroni, N.G.; Ratnayake, R.; Matthews, J.H.; Norwood, V.M., IV; Arnold, A.C.; Dang, L.H.; Luesch, H.; Huigens, R.W., III. A tryptoline ring-distortion strategy leads to complex and diverse biologically active molecules from the indole alkaloid yohimbine. Chem. Eur. J. 2017, 23, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Scheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL 2014/1; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Bruker AXS Inc. APEX-II (ver. 2008.1-0), SAINT (ver. 7.51A) and SADABS (ver. 2007/4); Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- The Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 20 September 2017).


| Formula | C10H10N4O3 |
| Formula weight (g·mol−1) | 234.22 |
| Temperature (K) | 296 |
| Wavelength (Å) | 0.71073 |
| Crystal system | orthorhombic |
| Space group | P212121 |
| Unit cell dimensions | |
| a (Å) | 8.1974(6) |
| b (Å) | 10.6696(7) |
| c (Å) | 12.9766(8) |
| Volume (Å3) | 1135.0(1) |
| Z | 4 |
| Density (calcd.) (g·cm–3) | 1.371 |
| Absorpt. coeff. (mm–1) | 0.10 |
| F(000) | 488 |
| Crystal size (mm3) | 0.31 × 0.06 × 0.06 |
| Refinement method | Full matrix least-squares on F2 |
| Data/restraints/param. | 2719/0/155 |
| Measured reflections | 18,484 |
| 2 θmax (°) | 28.0 |
| Rint | 0.069 |
| GoF on F2 | 0.99 |
| R1 [I > 2σ(I)] | 0.046 |
| wR2 (all data) | 0.131 |
| Compound 2 | Other Nα-substituted 4-nitrobenzoylhydrazines | ||||
|---|---|---|---|---|---|
| Range | Mean ± SEM | Median | n | ||
| Carbonyl-phenyl twist (°) | 87.9 | 23.74–66.42 | 44.37 ± 2.70 | 43.99 | 13 |
| Distance C1–O1 (Å) | 1.213(4) | 1.203–1.243 | 1.226 ± 0.004 | 1.231 | |
| Distance C1–C5 (Å) | 1.491(5) | 1.476–1.516 | 1.499 ± 0.003 | 1.498 | |
| Distance C1–N1 (Å) | 1.343(4) | 1.324–1.418 | 1.358 ± 0.009 | 1.358 | |
| Compound | Nα–Nβ Distance | Torsion of the N–N Bond 1 | Out-of-Plane Angle | CCDC Number | Ref. | |
|---|---|---|---|---|---|---|
| Nα | Nβ | |||||
| 2 | 1.404 Å | 85.7° | 2.40° | 19.4° | 1567206 | this work |
| I | 1.425 Å | 52.1° | 10.82° | 28.91° | 1315768 | [10] |
| II | 1.394 Å | 82.8° | 7.60° | 10.73° | 837002 | [14] |
| III | 1.396 Å | 85.6° | 2.33° | 9.56° | 1401837 | [15] |
| IV | 1.400 Å | 85.9° | 0° | 14.26° | 896854 | [16] |
 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löser, R.; Pitzschler, R.; Köckerling, M. Synthesis and X-ray Crystal Structure of N’-Cyano-N,N’-dimethyl-4-nitrobenzohydrazide. Crystals 2017, 7, 290. https://doi.org/10.3390/cryst7100290
Löser R, Pitzschler R, Köckerling M. Synthesis and X-ray Crystal Structure of N’-Cyano-N,N’-dimethyl-4-nitrobenzohydrazide. Crystals. 2017; 7(10):290. https://doi.org/10.3390/cryst7100290
Chicago/Turabian StyleLöser, Reik, Riccardo Pitzschler, and Martin Köckerling. 2017. "Synthesis and X-ray Crystal Structure of N’-Cyano-N,N’-dimethyl-4-nitrobenzohydrazide" Crystals 7, no. 10: 290. https://doi.org/10.3390/cryst7100290
APA StyleLöser, R., Pitzschler, R., & Köckerling, M. (2017). Synthesis and X-ray Crystal Structure of N’-Cyano-N,N’-dimethyl-4-nitrobenzohydrazide. Crystals, 7(10), 290. https://doi.org/10.3390/cryst7100290








