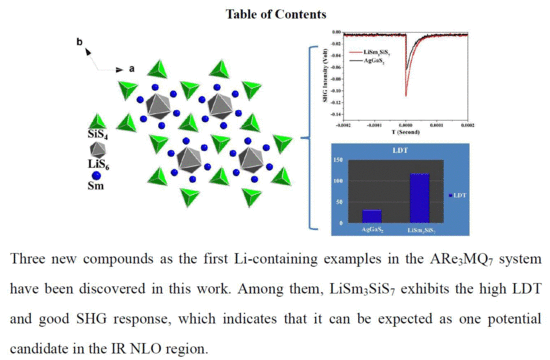
A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7
Abstract
:1. Introduction
2. Results and Discussion
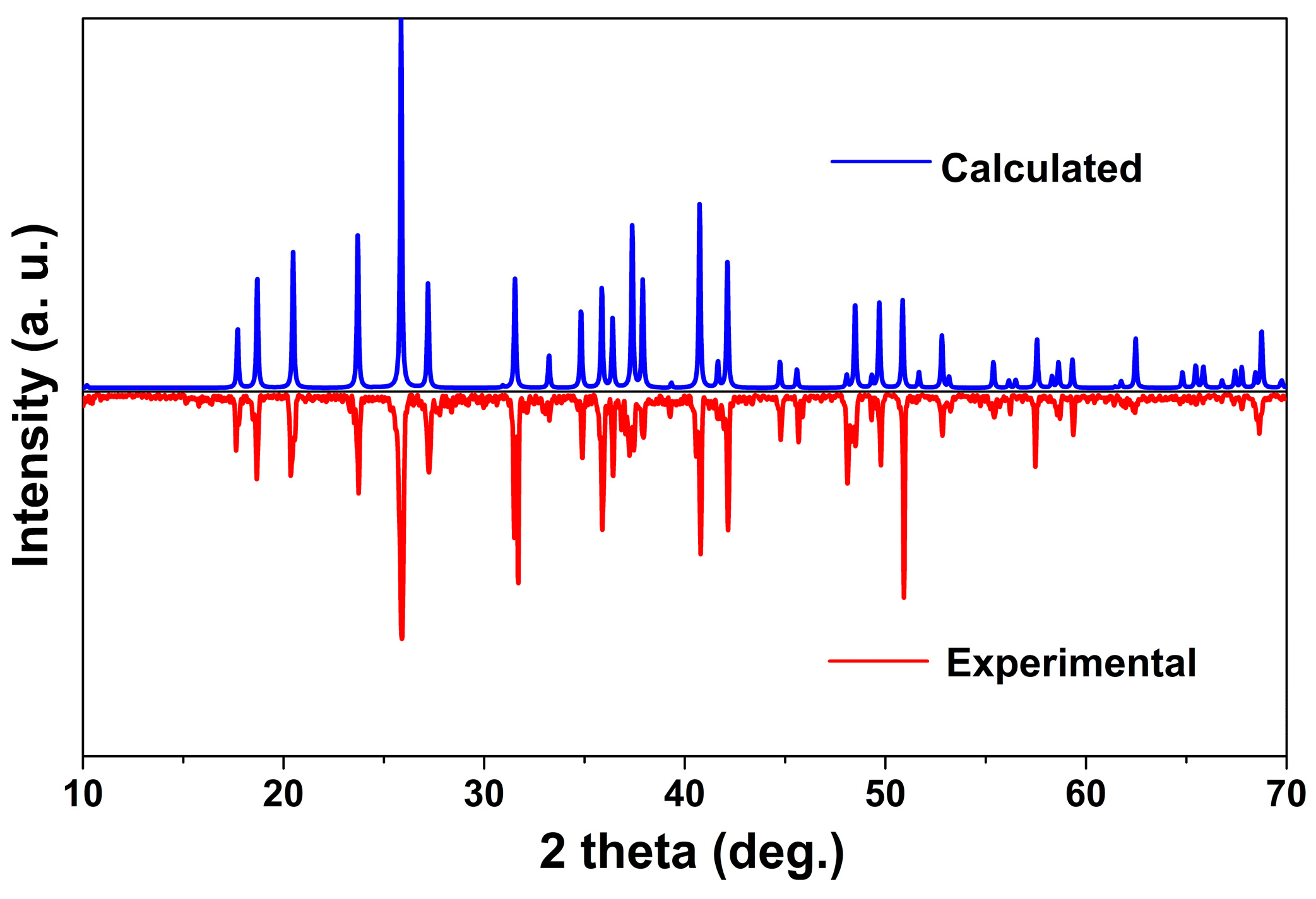
2.1. Crystal Structure
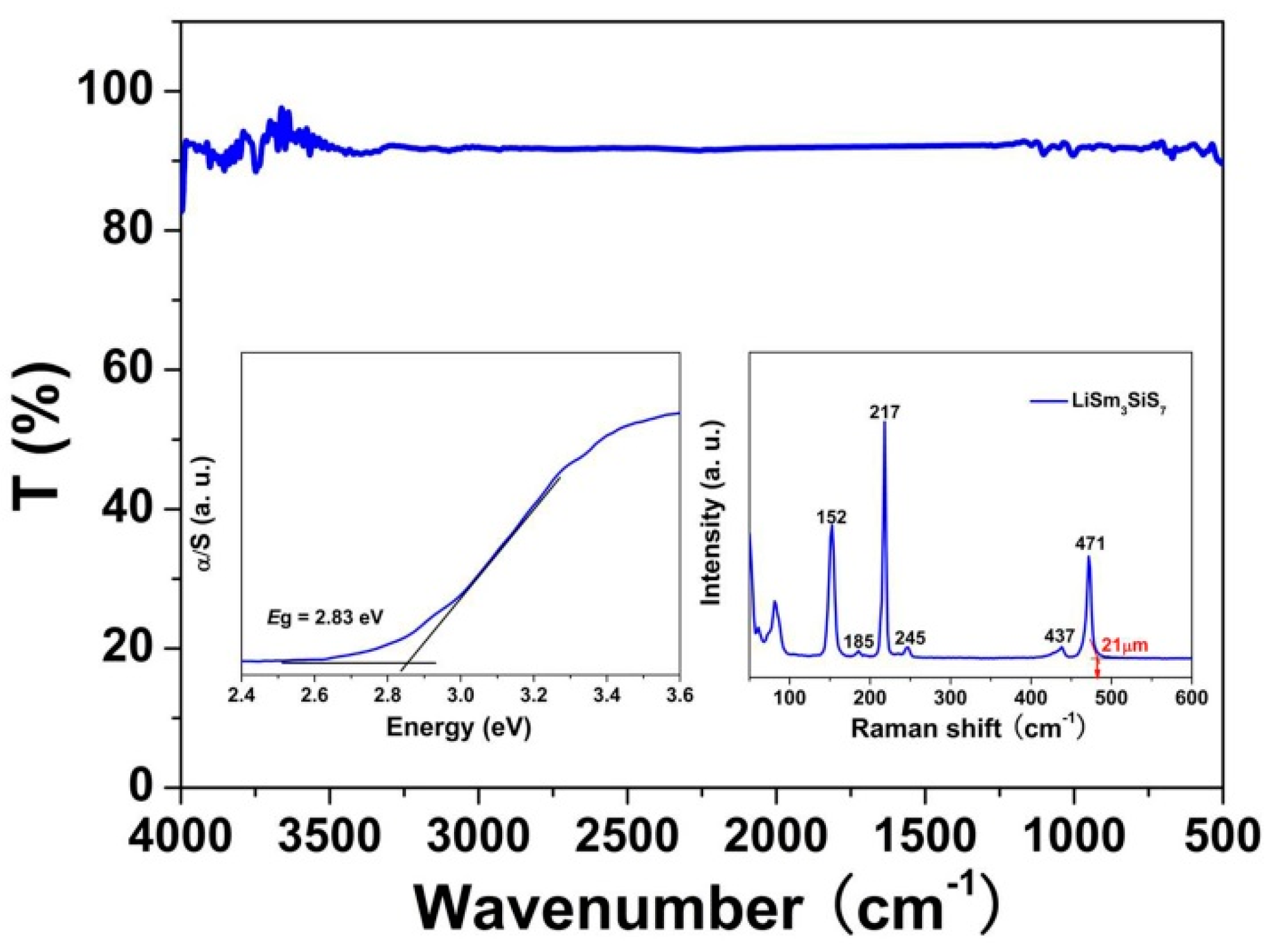
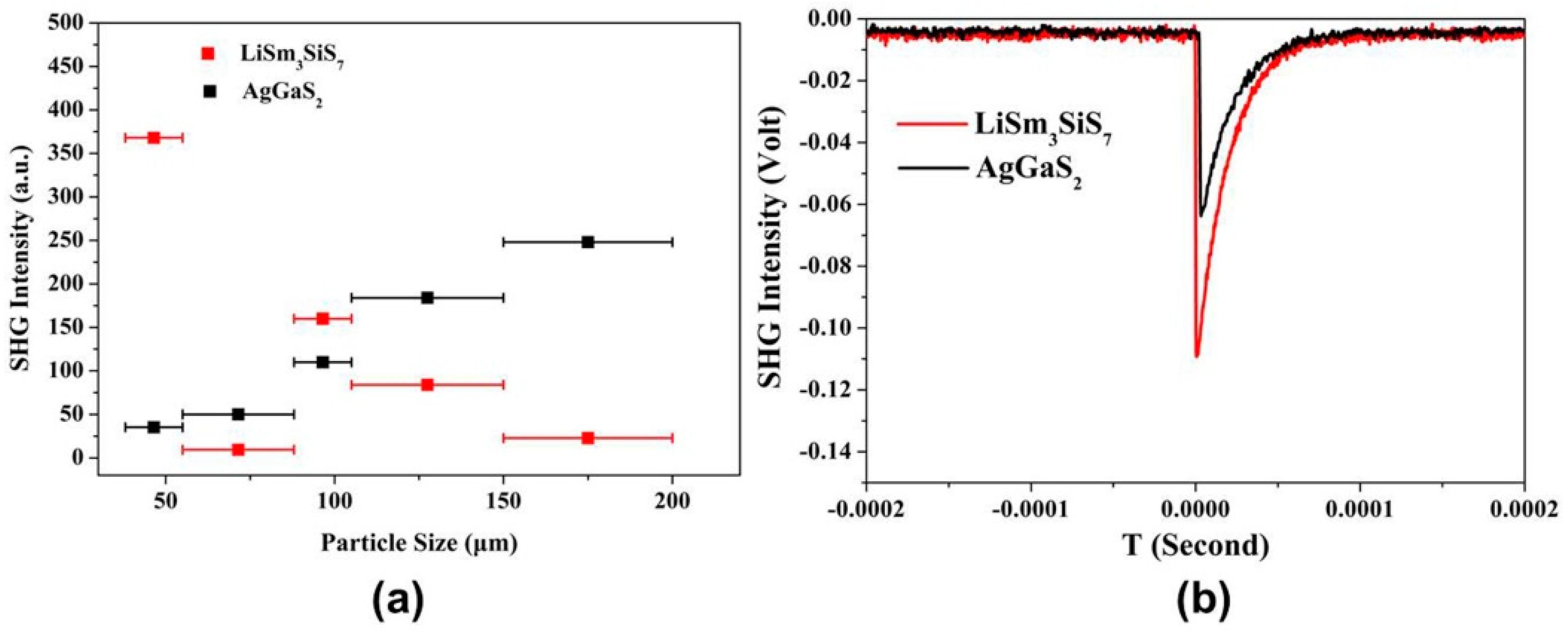
2.2. Optical Properties
2.3. Dipole Moment Calculation
3. Materials and Methods
3.1. Synthesis
3.1.1. LiSm3SiS7
3.1.2. LiSm3GeS7 and LiGd3GeS7
3.2. Structure Determination
3.3. Powder XRD Measurement
3.4. UV–Vis–NIR Diffuse-Reflectance Spectroscopy
3.5. Raman Spectroscopy
3.6. Infrared Spectroscopy
3.7. Second-Harmonic Generation (SHG) Measurement
3.8. LDT Measurement
3.9. Calculations of Group Dipole Moments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duarte, F.J. Tunable Laser Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; Chapters 2, 9, and 12. [Google Scholar]
- Nikogosyan, D.N. Nonlinear Optical Crystals: A Complete Survey, 1st ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New Nonlinear-Optical Crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A New-Type Ultraviolet SHG Crystal-β-BaB2O4. Sci. Sin. Ser. B (Engl. Ed.) 1985, 28, 235–243. [Google Scholar]
- Mei, L.; Wang, Y.; Chen, C.T.; Wu, B.C. Nonlinear Optical Materials Based on MBe2BO3F2 (M = Na, K). J. Appl. Phys. 1993, 74, 7014–7016. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhang, C.Q.; Chen, C.T.; Yao, A.Y.; Zhang, J.; Xu, Z.Y.; Wang, J.Y. High-Efficiency 266-nm Output of A KBe2BO3F2 Crystal. Appl. Opt. 2003, 42, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Becker, P. Borate Materials in Nonlinear Optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Sun, C.F.; Hu, C.L.; Xu, X.; Ling, J.B.; Hu, T.; Kong, F.; Long, X.F.; Mao, J.G. BaNbO(IO3)5: A New Polar Material with A Very Large SHG Response. J. Am. Chem. Soc. 2009, 131, 9486–9487. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.L.; Mao, J.G. Recent Advances on Second-Order NLO Materials Based on Metal Iodates. Coord. Chem. Rev. 2015, 288, 1–17. [Google Scholar] [CrossRef]
- Song, J.L.; Hu, C.L.; Xu, X.; Kong, F.; Mao, J.G. A Facile Synthetic Route to a New SHG Material with Two Types of Parallel π-Conjugated Planar Triangular Units. Angew. Chem. Int. Ed. 2015, 54, 3679–3682. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.H.; Ye, N.; Huang, L.; Lin, X.S. Alkaline-Alkaline Earth Fluoride Carbonate Crystals ABCO3F (A = K, Rb, Cs; B = Ca, Sr, Ba) as Nonlinear Optical Materials. J. Am. Chem. Soc. 2011, 133, 20001–20007. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.S.; Peng, G.; Ye, N.; Wang, J.Y.; Luo, M.; Yan, T.; Zhou, Y.Q. Structural Modulation of Anionic Group Architectures by Cations to Optimize SHG Effects: A Facile Route to New NLO Materials in the ATCO3F (A = K, Rb; T = Zn, Cd) Series. Chem. Mater. 2015, 27, 7520–7530. [Google Scholar] [CrossRef]
- Zou, G.H.; Huang, L.; Ye, N.; Lin, C.S.; Cheng, W.D.; Huang, H. CsPbCO3F: A Strong Second-Harmonic Generation Material Derived from Enhancement via p−π Interaction. J. Am. Chem. Soc. 2013, 135, 18560–18566. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Bidenticity-Enhanced Second Harmonic Generation from Pb Chelation in Pb3Mg3TeP2O14. J. Am. Chem. Soc. 2016, 138, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Design and Synthesis of the Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material Ba3(ZnB5O10)PO4. Adv. Mater. 2015, 27, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Tran, T.T.; Choi, W.; You, T.S.; Halasyamani, P.S.; Ok, K.M. Two New Non-centrosymmetric n = 3 Layered Dion–Jacobson Perovskites: Polar RbBi2Ti2NbO10 and Nonpolar CsBi2Ti2TaO10. Chem. Mater. 2016, 28, 2424–2432. [Google Scholar] [CrossRef]
- Zou, G.H.; Nam, G.; Kim, H.G.; Jo, H.; You, T.S.; Ok, K.M. ACdCO3F (A = K and Rb): New Noncentrosymmetric Materials with Remarkably Strong Second-Harmonic Generation (SHG) Responses Enhanced via π-Interaction. RSC Adv. 2015, 5, 84754–84761. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, Q.; Wu, H.Q.; Zhou, L.J.; Yang, G.Y. Ba3M2[B3O6(OH)]2[B4O7(OH)2] (M = Al, Ga): Two Novel UV Nonlinear Optical Metal Borates Containing Two Types of Oxoboron Clusters. Chem.—Eur. J. 2013, 19, 17662–17667. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, L.J.; Jin, S.F.; Yao, W.J.; Zhang, Y.H.; Chen, C.T. Deep-Ultraviolet Nonlinear Optical Materials: Na2Be4B4O11 and LiNa5Be12B12O33. J. Am. Chem. Soc. 2013, 135, 18319–18322. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hou, X.L.; Pan, S.L.; Wang, X.A. Growth, structure, and optical properties of a congruent melting oxyborate, Bi2ZnOB2O6. Chem. Mater. 2009, 21, 2846–2850. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.L.; Poeppelmeier, K.R.; Li, H.Y.; Jia, D.Z.; Chen, Z.H.; Fan, X.Y.; Yang, Y.; Rondinelli, J.M.; Luo, H.S. K3B6O10Cl: A New Structure Analogous to Perovskite with a Large Second Harmonic Generation Response and Deep UV Absorption Edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wu, H.P.; Pan, S.L.; Yang, Z.H.; Hou, X.L.; Su, X.; Jing, Q.; Poeppelmeier, K.R.; Rondinelli, J.M. Cs3Zn6B9O21: A Chemically Benign Member of the KBBF Family Exhibiting the Largest Second Harmonic Generation Response. J. Am. Chem. Soc. 2014, 136, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Okorogu, A.O.; Mirov, S.B.; Lee, W.; Crouthamel, D.I.; Jenkins, N.; Dergachev, A.Y.; Vodopyanov, K.L.; Badikov, V.V. Tunable Middle Infrared Downconversion in GaSe and AgGaS2. Opt. Commun. 1998, 155, 307–312. [Google Scholar] [CrossRef]
- Boyd, G.D.; Storz, F.G.; McFee, J.H.; Kasper, H.M. Linear and Nonlinear Optical Properties of Some Ternary Selenides. IEEE J. Quantum Electron. 1972, 8, 900–908. [Google Scholar] [CrossRef]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and Nonlinear Optical Properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Chung, I.; Kanatzidis, M.G. Metal chalcogenides: A rich source of nonlinear optical materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Jiang, X.M.; Guo, S.P.; Zeng, H.Y.; Zhang, M.J.; Guo, G.C. Large Crystal Growth and New Crystal Exploration of Mid-Infrared Second-Order Nonlinear Optical Materials. Struct. Bond. 2012, 145, 1–43. [Google Scholar]
- Bera, T.K.; Jang, J.I.; Ketterson, J.B.; Kanatzidis, M.G. Strong Second Harmonic Generation from the Tantalum Thioarsenates A3Ta2AsS11 (A = K and Rb). J. Am. Chem. Soc. 2009, 131, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Kim, S.H.; Halasyamani, P.S. Top-Seeded Solution Crystal Growth and Functional Properties of A Polar Material Na2TeW2O9. Cryst. Growth Des. 2010, 10, 4091–4095. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, L.J.; Chen, L. Sulfides with Strong Nonlinear Optical Activity and Thermochromism: ACd4Ga5S12 (A = K, Rb, Cs). Chem. Mater. 2012, 24, 3406–3414. [Google Scholar] [CrossRef]
- Chen, M.C.; Wu, L.M.; Lin, H.; Zhou, L.J.; Chen, L. Disconnection Enhances the Second Harmonic Generation Response: Synthesis and Characterization of Ba23Ga8Sb2S38. J. Am. Chem. Soc. 2012, 134, 6058–6060. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhou, L.J.; Chen, L. Noncentrosymmetric Inorganic Open-Framework Chalcohalides with Strong Middle IR SHG and Red emission: Ba3AGa5Se10Cl2 (A = Cs, Rb, K). J. Am. Chem. Soc. 2012, 134, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Li, L.H.; Chen, Y.B.; Chen, L. In-Phase Alignments of Asymmetric Building Units in Ln4GaSbS9 (Ln= Pr, Nd, Sm, Gd−Ho) and Their Strong Nonlinear Optical Responses in Middle IR. J. Am. Chem. Soc. 2011, 133, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Chen, M.C.; Zhou, L.J.; Chen, L.; Wu, L.M. Syntheses, Structures, and Nonlinear Optical Properties of Quaternary Chalcogenides: Pb4Ga4GeQ12 (Q = S, Se). Inorg. Chem. 2013, 52, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; Mei, D.J.; Bai, L.; Lin, Z.S.; Yin, W.L.; Fu, P.Z.; Wu, Y.C. BaGa4Se7: A New Congruent-Melting IR Nonlinear Optical Material. Inorg. Chem. 2010, 49, 9212–9216. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.S.; Zhang, G.; Ye, N. Growth and Characterization of BaGa4S7: A New Crystal for Mid-IR Nonlinear Optics. Cryst. Growth Des. 2009, 9, 1186–1189. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Lin, C.S.; Cui, H.H.; Zhang, W.L.; Zhang, H.; Chen, H.; He, Z.Z.; Cheng, W.D. PbGa2MSe6 (M = Si, Ge): Two Exceptional Infrared Nonlinear Optical Crystals. Chem. Mater. 2015, 27, 914–922. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Lin, C.S.; Cui, H.H.; Zhang, W.L.; Zhang, H.; He, Z.Z.; Cheng, W.D. SHG Materials SnGa4Q7 (Q = S, Se) Appearing with Large Conversion Efficiencies, High Damage Thresholds, and Wide Transparencies in the Mid-Infrared Region. Chem. Mater. 2014, 26, 2743–2749. [Google Scholar] [CrossRef]
- Geng, L.; Cheng, W.D.; Lin, C.S.; Zhang, W.L.; Zhang, H.; He, Z.Z. Syntheses and Characterization of New Mid-Infrared Transparency Compounds: Centric Ba2BiGaS5 and Acentric Ba2BiInS5. Inorg. Chem. 2011, 50, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Lin, C.S.; Zhang, W.L.; Zhang, H.; He, Z.Z.; Cheng, W.D. Ba8Sn4S15: A Strong Second Harmonic Generation Sulfide with Zero-Dimensional Crystal Structure. Chem. Mater. 2013, 26, 1093–1099. [Google Scholar] [CrossRef]
- Liu, B.W.; Zeng, H.Y.; Zhang, M.J.; Fan, Y.H.; Guo, G.C.; Huang, J.S.; Dong, Z.C. Syntheses, Structures, and Nonlinear-optical Properties of Metal Sulfides Ba2Ga8MS16 (M = Si, Ge). Inorg. Chem. 2014, 54, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Liu, B.W.; Zhang, M.J.; Fan, Y.H.; Zeng, H.Y.; Guo, G.C. Syntheses, Structures, and Nonlinear Optical Properties of Two Sulfides Na2In2MS6 (M = Si, Ge). Inorg. Chem. 2016, 55, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Meng, X.G.; Zhong, C.; Chen, X.G.; Qin, J.G. Rb2CdBr2I2: A New IR Nonlinear Optical Material with A Large Laser Damage Threshold. J. Am. Chem. Soc. 2014, 136, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Y.J.; Jiang, K.; Zeng, H.Y.; Liu, T.; Chen, X.G.; Qin, J.G.; Lin, Z.S.; Fu, P.Z.; Wu, Y.C.; et al. A New Mixed Halide, Cs2HgI2Cl2: Molecular Engineering for a New Nonlinear Optical Material in the Infrared Region. J. Am. Chem. Soc. 2012, 134, 14818–14822. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.S.; Saouma, F.O.; Otieno, C.O.; Clark, D.J.; Shoemaker, D.P.; Jang, J.I.; Kanatzidis, M.G. Phase-Change Behavior and Nonlinear Optical Second and Third Harmonic Generation of the One-Dimensional K(1–x)CsxPSe6 and Metastable β-CsPSe6. Chem. Mater. 2015, 27, 1837–1846. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na4MgM2Se6 (M = Si, Ge): The First Noncentrosymmetric Compounds with Special Ethane-Like [M2Se6]6− Units Exhibiting Large Laser-Damage Thresholds. Inorg. Chem. 2015, 54, 10108–10110. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na2Hg3M2S8 (M = Si, Ge, and Sn): New Infrared Nonlinear Optical Materials with Strong Second Harmonic Generation Effects and High Laser-Damage Thresholds. Chem. Mater. 2016, 28, 2795–2801. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na2BaMQ4 (M = Ge, Sn; Q = S, Se): Infrared Nonlinear Optical Materials with Excellent Performances and that Undergo Structural Transformations. Angew. Chem. Int. Ed. 2016, 128, 6825–6827. [Google Scholar] [CrossRef]
- Pan, M.Y.; Ma, Z.J.; Liu, X.C.; Xia, S.Q.; Tao, X.T.; Wu, K.C. Ba4AgGa5Pn8 (Pn = P, As): New Pnictide-Based Compounds with Nonlinear Optical Potential. J. Mater. Chem. C 2015, 3, 9695–9700. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, Y.M.; Chung, I.; Jang, J.I.; Her, B.H.; Yang, S.H.; Ketterson, J.B.; Kanatzidis, M.G.; Hsu, K.F. New Metal Chalcogenides Ba4CuGa5Q12 (Q = S, Se) Displaying Strong Infrared Nonlinear Optical Response. Chem. Mater. 2013, 25, 2427–2433. [Google Scholar] [CrossRef]
- Bera, T.K.; Jang, J.I.; Song, J.H.; Malliakas, C.D.; Freeman, A.J.; Ketterson, J.B.; Kanatzidis, M.G. Soluble Semiconductors AAsSe2 (A = Li, Na) with A Direct-Band-Gap and Strong Second Harmonic Generation: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc. 2010, 132, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Marking, G.M.; Hsu, K.F.; Matsushita, Y.; Ewbank, M.D.; Borwick, R.; Cunningham, P.; Rosker, M.J.; Kanatzidis, M.G. α- and β- A2Hg3M2S8 (A = K, Rb; M = Ge, Sn): Polar Quaternary Chalcogenides with Strong Nonlinear Optical Response. J. Am. Chem. Soc. 2003, 125, 9484–9493. [Google Scholar] [CrossRef] [PubMed]
- Parthé, E. Crystal Chemistry of Tetrahedral Structures; Gordon and Breach Science: New York, NY, USA, 1964. [Google Scholar]
- Aitken, J.A.; Larson, P.; Mahanti, S.D.; Kanatzidis, M.G. Li2PbGeS4 and Li2EuGeS4: Polar Chalcopyrites with a Severe Tetragonal Compression. Chem. Mater. 2001, 13, 4714–4721. [Google Scholar] [CrossRef]
- Bai, L.; Lin, Z.S.; Wang, Z.Z.; Chen, C.T. Mechanism of Linear and Nonlinear Optical Effects of Chalcopyrites LiGaX2 (X = S, Se, and Te) Crystals. J. Appl. Phys. 2008, 103, 083111. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, I.S.; Martin, S.W.; Baek, J.; Shiv Halasyamani, P.; Arumugam, N.; Steinfink, H. Characterization of New Infrared Nonlinear Optical Material with High Laser Damage Threshold, Li2Ga2GeS6. Chem. Mater. 2008, 20, 6048–6052. [Google Scholar] [CrossRef]
- Shi, Y.F.; Chen, Y.K.; Chen, M.C.; Wu, L.M.; Lin, H.; Zhou, L.J.; Chen, L. Strongest Second Harmonic Generation in the Polar R3MTQ7 Family: Atomic Distribution Induced Nonlinear Optical Cooperation. Chem. Mater. 2015, 27, 1876–1884. [Google Scholar] [CrossRef]
- Rudyk, B.W.; Stoyko, S.S.; Mar, A. Rare-earth transition-metal indium sulphides RE3FeInS7 (RE = La–Pr), RE3CoInS7 (RE = La, Ce), and La3NiInS7. J. Solid State Chem. 2013, 208, 78–85. [Google Scholar] [CrossRef]
- Choudhury, A.; Dorhout, P.K. Alkali-Metal Thiogermanates: Sodium Channels and Variations on the La3CuSiS7 Structure Type. Inorg. Chem. 2015, 54, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Poduska, K.M.; DiSalvo, F.J.; Min, K.; Halasyamani, P.S. Structure Determination of La3CuGeS7 and La3CuGeSe7. J. Alloys Compd. 2002, 335, 5–9. [Google Scholar] [CrossRef]
- Hwu, S.J.; Bucher, C.K.; Carpenter, J.D.; Taylor, S.P. A Solid-state Diastereomer, AgLa3GeS7. Inorg. Chem. 1995, 34, 1979–1980. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D.; Wołcyrz, M.; Stepien-Damm, J. The Crystal Structures of R3CuSnS7 (R = La–Nd, Sm, Gd–Ho). Z. Anorg. Allg. Chem. 2005, 631, 1919–1923. [Google Scholar] [CrossRef]
- Gulay, L.D.; Lychmanyuk, O.S.; Olekseyuk, I.D.; Daszkiewicz, M.; Stepien-Damm, J.; Pietraszko, A. Crystal Structures of the Compounds R3CuSiS7 (R = Ce, Pr, Nd, Sm, Tb, Dy and Er) and R3CuSiSe7 (R = La, Ce, Pr, Nd, Sm, Gd, Tb and Dy). J. Alloys Compd. 2007, 431, 185–190. [Google Scholar] [CrossRef]
- Yin, W.L.; Shi, Y.G.; Kang, B.; Deng, J.G.; Yao, J.Y.; Wu, Y.C. Rare-Earth Transition-Metal Chalcogenides Ln3MGaS7 (Ln = Nd, Sm, Dy, Er; M = Co, Ni) and Ln3MGaSe7 (Ln = Nd, Sm, Gd, Dy, M = Co; Ln = Nd, Gd, Dy, M = Ni). J. Solid State Chem. 2014, 213, 87–92. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solid. Acta Crystallogr. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model, 1st ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Preiser, C.; Losel, J.; Brown, I.D.; Kunz, M.; Skowron, A. Long Range Coulomb Forces and Localized Bonds. Acta Crystallogr. B 1999, 55, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Sanchez, A.; Garcia-Munoz, J.L.; Rodriguez-Carvajal, J.; Saez-Puche, R.; Martinez, J.L. Structural Characterization of R2BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) Oxides by X-ray and Neutron Diffraction. J. Solid State Chem. 1992, 100, 201–211. [Google Scholar] [CrossRef]
- Brant, J.A.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Zhang, J.H.; Aitken, J.A. Li2CdGeS4, A Diamond-Like Semiconductor with Strong Second-Order Optical Nonlinearity in the Infrared and Exceptional Laser Damage Threshold. Chem. Mater. 2014, 26, 3045–3048. [Google Scholar] [CrossRef]
- Maggard, P.A.; Nault, T.S.; Stern, C.L.; Poeppelmeier, K.R. Alignment of Acentric MoO3F33− Anions in a Polar Material: (Ag3MoO3F3)(Ag3MoO4)Cl. J. Solid State Chem. 2003, 175, 27–33. [Google Scholar] [CrossRef]
- Ok, K.M.; Halasyamani, P.S. Mixed-Metal Tellurites: Synthesis, Structure, and Characterization of Na1.4Nb3Te4.9O18 and NaNb3Te4O16. Inorg. Chem. 2005, 44, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Halasyamani, P.S. A Rare Multi-Coordinate Tellurite, NH4ATe4O9·2H2O (A = Rb or Cs): The Occurrence of TeO3, TeO4, and TeO5 Polyhedra in the Same Material. J. Solid State Chem. 2008, 181, 2108–2112. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, Version 6.14; Bruker Analytical X-ray Instruments, Inc.: Madison, WI, USA, 2008.
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]

| Compounds | Li+ | Sm/Gd3+ | Si/Ge4+ | S2− | GII |
|---|---|---|---|---|---|
| LiSm3SiS7 | 1.019 | 3.039 | 4.060 | 1.962–2.106 | 0.07 |
| LiSm3GeS7 | 0.993 | 3.034 | 4.114 | 1.933–2.102 | 0.08 |
| LiGd3GeS7 | 1.021 | 3.024 | 4.134 | 1.936–2.128 | 0.09 |
| Compounds | Damage Energy (mJ) | Spot Diameter (mm) | LDT (MW/cm2) |
|---|---|---|---|
| AgGaS2 | 0.35 | 0.375 | 32 |
| LiSm3SiS7 | 0.58 | 0.25 | 118 |
| Particle Size (μm) | |||||
|---|---|---|---|---|---|
| compounds | 33–55 | 55–88 | 88–105 | 105–150 | 150–200 |
| AgGaS2 | 35.2 | 50 | 110 | 185 | 248 |
| LiSm3SiS7 | 368 | 9.4 | 163 | 84 | 23 |
| Dipole Moment | |||||
|---|---|---|---|---|---|
| Species | x (a) | y (b) | z (c) | Magnitude | |
| Debye | ×10−4 esu·cm/Å3 | ||||
| LiSm3SiS7 | |||||
| LiS6 | 0.00 | 0.00 | 9.52 | 9.52 | 0.04 |
| SmS8 | 0.00 | 0.06 | 21.08 | 21.08 | 0.09 |
| SiS4 | 0.00 | 0.00 | −8.24 | 8.24 | 0.03 |
| Unit cell | 0.00 | 0.06 | 22.37 | 22.37 | 0.09 |
| LiSm3GeS7 | |||||
| LiS6 | 0.00 | 0.00 | 8.23 | 8.23 | 0.03 |
| SmS8 | 0.06 | 0.00 | 21.31 | 24.90 | 0.10 |
| GeS4 | 0.00 | 0.00 | −8.05 | 8.05 | 0.03 |
| Unit cell | 0.06 | 0.00 | 21.49 | 21.49 | 0.09 |
| LiGd3GeS7 | |||||
| LiS6 | 0.00 | 0.00 | 14.06 | 14.06 | 0.06 |
| GdS8 | 0.00 | 0.00 | 22.18 | 26.91 | 0.12 |
| GeS4 | 0.00 | 0.00 | −8.43 | 8.43 | 0.04 |
| Unit cell | 0.00 | 0.00 | 27.81 | 27.81 | 0.12 |
| Empirical Formula | LiSm3SiS7 | LiSm3GeS7 | LiGd3GeS7 |
|---|---|---|---|
| Formula weight | 710.50 | 755.00 | 775.70 |
| Temperature | 296 (2) K | ||
| Crystal system | Hexagonal | ||
| Space group | P63 | ||
| Unit cell dimensions | a = 10.007(2) Å c = 5.668(3) Å | a = 9.991(3) Å c = 5.752(3) Å | a = 9.900(7) Å c = 5.753(5) Å |
| Z, V | 2, 491.6(3) Å3 | 2, 497.3(4) Å3 | 2, 488.4(2) Å3 |
| Density (calculated) | 4.800 g/cm3 | 5.042 g/cm3 | 5.274 g/cm3 |
| crystal size (mm3) | 0.182 × 0.180 × 0.095 | 0.261 × 0.172 × 0.138 | 0.210 × 0.160 × 0.120 |
| Completeness to theta = 27.49 | 100 % | 100.0 % | 100 % |
| Goodness-of-fit on F2 | 1.003 | 1.183 | 1.150 |
| Final R indices [Fo2 > 2σ(Fo2)] [a] | R1 = 0.0150 wR2 = 0.01558 | R1 = 0.0162 wR2 = 0.0169 | R1 = 0.0179 wR2 = 0.0180 |
| R indices (all data) [a] | R1 = 0.0303 wR2 = 0.0305 | R1 = 0.0357 wR2 = 0.0359 | R1 = 0.0399 wR2 = 0.0399 |
| Absolute structure parameter | 0.02(2) | 0.011(18) | −0.02 (2) |
| Extinction coefficient | 0.0150(3) | 0.0277(6) | 0.0350(8) |
| Largest diff. peak and hole | 0.564 and −0.614 e Å−3 | 0.661 and −0.648 e Å−3 | 1.052 and −1.291 e Å−3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, N.; Nian, L.; Li, G.; Wu, K.; Pan, S. A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7. Crystals 2016, 6, 121. https://doi.org/10.3390/cryst6100121
Zhen N, Nian L, Li G, Wu K, Pan S. A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7. Crystals. 2016; 6(10):121. https://doi.org/10.3390/cryst6100121
Chicago/Turabian StyleZhen, Ni, Leyan Nian, Guangmao Li, Kui Wu, and Shilie Pan. 2016. "A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7" Crystals 6, no. 10: 121. https://doi.org/10.3390/cryst6100121
APA StyleZhen, N., Nian, L., Li, G., Wu, K., & Pan, S. (2016). A High Laser Damage Threshold and a Good Second-Harmonic Generation Response in a New Infrared NLO Material: LiSm3SiS7. Crystals, 6(10), 121. https://doi.org/10.3390/cryst6100121