Synthesis and Molecular Structures of the Lowest Melting Odd- and Even-Numbered α,β-Unsaturated Carboxylic Acids—(E)-Hept-2-Enoic Acid and (E)-Oct-2-Enoic Acid
Abstract
:1. Introduction
2. Results and Discussion
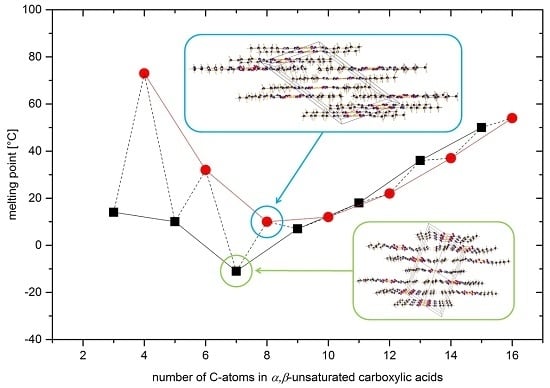
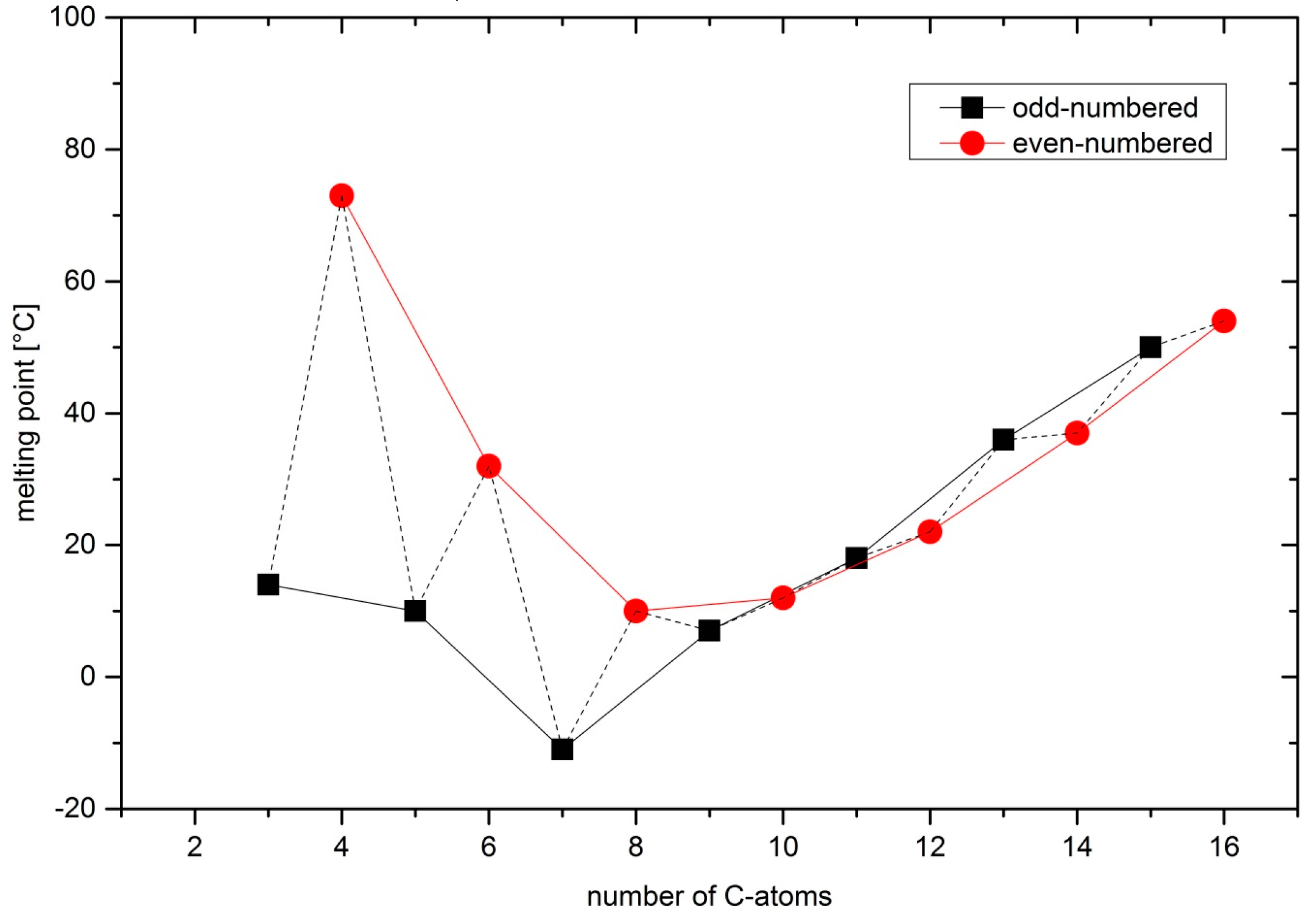
2.1. Synthesis and Melting Points
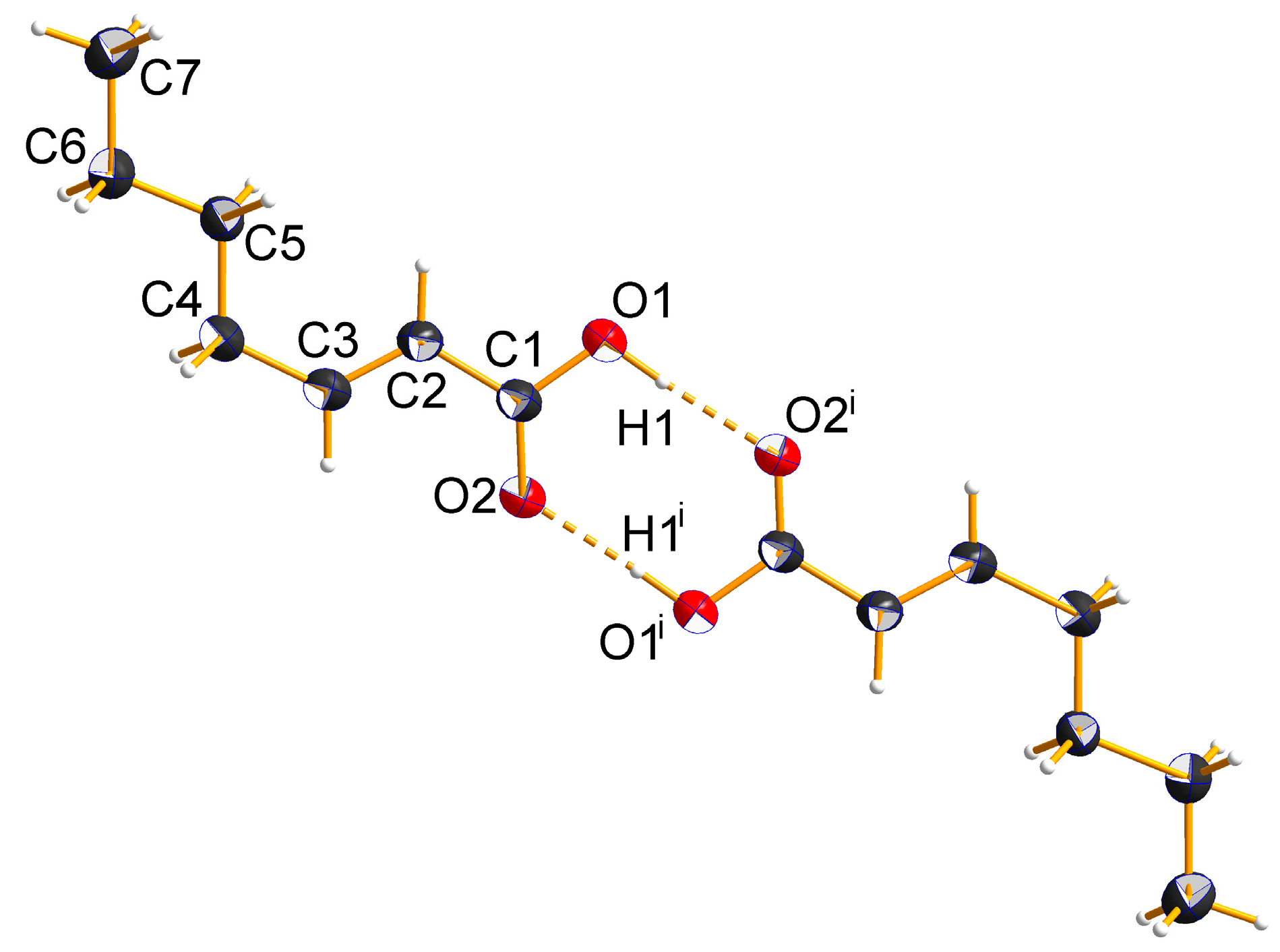
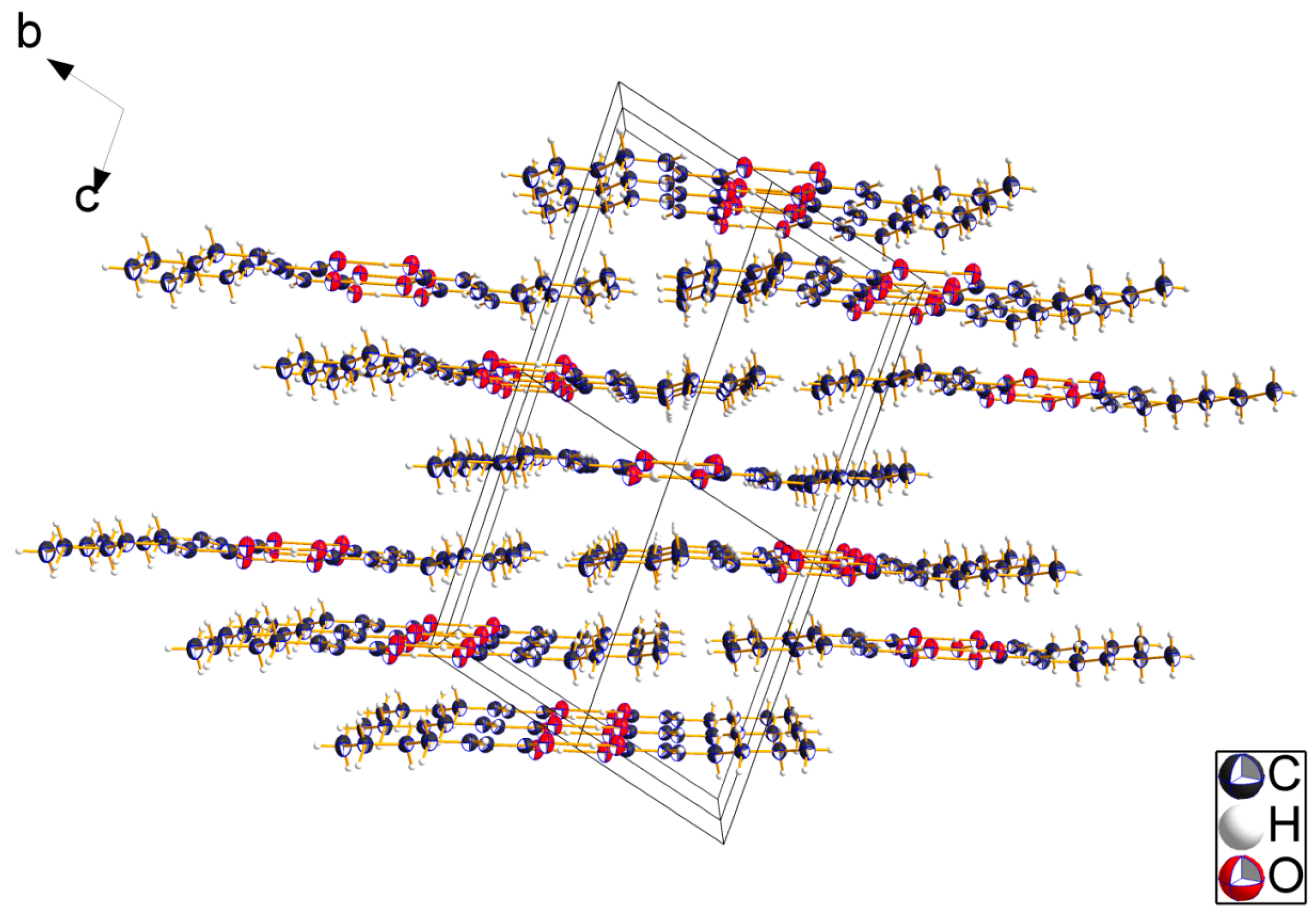
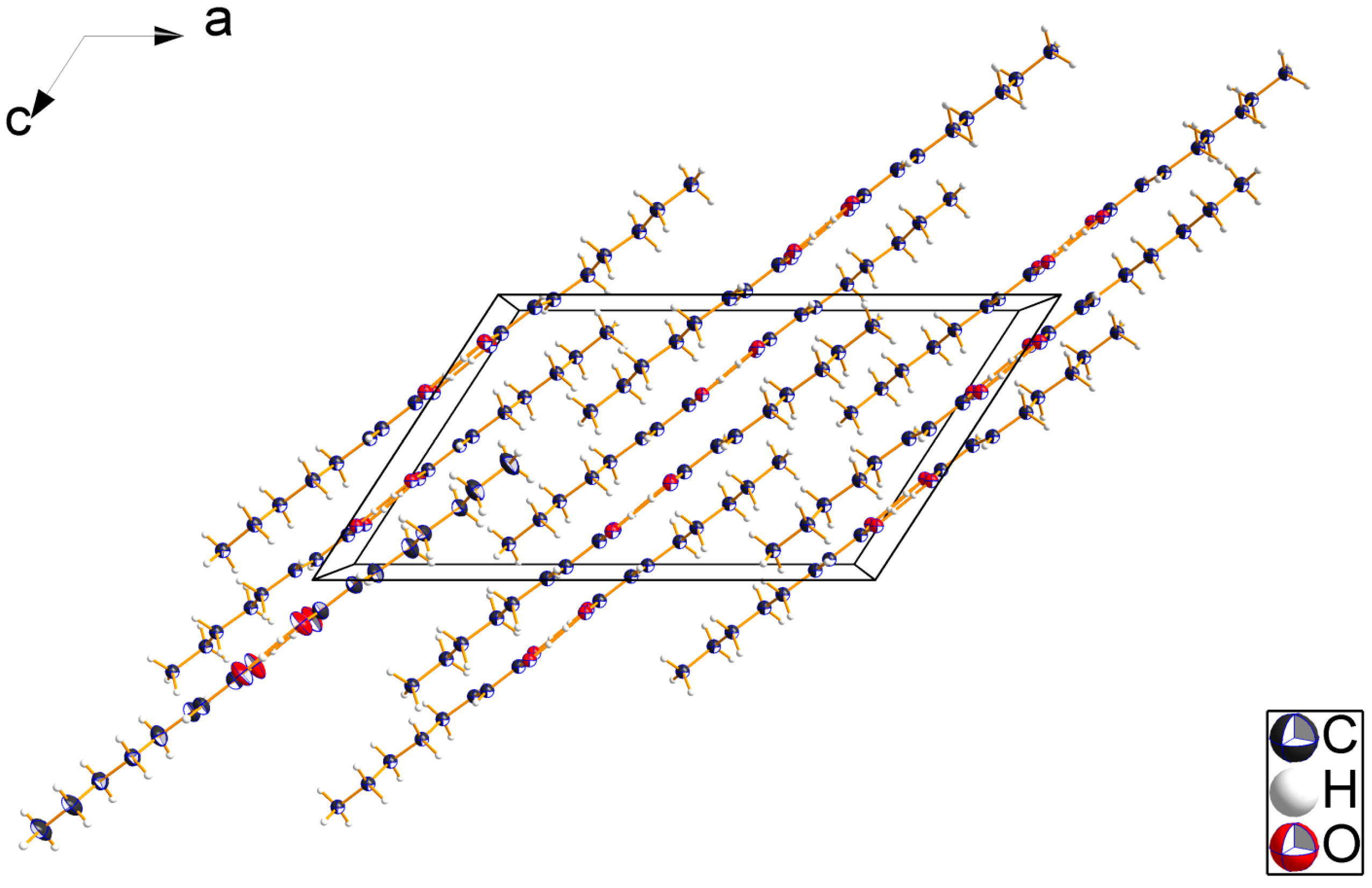
2.2. Crystal Structures
3. Experimental Section
3.1. General Considerations
3.2. Materials
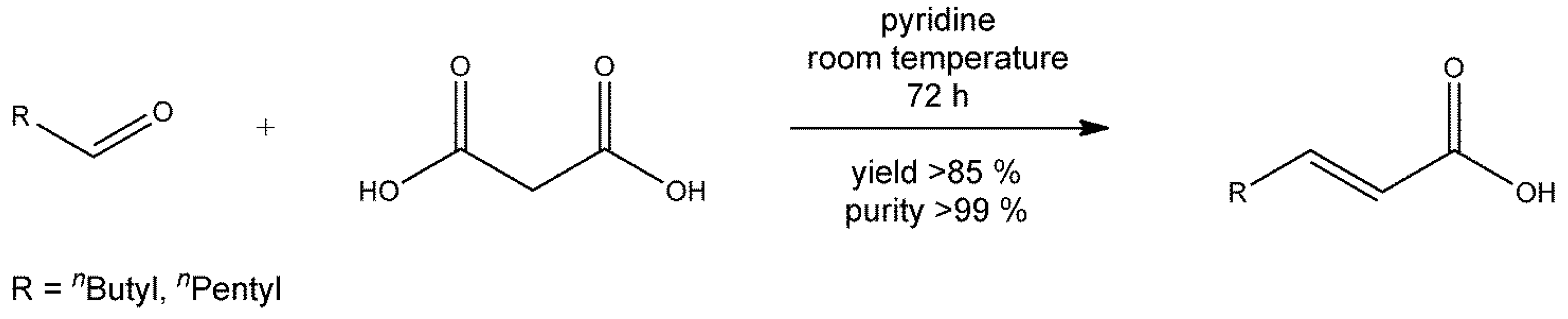
3.3. General Synthesis and Crystallization of α,β-Unsaturated Carboxylic Acids
3.4. Crystal Structure Determinations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burr, G.O.; Burr, M.M.; Miller, E.S. On the fatty acids essential in nutrition. III. J. Biol. Chem. 1932, 97, 1–9. [Google Scholar]
- Damude, H.G.; Kinney, A.J. Enhancing Plant Seed Oils for Human Nutrition. Plant Physiol. 2008, 147, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Lands, B. Consequences of Essential Fatty Acids. Nutrients 2012, 4, 1338–1357. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Kim, H.-Y. Discovery of essential fatty acids. J. Lipid Res. 2015, 56, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Beare-Rogers, J.L.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 685–744. [Google Scholar] [CrossRef]
- Cork, A.; Hall, D.R.; Blaney, W.M.; Simmonds, M.S.J. Identification of a component of the female sex pheromone of callosobruchus analis (coleoptera: bruchidae). Tetrahedron Lett. 1991, 32, 129–132. [Google Scholar] [CrossRef]
- Shu, S.; Mbata, G.N.; Cork, A.; Ramaswamy, S.B. Sex Pheromone of Callosobruchus subinnotatus. J. Chem. Ecol. 1999, 25, 2715–2727. [Google Scholar] [CrossRef]
- Martin, J.D.; Ito, Y.; Homann, V.V.; Haygood, M.G.; Butler, A. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. JBIC J. Biol. Inorg. Chem. 2006, 11, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Okujo, N.; Sakakibara, Y.; Yoshida, T.; Yamamoto, S. Structure of acinetoferrin, a new citrate-based dihydroxamate siderophore fromAcinetobacter haemolyticus. Biometals 1994, 7, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shinohara, C.; Kojima, S.; Sodeoka, M.; Tsuji, T. (2E,6R)-8-Hydroxy-2,6-dimethyl-2-octenoic Acid, a Novel Anti-osteoporotic Monoterpene, Isolated from Cistanche salsa. Biosci. Biotechnol. Biochem. 1999, 63, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Jacyno, J.M.; Harwood, J.S.; Cutler, H.G.; Dulik, D.M. Structure and solution-state conformation of botcinolide, a new biologically active metabolite from the fungus Botrytis cinerea. Tetrahedron 1994, 50, 11585–11592. [Google Scholar] [CrossRef]
- Boese, R.; Bläser, D.; Steller, I.; Latz, R.; Bäumen, A. Redetermination of 2-propenoic acid at 125K. Acta Crystallogr. Sect. C 1999, 55. [Google Scholar] [CrossRef]
- Chatani, Y.; Sakata, Y.; Nitta, I. Crystal structures of monomers polymerizable in their solid states. I. Acrylic acid. J. Polym. Sci. 1963, Pt.B 1, 419–421. [Google Scholar] [CrossRef]
- Higgs, M.A.; Sass, R.L. The crystal structure of acrylic acid. Acta Crystallogr. 1963, 16, 657–661. [Google Scholar] [CrossRef]
- Oswald, I.D.H.; Urquhart, A.J. Polymorphism and polymerisation of acrylic and methacrylic acid at high pressure. Cryst. Eng. Comm. 2011, 13, 4503–4507. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Kekka, S.; Kashino, S.; Haisa, M. Topochemical Studies. III. The Crystal and Molecular Structures of Crotonic Acid, CH3CH=CHCOOH, and Crotonamide, CH3CH=CHCONH2. Bull. Chem. Soc. Jpn. 1974, 47, 1627–1631. [Google Scholar] [CrossRef]
- Peppel, T.; Sonneck, M.; Spannenberg, A.; Wohlrab, S. Crystal structure of ((E))-pent-2-enoic acid. Acta Crystallogr. Sect. E 2015, 71, o316. [Google Scholar] [CrossRef] [PubMed]
- Peppel, T.; Sonneck, M.; Spannenberg, A.; Wohlrab, S. Crystal structure of ((E))-hex-2-enoic acid. Acta Crystallogr. Sect. E 2015, 71, o323. [Google Scholar] [CrossRef] [PubMed]
- Sonneck, M.; Peppel, T.; Spannenberg, A.; Wohlrab, S. Synthesis and Molecular Structures of ((E))-non-2-enoic Acid and ((E))-dec-2-enoic Acid. Crystals 2015, 5, 466–474. [Google Scholar] [CrossRef]
- Sonneck, M.; Peppel, T.; Spannenberg, A.; Wohlrab, S. Crystal structure of ((E))-undec-2-enoic acid. Acta Crystallogr. Sect. E 2015, 71, o426–o427. [Google Scholar] [CrossRef] [PubMed]
- Sonneck, M.; Peppel, T.; Spannenberg, A.; Wohlrab, S. Crystal structure of ((E))-dodec-2-enoic acid. Acta Crystallogr. Sect. E 2015, 71, o528–o529. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A.; Nieuwenhuyzen, M. Do Polymorphic Compounds Make Good Cocrystallizing Agents? A Structural Case Study that Demonstrates the Importance of Synthon Flexibility. Cryst. Growth Des. 2003, 3, 159–165. [Google Scholar] [CrossRef]
- Stanton, M.K.; Bak, A. Physicochemical Properties of Pharmaceutical Co-Crystals: A Case Study of Ten AMG 517 Co-Crystals. Cryst. Growth Des. 2008, 8, 3856–3862. [Google Scholar] [CrossRef]
- Bond, A.D. On the crystal structures and melting point alternation of the n-alkyl carboxylic acids. New J. Chem. 2004, 28, 104–114. [Google Scholar] [CrossRef]
- Scheuerman, R.F.; Sass, R.L. The crystal structure of valeric acid. Acta Crystallogr. 1962, 15, 1244–1247. [Google Scholar] [CrossRef]
- Strieter, F.J.; Templeton, D.H. Crystal structure of butyric acid. Acta Crystallogr. 1962, 15, 1240–1244. [Google Scholar] [CrossRef]
- Strieter, F.J.; Templeton, D.H.; Scheuerman, R.F.; Sass, R.L. The crystal structure of propionic acid. Acta Crystallogr. 1962, 15, 1233–1239. [Google Scholar] [CrossRef]
- Allan, D.R.; Clark, S.J.; Parsons, S.; Ruf, M. A high-pressure structural study of propionic acid and the application of CCD detectors in high-pressure single-crystal X-ray diffraction. J. Phy.: Condens. Matter 2000, 12, L613. [Google Scholar] [CrossRef]
- Schneegans, A. Die Perkin’sche Reaction in der Fettkörperreihe. Justus Liebigs Ann. Chem. 1885, 227, 79–96. [Google Scholar] [CrossRef]
- Harding, V.J.; Weizmann, C. XXXII.-[capital Delta]1-Nonylenic acid. J. Chem. Soc. Trans. 1910, 97, 299–304. [Google Scholar] [CrossRef]
- Bennani, Y.L.; Sharpless, K.B. Asymmetric Synthesis of γ-Hydroxy α,β-Unsaturated Amides via an AD-elimination Process; Synthesis of (+)-Coriolic Acid. Tetrahedron Lett. 1993, 34, 2083–2086. [Google Scholar] [CrossRef]
- Böhme, R.; Jung, G.; Breitmaier, E. Synthesis of the Antibiotic ((R))-Reutericyclin via Dieckmann Condensation. Helv. Chim. Acta 2005, 88, 2837–2841. [Google Scholar] [CrossRef]
- Doebner, O. Ueber die der Sorbinsäure homologen, ungesättigten Säuren mit zwei Doppelbindungen. Ber. Deutsch. Chem. Ges. 1902, 35, 1136–1147. [Google Scholar] [CrossRef]
- Knoevenagel, E. Condensation von Malonsäure mit aromatischen Aldehyden durch Ammoniak und Amine. Ber. Deutsch. Chem. Ges. 1898, 31, 2596–2619. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zargarani, D.; Shoar, R.H.; Khaleghi, S. Hydrolysis of amides to carboxylic acids using phthalic anhydride under microwave irradiation and solvent-free conditions. J. Chem. Res. 2005, 119–120. [Google Scholar] [CrossRef]
- Dalcanale, E.; Montanari, F. Selective oxidation of aldehydes to carboxylic acids with sodium chlorite-hydrogen peroxide. J. Org. Chem. 1986, 51, 567–569. [Google Scholar] [CrossRef]
- Stoll, M. Sur la formation de chaînes normales dans la condensation d’aldéhydes saturés Synthèse du n-tridécylène-2-al et du n-pentadécadiène-2,4-al. Helv. Chim. Acta 1947, 30, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Savary, P. Sur la détermination des acides gras α.β-insaturés. Bull. Soc. Chim. Fr. 1950, 624–627. [Google Scholar]
- Grimmer, G.; Hildebrandt, A. Synthese von cis-Formen α.β-ungesättigter Carbonsäuren. Justus Liebigs Ann. Chem. 1965, 685, 154–160. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Shamim Ahmad, M.; Osman, S.M. Allylic halogenation and oxidation of long chain α,β-unsaturated ester. J. Am. Oil Chem. Soc. 2013, 55, 491–495. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Morton, A.A.; Marsh, F.D.; Coombs, R.D.; Lyons, A.L.; Penner, S.E.; Ramsden, H.E.; Baker, V.B.; Little, E.L.; Letsinger, R.L. Polymerization. XII. The Metalation of Olefins and Dienes and their Use in Alfin Polymerization of Butadiene. J. Am. Chem. Soc. 1950, 72, 3785–3792. [Google Scholar] [CrossRef]
- Colonge, J.; Lartigau, G. 1.1.1.4-Tetrachlor-buten-(2). Justus Liebigs Ann. Chem. 1965, 684, 10–14. [Google Scholar] [CrossRef]
- Bourguel, M.M. Sur l’isomérie cis-trans éthylénique. Addition de deux atomes d’hydrogène à la liaison acétylénique. Bull. Soc. Chim. Fr. 1929, 45, 1067–1091. [Google Scholar]
- Bruker. APEX II; Bruker AXS Inc.: Madion, WI, USA, 2007. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2001. [Google Scholar]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]

| Compound | C7 | C8 |
|---|---|---|
| Chemical formula | C7H12O2 | C8H14O2 |
| Formula weight | 128.17 | 142.19 |
| Crystal system | triclinic | monoclinic |
| a | 5.3049(2) Å | 19.032(10) Å |
| b | 6.6322(3) Å | 9.368(5) Å |
| c | 11.1428(5) Å | 11.520(6) Å |
| α | 103.972(3)° | 90° |
| β | 97.542(3)° | 123.033(11)° |
| γ | 90.104(3)° | 90° |
| Unit cell volume | 376.92(3) Å3 | 1721.8(16) Å3 |
| Temperature | 150(2) K | 200(2) K |
| Space group | C2/c | |
| Z (Z’) | 2 (1) | 8 (1) |
| μ | 0.661 mm−1 | 0.077 mm−1 |
| No. of reflections measured | 5233 | 13812 |
| No. of independent reflections | 1325 | 1883 |
| Rint | 0.0194 | 0.0293 |
| Final R1 values (I > 2σ(I)) | 0.0299 | 0.0433 |
| Final wR(F2) values (I > 2σ(I)) | 0.0837 | 0.1071 |
| Final R1 values (all data) | 0.0328 | 0.0634 |
| Final wR(F2) values (all data) | 0.0861 | 0.1238 |
| Goodness of fit on F2 | 1.068 | 1.047 |
| Density | 1.129 g/cm3 | 1.097 g/cm3 |
| Compound | C7 | C8 |
|---|---|---|
| Atoms | Distance | Distance |
| C1–O1 | 1.3178(12) | 1.2655(18) |
| C1–O2 | 1.2265(12) | 1.2644(18) |
| C1–C2 | 1.4672(15) | 1.4712(19) |
| C2–C3 | 1.3240(15) | 1.3140(20) |
| C3–C4 | 1.4905(15) | 1.4920(20) |
| average C–C (alkyl chain) | 1.519 | 1.519 |
| H-bonds in: | D–H∙∙∙A | D∙∙∙A |
| C7 | O1–H1∙∙∙O2i | 2.637 |
| C8 | O1–H1∙∙∙O1ii | 2.651 # |
| O2–H2A∙∙∙O2ii | 2.595 # | |
| Atoms | Angle | Angle |
| O1–C1–O2 | 122.91(9) | 122.36(13) |
| O1–C1–C2 | 113.37(9) | 118.88(12) |
| O2–C1–C2 | 123.71(9) | 118.76(13) |
| C1–C2–C3 | 121.41(9) | 121.69(13) |
| C2–C3–C4 | 126.47(9) | 127.87(13) |
| H-bonds in: | D–H∙∙∙A | Angle |
| C7 | O1–H1∙∙∙O2i | 176.8 |
| C8 | O1–H1∙∙∙O1ii | 177.3 # |
| O2–H2A∙∙∙O2ii | 171.7 # | |
| Symmetry codes: | (i) −x, −y, −z | |
| (ii) –x, y, –z + 2.5 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonneck, M.; Spannenberg, A.; Wohlrab, S.; Peppel, T. Synthesis and Molecular Structures of the Lowest Melting Odd- and Even-Numbered α,β-Unsaturated Carboxylic Acids—(E)-Hept-2-Enoic Acid and (E)-Oct-2-Enoic Acid. Crystals 2016, 6, 66. https://doi.org/10.3390/cryst6060066
Sonneck M, Spannenberg A, Wohlrab S, Peppel T. Synthesis and Molecular Structures of the Lowest Melting Odd- and Even-Numbered α,β-Unsaturated Carboxylic Acids—(E)-Hept-2-Enoic Acid and (E)-Oct-2-Enoic Acid. Crystals. 2016; 6(6):66. https://doi.org/10.3390/cryst6060066
Chicago/Turabian StyleSonneck, Marcel, Anke Spannenberg, Sebastian Wohlrab, and Tim Peppel. 2016. "Synthesis and Molecular Structures of the Lowest Melting Odd- and Even-Numbered α,β-Unsaturated Carboxylic Acids—(E)-Hept-2-Enoic Acid and (E)-Oct-2-Enoic Acid" Crystals 6, no. 6: 66. https://doi.org/10.3390/cryst6060066
APA StyleSonneck, M., Spannenberg, A., Wohlrab, S., & Peppel, T. (2016). Synthesis and Molecular Structures of the Lowest Melting Odd- and Even-Numbered α,β-Unsaturated Carboxylic Acids—(E)-Hept-2-Enoic Acid and (E)-Oct-2-Enoic Acid. Crystals, 6(6), 66. https://doi.org/10.3390/cryst6060066