Crystal Growth and Associated Properties of a Nonlinear Optical Crystal—Ba2Zn(BO3)2
Abstract
:1. Introduction
2. Results and Discussion
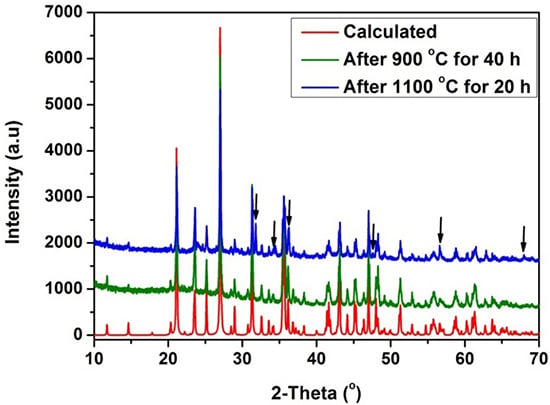
2.1. Polycrystalline Powder Synthesis and Characterization
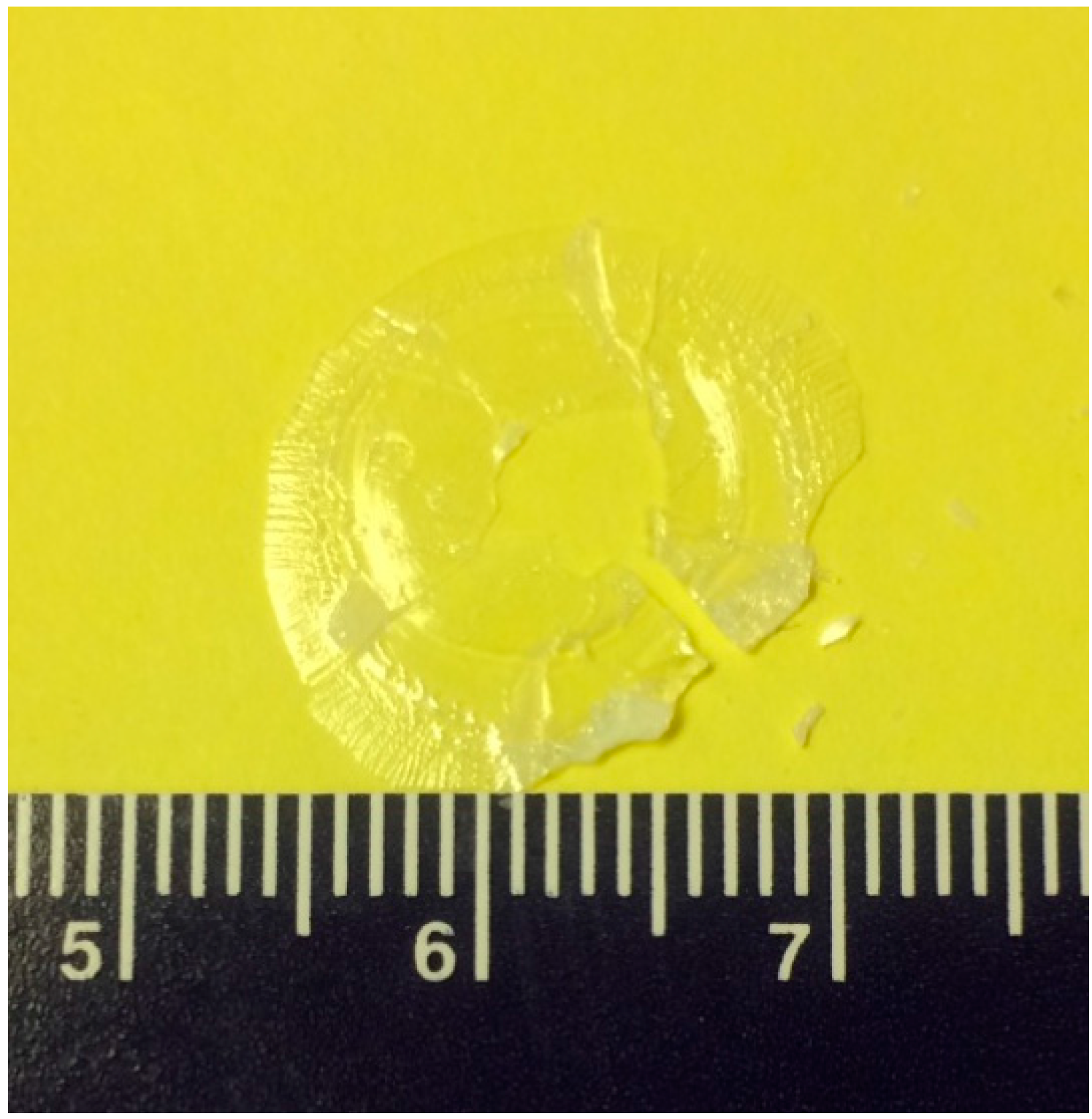
2.2. Crystal Growth
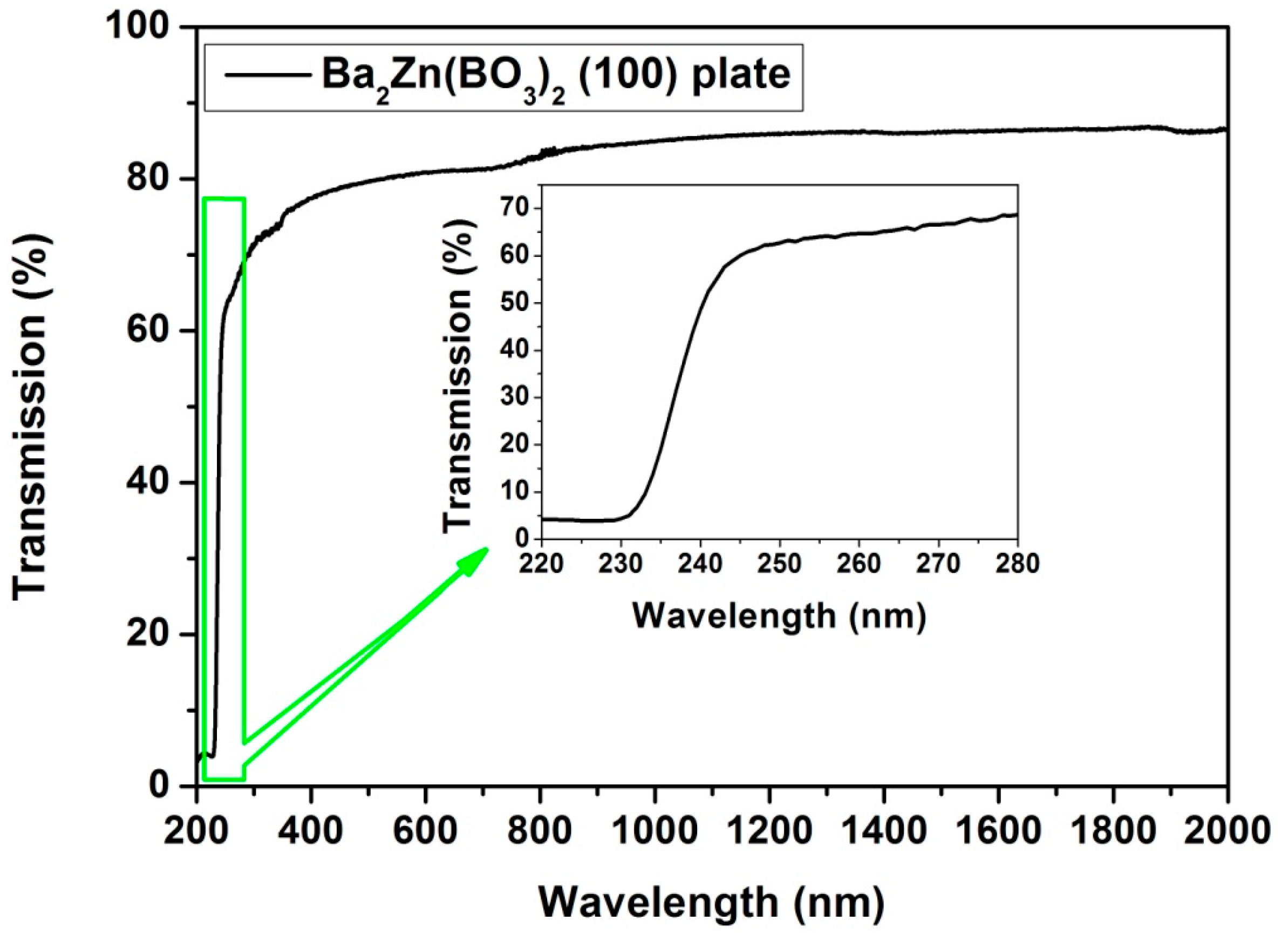
2.3. UV-Visible-Near Infrared Transmission Spectra
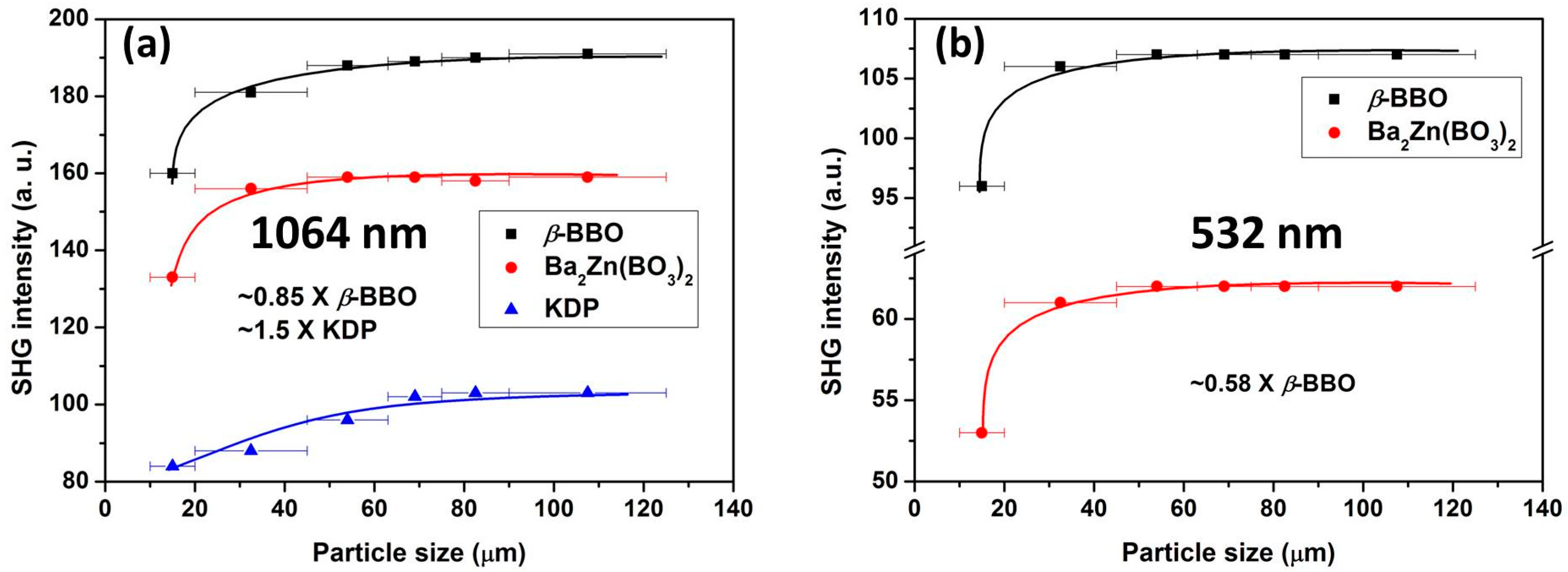
2.4. Powder SHG Measurements
3. Materials and Methods
3.1. Materials and Polycrystalline Powder Synthesis
3.2. Powder X-Ray Diffraction
3.3. Single Crystal Growth of Ba2Zn(BO3)2
3.4. Optical Spectra Measurement
3.5. Powder Second Harmonic Generation Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, C.; Lin, Z.; Wang, Z. The development of new borate-based UV nonlinear optical crystals. Appl. Phys. B 2005, 80, 1–25. [Google Scholar] [CrossRef]
- Becker, P. Borate Materials in Nonlinear Optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Petrov, V.; Rempel, C.; Stolberg, K.-P.; Schade, W. Widely tunable continuous-wave mid-infrared laser source based on difference-frequency generation in AgGaS2. Appl. Opt. 1998, 37, 4925–4928. [Google Scholar] [CrossRef] [PubMed]
- Gettemy, D.J.; Harker, W.C.; Lindholm, G.; Barnes, N.P. Some Optical Properties of KTP, LiIO3, and LiNbO3. IEEE J. Quantum Electron. 1988, 24, 2231–2237. [Google Scholar] [CrossRef]
- Arun Kumar, R. Borate Crystals for Nonlinear Optical and Laser Applications: A Review. J. Chem. 2013, 2013, 154862. [Google Scholar] [CrossRef]
- Dewey, C.F.; Cook, W.R.; Hodgson, R.T.; Wynne, J.J. Frequency doubling in KB5O8·4H2O and NH4B5O8·4H2O to 217.3 nm. Appl. Phys. Lett. 1975, 26, 714–716. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Jiang, A.; Wu, B.; You, G.; Li, R.; Lin, S. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Chen, C.; Wu, B.; Jiang, A.; You, G. A new-type ultraviolet SHG crystal—β-BaB2O4. Sci. China Ser. B 1985, 28, 235–243. [Google Scholar]
- Smith, R.W.; Keszler, D.A. The noncentrosymmetric orthoborate BaZn2(BO3)2. J. Solid State Chem. 1992, 100, 325–330. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Nakai, S.; Yokotani, A.; Tang, H.; Chen, C. CsB3O5: A new nonlinear optical crystal. Appl. Phys. Lett. 1993, 62, 2614–2615. [Google Scholar] [CrossRef]
- Smith, R.W.; Koliha, L.J. A new noncentrosymmetric orthoborate [Ba2Zn(BO3)2]. Mater. Res. Bull. 1994, 29, 1203–1210. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Xia, Y.; Wu, B.; Tang, D.; Wu, K.; Wenrong, Z.; Yu, L.; Mei, L. New development of nonlinear optical crystals for the ultraviolet region with molecular engineering approach. J. Appl. Phys. 1995, 77, 2268–2272. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.B.; Wu, B.C.; Wu, K.C.; Zeng, W.L.; Yu, L.H. Design and synthesis of an ultraviolet-transparent nonlinear-optical crystal Sr2Be2B2O7. Nature 1995, 373, 322–324. [Google Scholar] [CrossRef]
- Tu, J.-M.; Keszler, D.A. CsLiB6O10: A noncentrosymmetric polyborate. Mater. Res. Bull. 1995, 30, 209–215. [Google Scholar] [CrossRef]
- Ye, N.; Zeng, W.; Wu, B.; Chen, C. Two new nonlinear optical crystals: BaAl2B2O7 and K2Al2B2O7. SPIE 1998, 3556, 21–23. [Google Scholar]
- Ono, Y.; Nakaya, M.; Sugawara, T.; Watanabe, N.; Siraishi, H.; Komatsu, R.; Kajitani, T. Structural study of LiKB4O7 and LiRbB4O7: New nonlinear optical crystals. J. Cryst. Growth 2001, 229, 472–476. [Google Scholar] [CrossRef]
- Plachinda, P.A.; Dolgikh, V.A.; Stefanovich, S.Y.; Berdonosov, P.S. Nonlinear-optical susceptibility of hilgardite-like borates M2B5O9X (M = Pb, Ca, Sr, Ba; X = Cl, Br). Solid State Sci. 2005, 7, 1194–1200. [Google Scholar] [CrossRef]
- Barbier, J.; Cranswick, L.M. D. The non-centrosymmetric borate oxides, MBi2B2O7 (M = Ca, Sr). J. Solid State Chem. 2006, 179, 3958–3964. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Fu, P. Growth and characterization of a new nonlinear optical crystal Ca5(BO3)3F. J. Cryst. Growth 2006, 292, 449–453. [Google Scholar] [CrossRef]
- Wang, S.; Ye, N. Nonlinear optical crystal BiAlGa2(BO3)4. Solid State Sci. 2007, 9, 713–717. [Google Scholar] [CrossRef]
- Li, F.; Hou, X.; Pan, S.; Wang, X. Growth, Structure, and Optical Properties of a Congruent Melting Oxyborate, Bi2ZnOB2O6. Chem. Mater. 2009, 21, 2846–2850. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Zhang, J.; Fan, F.; Liu, Y.; Fu, P. Crystal Growth, Structure, and Properties of a Non-Centrosymmetric Fluoride Borate, Ba3Sr4(BO3)3F5. Cryst. Growth Des. 2009, 9, 3137–3141. [Google Scholar] [CrossRef]
- Li, R.K.; Chen, P. Cation Coordination Control of Anionic Group Alignment to Maximize SHG Effects in the BaMBO3F (M = Zn, Mg) Series. Inorg. Chem. 2010, 49, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- McMillen, C.D.; Stritzinger, J.T.; Kolis, J.W. Two Novel Acentric Borate Fluorides: M3B6O11F2 (M = Sr, Ba). Inorg. Chem. 2012, 51, 3953–3955. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, S.; Poeppelmeier, K.R.; Li, H.; Jia, D.; Chen, Z.; Fan, X.; Yang, Y.; Rondinelli, J.M.; Luo, H. K3B6O10Cl: A new structure analogous to perovskite with a large second harmonic generation response and deep UV absorption edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pan, S.; Hou, X.; Wang, C.; Poeppelmeier, K.R.; Chen, Z.; Wu, H.; Zhou, Z. A congruently melting and deep UV nonlinear optical material: Li3Cs2B5O10. J. Mater. Chem. 2011, 21, 2890–2894. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, S.; Li, H.; Han, J.; Chen, Z.; Zhao, W.; Zhou, Z. Li4Cs3B7O14: Synthesis, crystal structure, and optical properties. Inorg. Chem. 2011, 50, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pan, S.; Zhao, W.; Yu, H.; Wu, H.; Yang, Z.; Yang, Y. A new noncentrosymmetric vanadoborate: Synthesis, crystal structure and characterization of K2SrVB5O12. Dalton. Trans. 2012, 41, 9202–9208. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, S.; Wu, H.; Zhao, W.; Zhang, F.; Li, H.; Yang, Z. A new congruent-melting oxyborate, Pb4O(BO3)2 with optimally aligned BO3 triangles adopting layered-type arrangement. J. Mater. Chem. 2012, 22, 2105–2110. [Google Scholar] [CrossRef]
- Zhao, W.; Pan, S.; Wang, Y.; Yang, Z.; Wang, X.; Han, J. Structure, growth and properties of a novel polar material, KSr4B3O9. J. Solid State Chem. 2012, 195, 73–78. [Google Scholar] [CrossRef]
- Wu, H.; Yu, H.; Yang, Z.; Hou, X.; Su, X.; Pan, S.; Poeppelmeier, K.R.; Rondinelli, J.M. Designing a Deep Ultraviolet Nonlinear Optical Material with Large Second Harmonic Generation Response. J. Am. Chem. Soc. 2013, 135, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, H.; Pan, S.; Yang, Z.; Hou, X.; Su, X.; Jing, Q.; Poeppelmeier, K.R.; Rondinelli, J.M. Cs3Zn6B9O21: A Chemically Benign Member of the KBBF Family Exhibiting the Largest Second Harmonic Generation Response. J. Am. Chem. Soc. 2014, 136, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, S.; Han, J.; Yang, Z.; Su, X.; Zhao, W. Li2Sr4B12O23: A new alkali and alkaline-earth metal mixed borate with [B10O18]6− network and isolated [B2O5]4− unit. J. Solid State Chem. 2012, 190, 92–97. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, P.; Bai, L.; Xu, X.; Zhang, S.; Sun, Z.; Lin, Z.; Hong, M.; Chen, C.; Luo, J. Beryllium-free Li4Sr(BO3)2 for deep-ultraviolet nonlinear optical applications. Nat. Comm. 2014, 5, 4019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kang, L.; Shen, Y.; Wang, X.; Asghar, M.A.; Lin, Z.; Xu, Y.; Zeng, S.; Hong, M.; Luo, J. Designing a Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material without a Structural Instability Problem. J. Am. Chem. Soc. 2016, 138, 2961–2964. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, R. The anionic group theory of the non-linear optical effect and its applications in the development of new high-quality NLO crystals in the borate series. Int. Rev. Phys. Chem. 1989, 8, 65–91. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, C.; Tang, D.; Wu, B. New nonlinear optical crystals for UV and VUV harmonic generation. Adv. Mater. 1995, 7, 79–81. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, C.Q.; Chen, C.T.; Xu, Z.Y.; Wang, J.Y. Determination of nonlinear optical coefficients of KBe2BO3F2 crystals. Chin. Phys. Lett. 2003, 20, 243–245. [Google Scholar]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Xia, M.; Li, R.K. Growth, structure and optical properties of nonlinear optical crystal BaZnBO3F. J. Solid State Chem. 2016, 233, 58–61. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, M.; Li, R.K. Flux growth of a potential nonlinear optical crystal BaMgBO3F. J. Cryst. Growth 2011, 318, 971–973. [Google Scholar] [CrossRef]
- Kurtz, S.K.; Perry, T.T. A Powder Technique for the Evaluation of Nonlinear Optical Materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Ok, K.M.; Chi, E.O.; Halasyamani, P.S. Bulk characterization methods for non-centrosymmetric materials: Second-harmonic generation, piezoelectricity, pyroelectricity, and ferroelectricity. Chem. Soc. Rev. 2006, 35, 710–717. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yu, H.; Wu, H.; Halasyamani, P.S. Crystal Growth and Associated Properties of a Nonlinear Optical Crystal—Ba2Zn(BO3)2. Crystals 2016, 6, 68. https://doi.org/10.3390/cryst6060068
Zhang W, Yu H, Wu H, Halasyamani PS. Crystal Growth and Associated Properties of a Nonlinear Optical Crystal—Ba2Zn(BO3)2. Crystals. 2016; 6(6):68. https://doi.org/10.3390/cryst6060068
Chicago/Turabian StyleZhang, Weiguo, Hongwei Yu, Hongping Wu, and P. Shiv Halasyamani. 2016. "Crystal Growth and Associated Properties of a Nonlinear Optical Crystal—Ba2Zn(BO3)2" Crystals 6, no. 6: 68. https://doi.org/10.3390/cryst6060068
APA StyleZhang, W., Yu, H., Wu, H., & Halasyamani, P. S. (2016). Crystal Growth and Associated Properties of a Nonlinear Optical Crystal—Ba2Zn(BO3)2. Crystals, 6(6), 68. https://doi.org/10.3390/cryst6060068