Raman Identification of Polymorphs in Pentacene Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Instrumentation
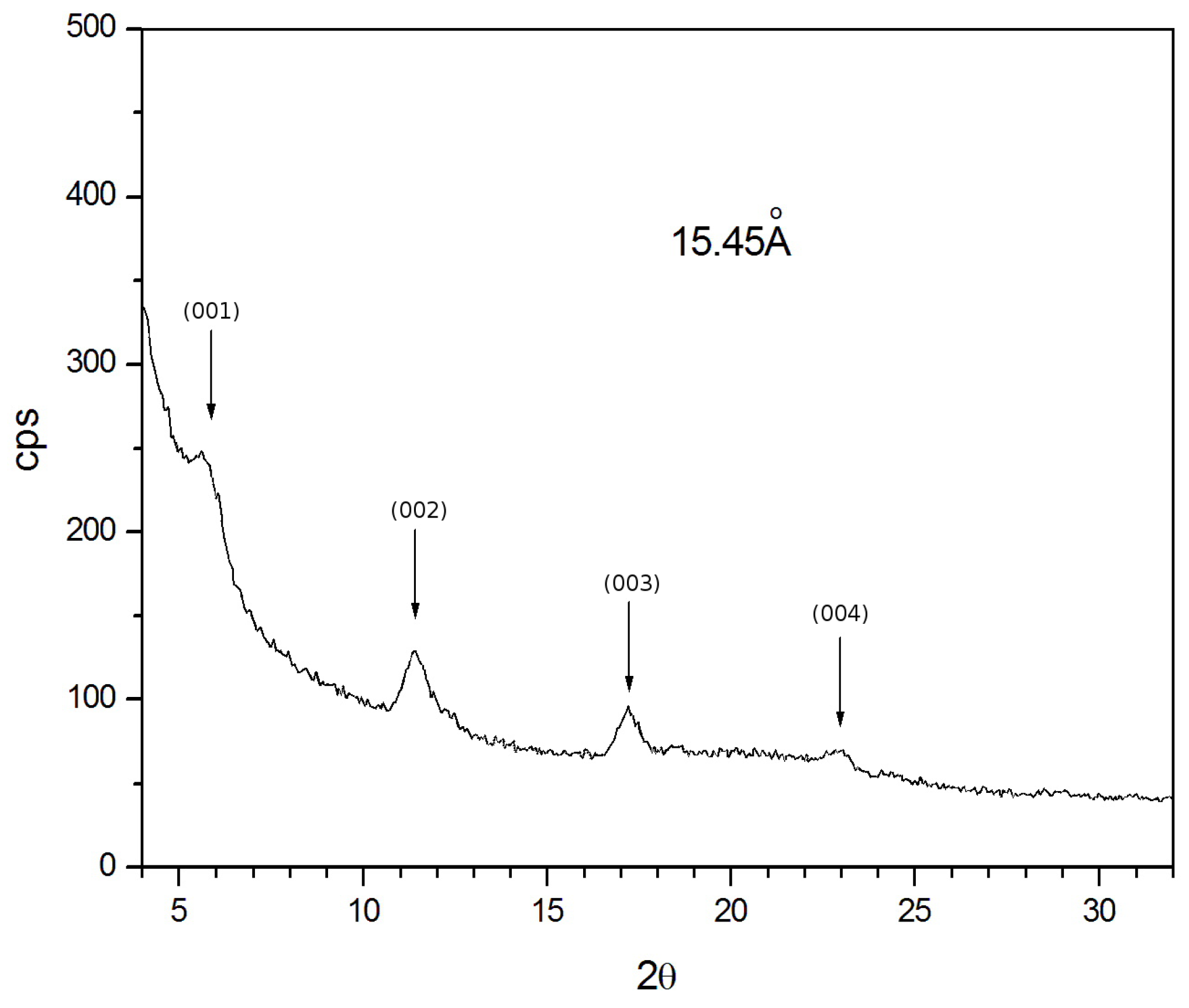
2.2. Film Preparation and Characterization
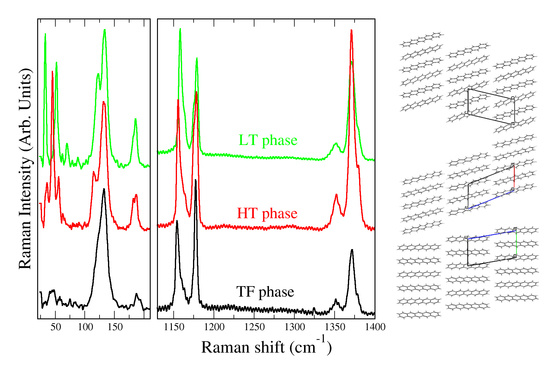
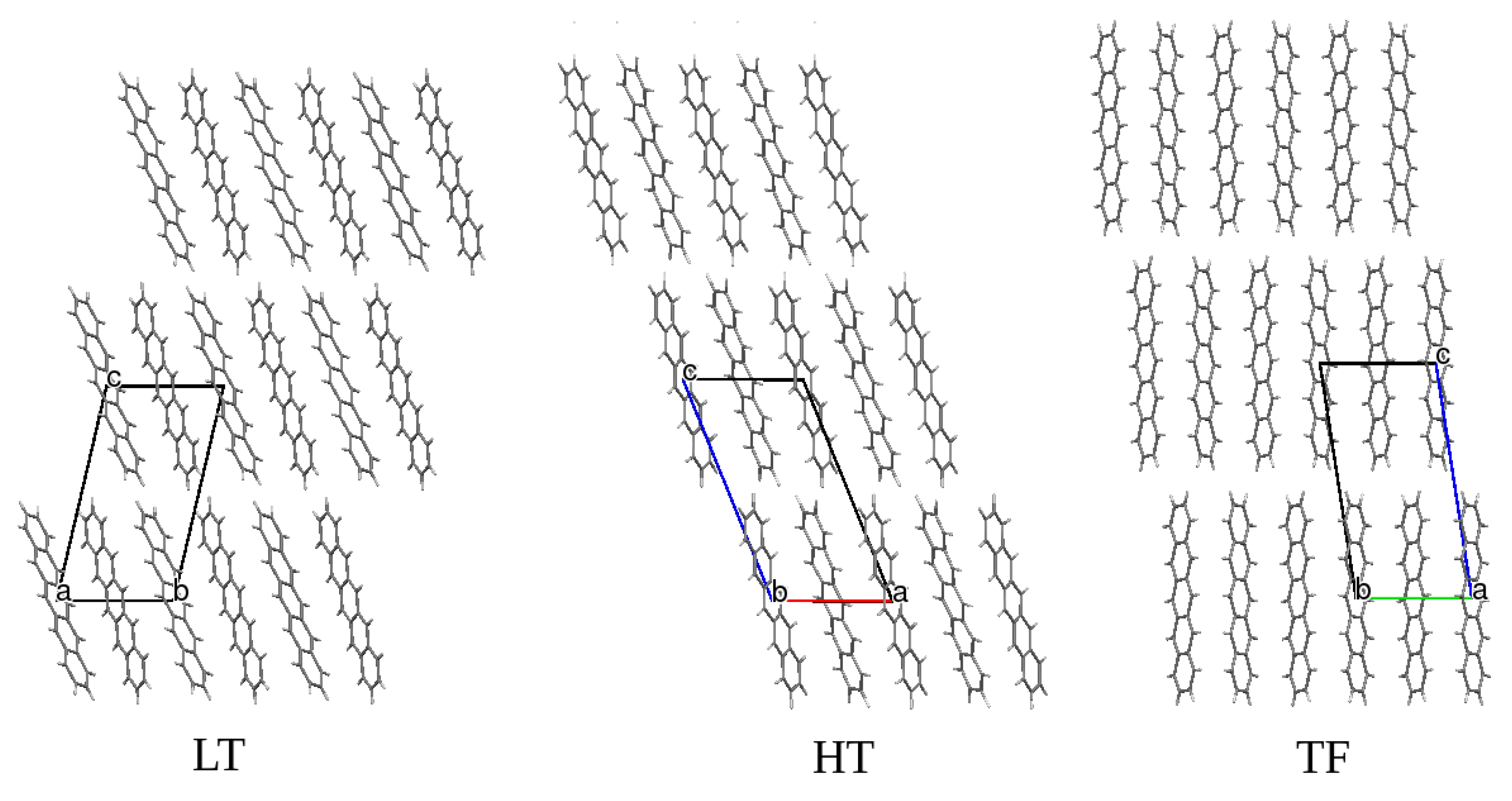
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dimitrakopoulos, C.D.; Malenfant, P.R.L. Organic Thin Film Transistors for Large Area Electronics. Adv. Mater. 2002, 14, 99–117. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.; Liu, J.; Wang, Z.; Hu, W. 25th Anniversary Article: Key Points for High-Mobility Organic Field-Effect Transistors. Adv. Mater. 2013, 25, 6158–6183. [Google Scholar] [CrossRef] [PubMed]
- Jurchescu, O.D.; Baas, J.; Palstra, T.T.M. Effect of impurities on the mobility of single crystal pentacene. Appl. Phys. Lett. 2004, 84, 3061–3063. [Google Scholar] [CrossRef]
- Jurchescu, O.D.; Mourey, D.A.; Subramanian, S.; Parkin, S.R.; Vogel, B.M.; Anthony, J.E.; Jackson, T.N.; Gundlach, D.J. Effect of polymorphysm on charge transport in organic semiconductors. Phys. Rev. B 2009, 80, 085201. [Google Scholar] [CrossRef]
- Brillante, A.; Bilotti, I.; Della Valle, R.G.; Venuti, E.; Masino, M.; Girlando, A. Characterization of phase purity in organic semiconductors by lattice phonons confocal Raman mapping: Application to pentacene. Adv. Mater. 2005, 17, 2549–2553. [Google Scholar] [CrossRef]
- Venuti, E.; Della Valle, R.G.; Brillante, A.; Masino, M.; Girlando, A. Probing Pentacene Polymorphs by Lattice Dynamics Calculations. J. Am. Chem. Soc. 2002, 124, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Choudhary, D.; Nickel, B.; Toccoli, T.; Chang, K.C.; Mayer, A.C.; Clancy, P.; Blakely, J.M.; Headrick, R.L.; Iannotta, S.; et al. Pentacene Thin Film Growth. Chem. Mater. 2004, 16, 4497–4508. [Google Scholar] [CrossRef]
- Duffy, C.M.; Andreasen, J.W.; Breiby, D.W.; Nielsen, M.M.; Ando, M.; Minakata, T.; Sirringhaus, H. High-Mobility Aligned Pentacene Films Grown by Zone-Casting. Chem. Mater. 2008, 20, 7252–7259. [Google Scholar] [CrossRef]
- Della Valle, R.G.; Venuti, E.; Brillante, A.; Girlando, A. Molecular dynamics simulations for a pentacene monolayer on amorphous silica. ChemPhysChem 2009, 10, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Yoneya, M.; Kawasaki, M.; Ando, M. Molecular dynamics simulations of pentacene thin films: The effect of surface on polymorph selection. J. Mater. Chem. 2010, 20, 10397–10402. [Google Scholar] [CrossRef]
- Holmes, D.; Kumaraswamy, S.; Matzger, A.J.; Vollhardt, K.P. On the Nature of Nonplanarity in the [N]Phenylenes. Chem. Eur. J. 1999, 5, 3399–3412. [Google Scholar] [CrossRef]
- Mattheus, C.C.; Dros, A.B.; Baas, J.; Meetsma, A.; de Boer, J.L.; Palstra, T.T.M. Polymorphism in pentacene. Acta Cryst. 2001, C57, 939–941. [Google Scholar] [CrossRef]
- Siegrist, T.; Kloc, C.; Schön, J.H.; Batlogg, B.; Haddon, R.C.; Berg, S.; Thomas, G.A. Enhanced Physical Properties in a Pentacene Polymorph. Angew. Chem. Int. Ed. 2001, 40, 1732–1736. [Google Scholar] [CrossRef]
- Campbell, R.B.; Roberston, J.M.; Trotter, J. The crystal and molecular structure of pentacene. Acta Crystallogr. 1962, 14, 705–711. [Google Scholar] [CrossRef]
- Farina, L.; Brillante, A.; Della Valle, R.G.; Venuti, E.; Amboage, M.; Syassen, K. Pressure-induced phase transition in pentacene. Chem. Phys. Lett. 2003, 375, 490–494. [Google Scholar] [CrossRef]
- Siegrist, T.; Besnard, C.; Haas, S.; Schiltz, M.; Pattison, P.; Chernyshov, D.; Batlogg, B.; Kloc, C. A Polymorph Lost and Found: The High-Temperature Crystal Structure of Pentacene. Adv. Mater. 2007, 19, 2079–2082. [Google Scholar] [CrossRef]
- Chou, W.-Y.; Chang, M.-H.; Cheng, H.-L.; Lee, Y.-C.; Chang, C.-C.; Sheu, H.-S. New Pentacene Crystalline phase Induced by Nanoimprinted Polyimide Gratings. J. Phys. Chem. C 2012, 116, 8619–8626. [Google Scholar] [CrossRef]
- Cheng, H.L.; Mai, Y.S.; Chou, W.Y.; Chang, L.R.; Liang, X.W. Thickness-dependent structural evolution and growth models in relation to carrier transport properties in polycrystalline pentacene films. Adv. Funct. Mater. 2007, 17, 3639–3649. [Google Scholar] [CrossRef]
- Mattheus, C.C.; Dros, A.B.; Baas, J.; Oostergetel, G.T.; Meetsma, A.; de Boer, J.L.; Palstra, T.T.M. Identification of polymorphs of pentacene. Synth. Met. 2003, 138, 475–481. [Google Scholar] [CrossRef]
- Schiefer, S.; Huth, M.; Dobrinevski, A.; Nickel, B. Determination of the Crystal Structure of Substrate-Induced Pentacene Polymorphs in Fiber Structured Thin Films. J. Am. Chem. Soc. 2007, 129, 10316–10317. [Google Scholar] [CrossRef] [PubMed]
- Drummy, L.F.; Martin, D.C. Thickness-Driven Orthorhombic to Triclinic Phase Transformation in Pentacene Thin Films. Adv. Mater. 2005, 17, 903–907. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.B.; Virkar, A.; Reese, C.; Toney, M.F.; Bao, Z. Precise Structure of Pentacene Monolayers on Amorphous Silicon Oxide and Relation to Charge Transport. Adv. Mater. 2009, 21, 2294–2298. [Google Scholar] [CrossRef]
- Brillante, A.; Bilotti, I.; Della Valle, R.G.; Venuti, E.; Girlando, A.; Masino, M.; Liscio, F.; Milita, S.; Albonetti, C.; D’Angelo, P.; et al. Structure and dynamics of pentacene on SiO2 : From monolayer to bulk structure. Phys. Rev. B 2012, 85, 195308. [Google Scholar] [CrossRef]
- Ando, M.; Kehoe, T.B.; Yoneya, M.; Ishii, H.; Kawasaki, M.; Duffy, C.M.; Minakata, T.; Phillips, R.T.; Sirringhaus, H. Evidence for Charge-Trapping Inducing Polymorphic Structural-Phase Transition in Pentacene. Adv. Mater. 2015, 27, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Brillante, A.; Bilotti, I.; Della Valle, R.G.; Venuti, E.; Girlando, A. Probing polymorphs of organic semiconductors by lattice phonon Raman microscopy. CrystEngComm 2008, 10, 937–946. [Google Scholar] [CrossRef]
- Brillante, A.; Della Valle, R.G.; Farina, L.; Girlando, A.; Masino, M.; Venuti, E. Raman phonon spectra of pentacene polymorphs. Chem. Phys. Lett. 2002, 357, 32–36. [Google Scholar] [CrossRef]
- He, R.; Tassi, N.G.; Blanchet, G.B.; Pinczuk, A. Low-lying lattice modes of highly uniform pentacene monolayers. Appl. Phys. Lett. 2009, 94, 223310. [Google Scholar] [CrossRef]
- Wong, F.; He, R.; Pinkzuk, A. An Optical and Structural Study of Pentacene Thin-film Polymorphs. Columbia Undergrad. Sci. J. 2006, 1, 50–55. [Google Scholar]
- Wu, Y.; Toccoli, T.; Koch, N.; Iacob, E.; Pallaoro, A.; Rudolf, P.; Iannotta, S. Controlling the Early Stages of Pentacene Growth by Supersonic Molecular Beam Deposition. Phys. Rev. Lett. 2007, 98, 076601. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, S.; Toccoli, T.; Wu, Y.; Iannotta, S.; Rudolf, P. Growth dynamics in supersonic molecular beam deposition of pentacene sub-monolayers on SiO2. Chem. Commun. 2014, 50, 7694–7697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, R.; Tassi, N.G.; Blanchet, G.B.; Pinczuk, A. Intense photoluminescence from pentacene monolayers. Appl. Phys. Lett. 2010, 96, 263303. [Google Scholar] [CrossRef]
- Stenger, I.; Frigout, A.; Tondelier, D.; Geffroy, B.; Ossikovski, R.; Bonassieux, Y. Polarized micro-Raman spectroscopy of pentacee thin-film. Appl. Phys. Lett. 2009, 94, 133301. [Google Scholar] [CrossRef]
- Cheng, H.-L.; Liang, X.-W.; Chou, W.-Y.; Mai, Y.-S.; Yang, C.-Y.; Chang, L.-R.; Tang, F.-C. Raman spectroscopy applied to reveal polycrystalline grain structures and carrier transport properties of organic semiconductors films: Application to pentacene-based organic transistors. Org. Electr. 2009, 10, 289–298. [Google Scholar] [CrossRef]
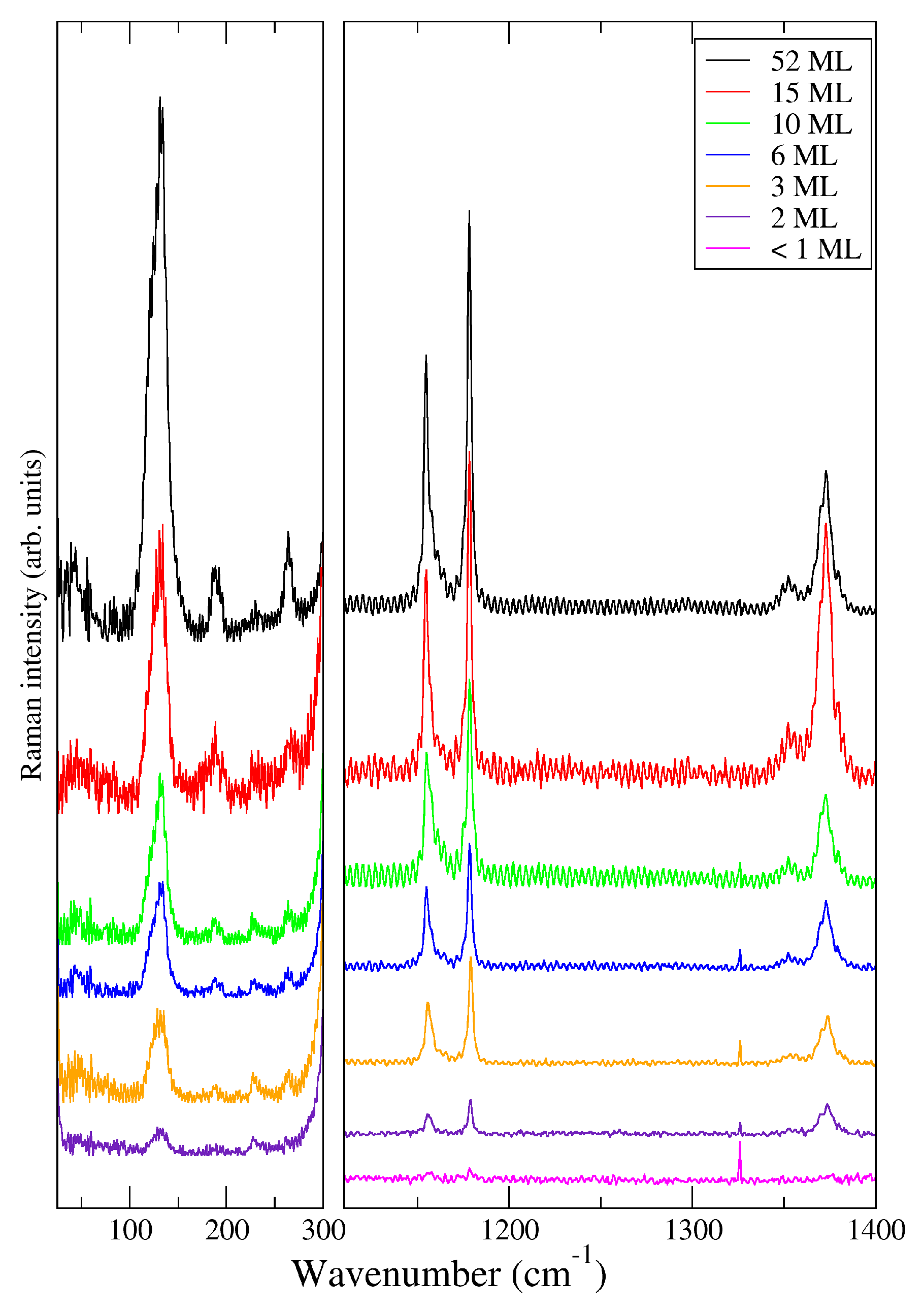
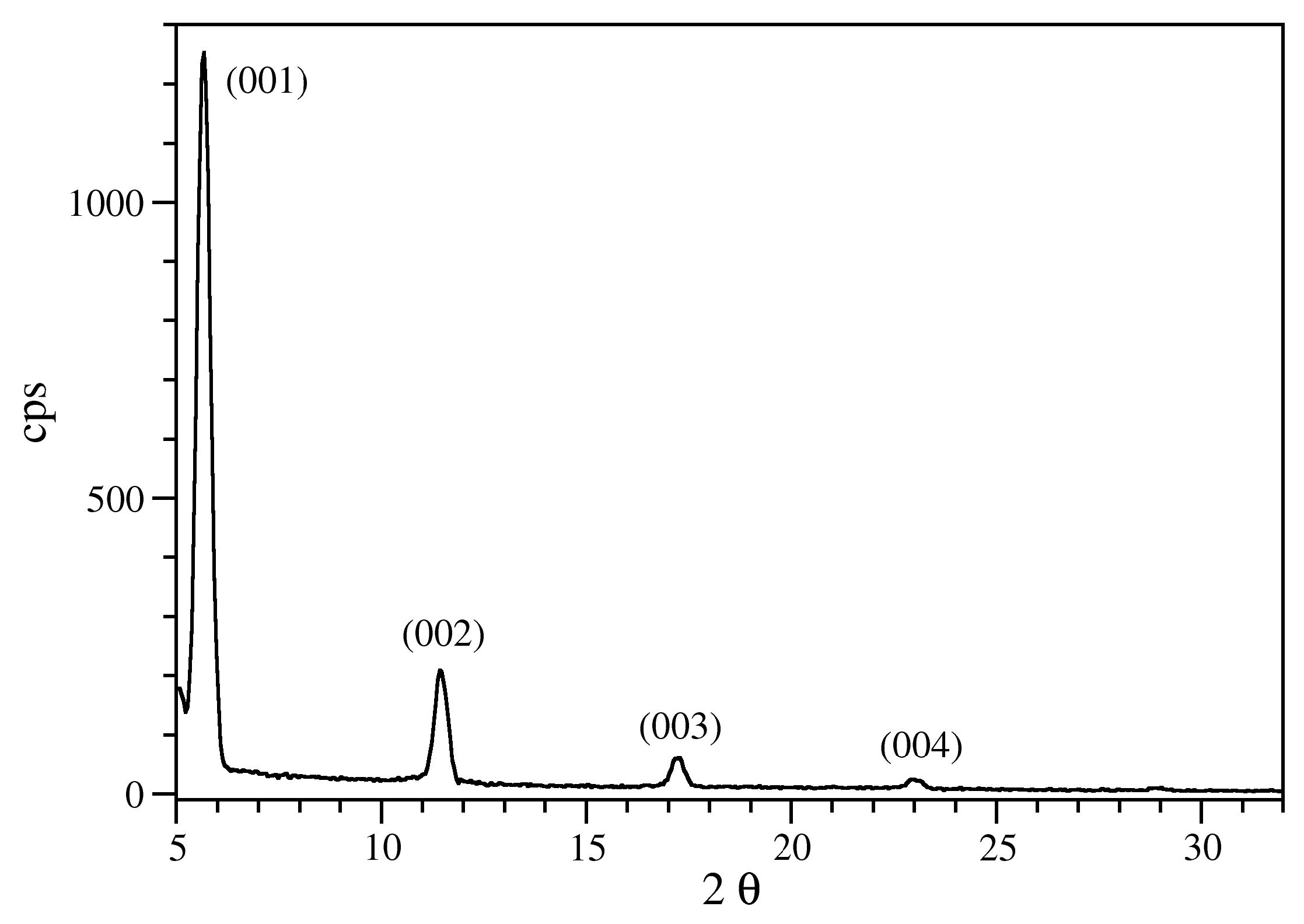
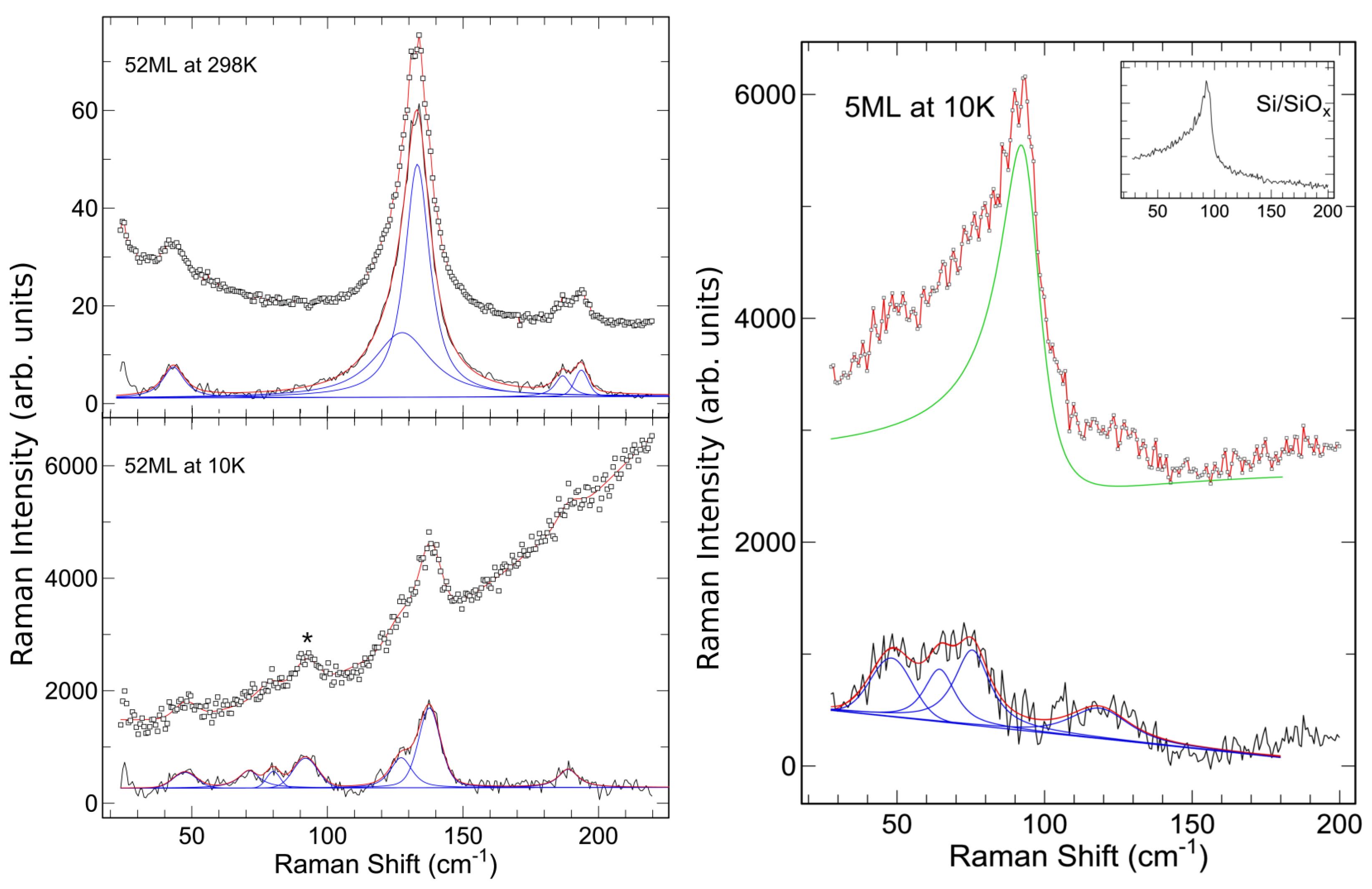

| Label | Thickness (Å) | Thickness (ML) |
|---|---|---|
| 10-02 | 781 ± 15 | 52 |
| 10-01 | 226 ± 5 | 15 |
| 10-016 | 145 ± 3 | 10 |
| 10-017 | 88.2 ± 2.8 | 6 |
| 10-018 | 80.8 ± 2.6 | 5 |
| 10-019 | 49.2 ± 2.9 | 3 |
| 10-020 | 23.0 ± 1.6 | ∼1–2 |
| 10-021 | 11–12 | <1 |
| Pentacene TF | Pentacene HT | Pentacene LT | |||
|---|---|---|---|---|---|
| Expt. | Calc. | Expt. | Calc. | Expt. | Calc. |
| 30.4 | 36.4 | 46.7 | 33.1 | 33.5 | |
| 43.2 | 44.5 | 45.5 | 57.2 | 52.2 | 64.1 |
| 70.0 | 70.7 | 55.7 | 75.5 | 69.8 | 76.9 |
| 88.5 | 115.0 | 88.1 | 110.2 | ||
| 122.3 | 133.8 | 115.1 | 155.8 | 122.5 | 157.9 |
| 132.3 | 140.8 | 132.3 | 164.8 | 133.2 | 164.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girlando, A.; Masino, M.; Brillante, A.; Toccoli, T.; Iannotta, S. Raman Identification of Polymorphs in Pentacene Films. Crystals 2016, 6, 41. https://doi.org/10.3390/cryst6040041
Girlando A, Masino M, Brillante A, Toccoli T, Iannotta S. Raman Identification of Polymorphs in Pentacene Films. Crystals. 2016; 6(4):41. https://doi.org/10.3390/cryst6040041
Chicago/Turabian StyleGirlando, Alberto, Matteo Masino, Aldo Brillante, Tullio Toccoli, and Salvatore Iannotta. 2016. "Raman Identification of Polymorphs in Pentacene Films" Crystals 6, no. 4: 41. https://doi.org/10.3390/cryst6040041
APA StyleGirlando, A., Masino, M., Brillante, A., Toccoli, T., & Iannotta, S. (2016). Raman Identification of Polymorphs in Pentacene Films. Crystals, 6(4), 41. https://doi.org/10.3390/cryst6040041