Structure and Magnetic Properties of Ce3(Ni/Al/Ga)11—A New Phase with the La3Al11 Structure Type
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
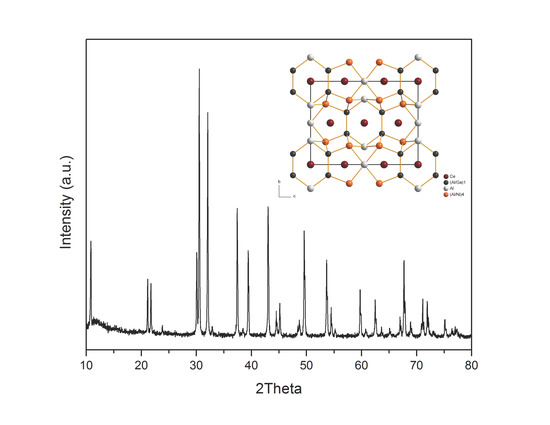
2.2. Crystal Structure

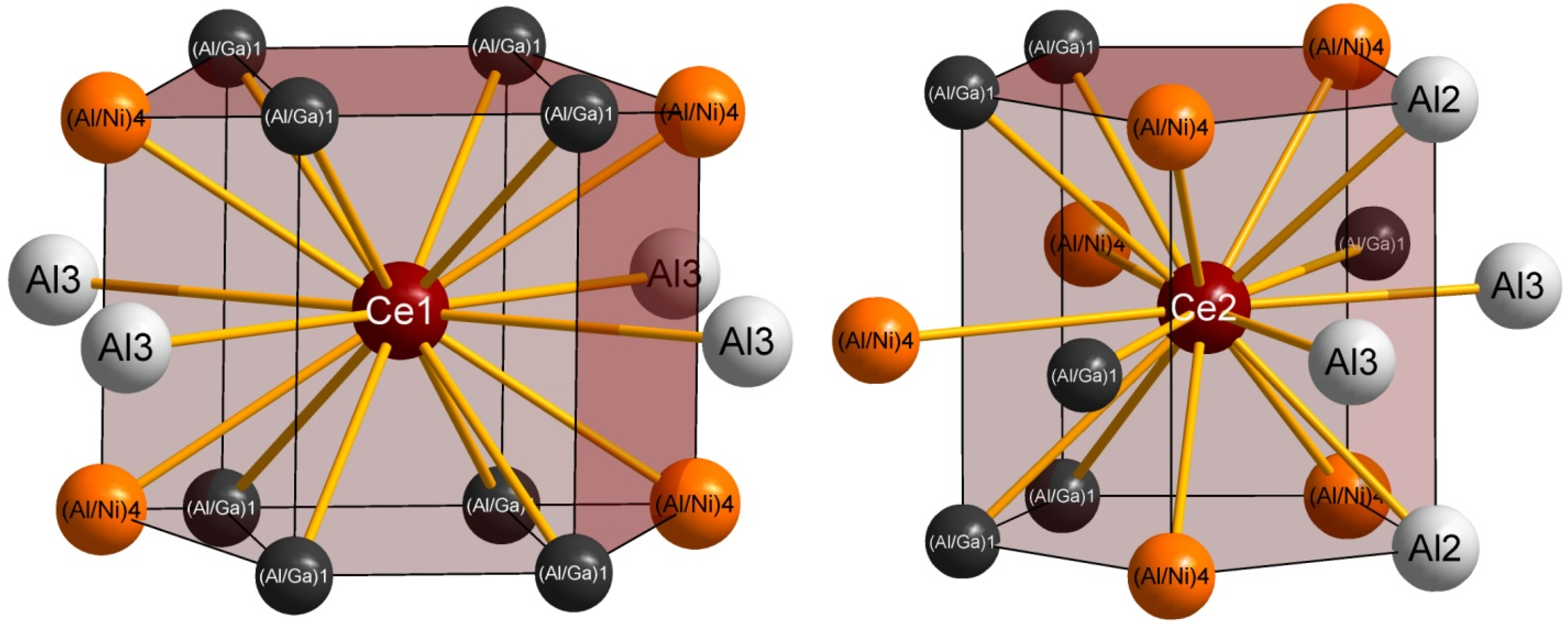
| Ce1– | Count | d (in pm) | Ce2– | Count | d (in pm) |
|---|---|---|---|---|---|
| (Al/Ni)4 | 4× | 328.51(12) | (Al/Ni)4 | 4× | 320.65(9) |
| (Al/Ga)1 | 8× | 330.61(7) | Al3 | 2× | 320.71(11) |
| Al3 | 4× | 360.38(13) | (Al/Ga)1 | 4× | 321.04(7) |
| – | – | – | Al2 | 2× | 322.59(7) |
| – | – | – | (Al/Ni)4 | 2× | 356.68(12) |
| – | – | – | (Al/Ga)1 | 2× | 373.95(13) |
| (Al/Ga)1– | Count | d (in pm) | Al2– | Count | d (in pm) |
| (Al/Ga)1 | 1× | 258.29(13) | (Al/Ni)4 | 4× | 291.13(11) |
| (Al/Ni)4 | 2× | 263.85(9) | Al3 | 4× | 306.60(11) |
| Al3 | 1× | 264.35(11) | Ce2 | 4× | 322.59(7) |
| (Al/Ni)4 | 1× | 272.17(12) | – | – | – |
| Ce2 | 2× | 321.04(7) | – | – | – |
| Ce1 | 2× | 330.61(7) | – | – | – |
| Ce2 | 1× | 373.95(13) | – | – | – |
| Al3– | Count | d (in pm) | (Al/Ni)4– | Count | d (in pm) |
| (Al/Ga)1 | 2× | 264.35(11) | (Al/Ga)1 | 3× | 263.85(9) |
| (Al/Ni)4 | 4× | 283.76(8) | Al3 | 2× | 283.76(8) |
| Al2 | 2× | 306.60(11) | Al2 | 1× | 291.13(11) |
| Ce2 | 2× | 320.71(11) | Ce2 | 2× | 320.65(9) |
| Ce1 | 2× | 360.39(13) | Ce1 | 1× | 328.51(12) |
| – | – | – | Ce2 | 1× | 356.68(12) |
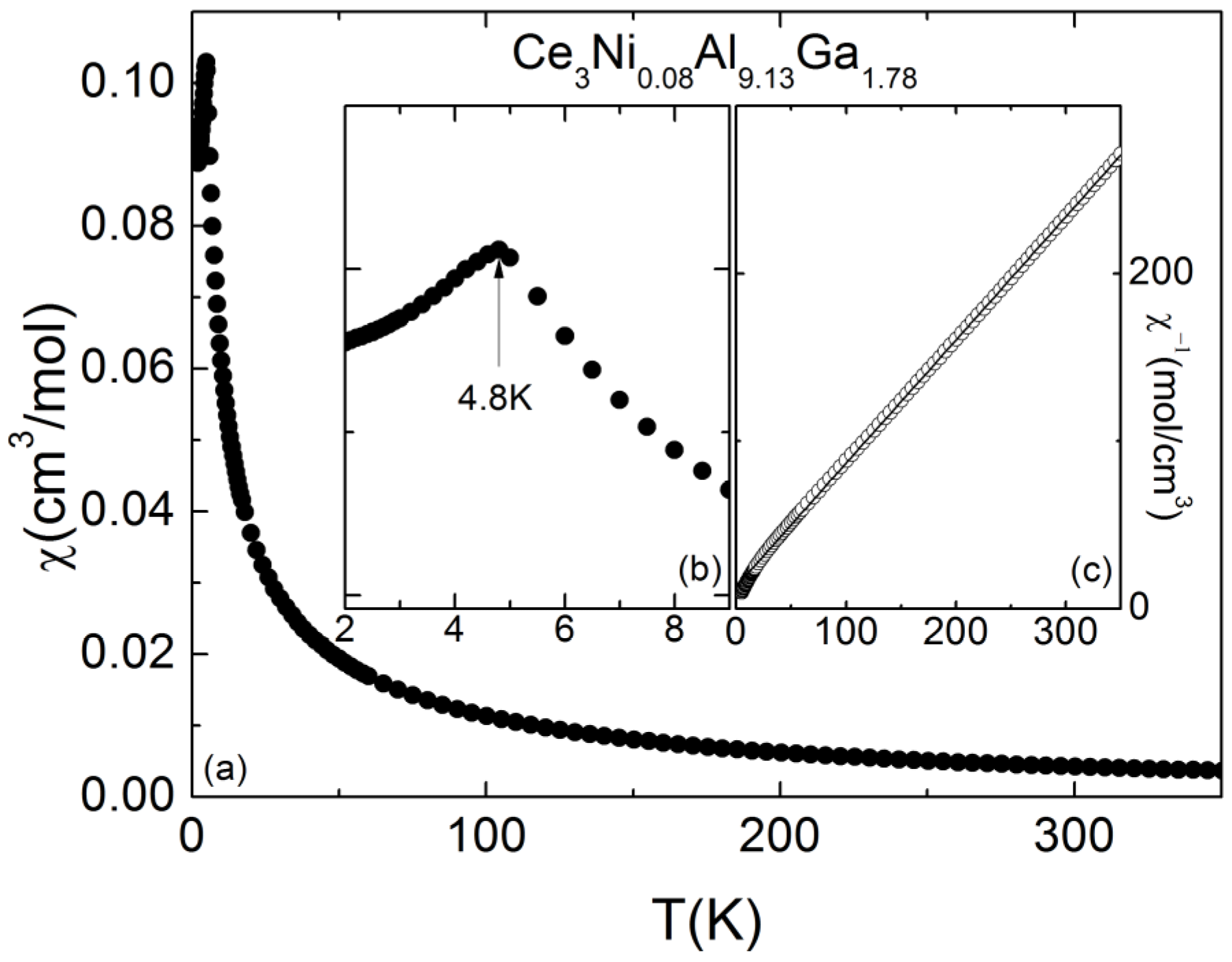
2.3. Magnetic Properties
3. Experimental Section
3.1. Synthesis
3.2. X-ray Crystallography
| Empirical formula | Ce3Ni0.08Al9.13Ga1.78 |
|---|---|
| Temperature | 90(2) K |
| Crystal dimensions (μm3) | 80 × 120 × 220 |
| Crystal system, space group | Orthorhombic, Immm (No. 71) |
| Lattice constants, a (pm) b (pm) c (pm) | 436.38(14) 1004.5(3) 1293.4(4) |
| Formula units (Z) | 2 |
| Calculated density, Dx (g/cm3) | 4.702 |
| Volume, V (Å3) | 566.9(3) |
| Diffractometer, radiation and wavelength | Bruker SMART 1000 CCD, Mo-Kα, λ = 71.073 pm |
| ±hmax; ±kmax; ±lmax | 5, 12, 16 |
| Θmax (°) | 27.09 |
| F(000) | 706 |
| Absorption coefficient μ (mm−1) | 17.13 |
| Data correction | Background, polarization and Lorentz factors |
| Reflections, unique | 3007, 389 |
| Rint/Rσ | 0.030/0.018 |
| Reflections with |Fo| ≥ 4σ(Fo) | 377 |
| Structure determination and refinement | Programs SHELXS–97 and SHELXL–97 [13] |
| Scattering factors | International Tables, Vol. C [14] |
| R1/R1 with |Fo| ≥ 4σ(Fo) | 0.012/0.011 |
| wR2/Goodness of Fit (GooF) | 0.025/1.114 |
| Extinction (g) | 0.0016(1) |
| Residual electron density, max. ρ (e− · 106 pm−3) min. | 0.64, −0.52 |
| Atom | x/a | y/b | z/c | Ueq/pm2 |
|---|---|---|---|---|
| Ce1 | 0 | 0 | 0 | 40(1) |
| Ce2 | 0 | 0 | 0.31629(2) | 43(1) |
| (Al/Ga)1 a | 0 | 0.37143(5) | 0.33596(3) | 60(2) |
| Al2 | 1/2 | 0 | 1/2 | 79(3) |
| Al3 | 0 | 0.21444(12) | 1/2 | 63(2) |
| (Al/Ni)4 b | 0 | 0.27297(9) | 0.13992(7) | 70(3) |
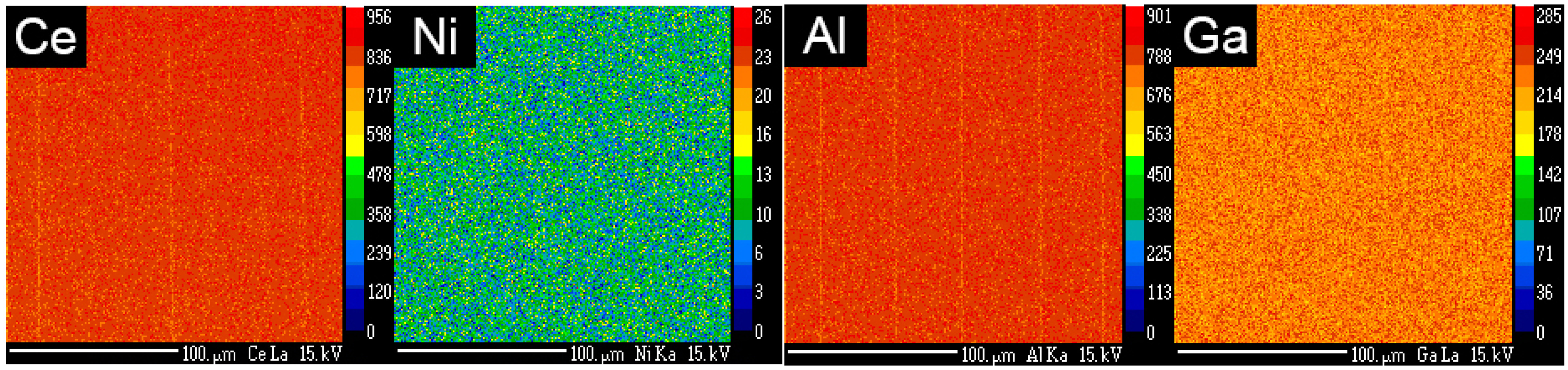
3.3. Electron Microprobe Analysis (EMPA)
3.4. Magnetization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buschow, K.H.J.; van Daal, H.J. Evidence for the presence of the Kondo effect in the compound CeAl2. Phys. Rev. Lett. 1969, 23, 408–409. [Google Scholar] [CrossRef]
- Borchi, E.; de Gennaro, S.; Taddei, C. Magnetic susceptibility of the dense Kondo system CeAl3. Phys. Rev. B 1977, 15, 4528–4531. [Google Scholar] [CrossRef]
- Boucherle, J.X.; Givord, F.; Lapertot, G.; Muñoz, A.; Schweizer, J. Unusual magnetic structures in Ce3Al11. J. Magn. Magn. Mater. 1995, 140–144, 1229–1230. [Google Scholar] [CrossRef]
- Van Daal, H.J.; Buschow, K.H.J. Anomalous behaviour of the electrical resistivity in the compound Ce3Al11. Phys. Lett. A 1970, 31, 103–104. [Google Scholar] [CrossRef]
- Berton, A.; Chaussy, J.; Cornut, B.; Lasjaunias, J.L.; Odin, J.; Peyrard, J. Specific heat of Ce3Al11 and CeAl3 compounds. J. Magn. Magn. Mater. 1980, 15–18, 379–380. [Google Scholar] [CrossRef]
- Boucherle, J.X.; Givord, F.; Lapertot, G.; Muñoz, A.; Schweizer, J. The magnetic structures of Ce3Al11: A single crystal study. J. Magn. Magn. Mater. 1995, 148, 397–408. [Google Scholar] [CrossRef]
- Ebihara, T.; Uwatoko, Y.; Mohri, N. Electrical resistivity in Ce3Al11 under high pressure. J. Magn. Magn. Mater. 2004, 272–276, E83–E84. [Google Scholar] [CrossRef]
- Ebihara, T.; Takahashi, T.; Tezuka, K.; Hedo, M.; Uwatoko, Y.; Uji, S. Magnetic field and pressure effects in Ce3Al11. Phys. B 2005, 359–361, 272–274. [Google Scholar] [CrossRef]
- Garde, C.S.; Takeuchi, T.; Nakano, Y.; Takeda, Y.; Ota, Y.; Miyauchi, Y.; Sugiyama, K.; Hagiwara, M.; Kindo, K.; Honda, F.; et al. Electrical and magnetic properties of R3Al11 (R = La, Ce, Pr, and Nd). J. Phys. Soc. Jpn. 2008, 77. [Google Scholar] [CrossRef]
- Kobayashi, R.; Inadomi, T.; Nishioka, T.; Kato, H.; Matsumura, M.; Kodama, K. Magnetic properties of R3Al11 (R = La, Ce, Pr, Nd, Sm) single crystals. J. Phys. Conf. Ser. 2009, 150. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (on Digital Versatile Disc); ASM International: Materials Park, OH, USA, 2013; Release 2013/2014. [Google Scholar]
- Gomes de Mesquita, A.H.; Buschow, K.H.J. The crystal structure of so-called α-LaAl4 (La3Al11). Acta Crystallogr. 1967, 22, 497–501. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Hahn, T.; Wilson, A.J.C. International Tables for Crystallography; Kluwer Academic Publishing: Dordrecht, The Netherlands, 1992; Volume C. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janka, O.; Shang, T.; Baumbach, R.E.; Bauer, E.D.; Thompson, J.D.; Kauzlarich, S.M. Structure and Magnetic Properties of Ce3(Ni/Al/Ga)11—A New Phase with the La3Al11 Structure Type. Crystals 2015, 5, 1-8. https://doi.org/10.3390/cryst5010001
Janka O, Shang T, Baumbach RE, Bauer ED, Thompson JD, Kauzlarich SM. Structure and Magnetic Properties of Ce3(Ni/Al/Ga)11—A New Phase with the La3Al11 Structure Type. Crystals. 2015; 5(1):1-8. https://doi.org/10.3390/cryst5010001
Chicago/Turabian StyleJanka, Oliver, Tian Shang, Ryan E. Baumbach, Eric D. Bauer, Joe D. Thompson, and Susan M. Kauzlarich. 2015. "Structure and Magnetic Properties of Ce3(Ni/Al/Ga)11—A New Phase with the La3Al11 Structure Type" Crystals 5, no. 1: 1-8. https://doi.org/10.3390/cryst5010001
APA StyleJanka, O., Shang, T., Baumbach, R. E., Bauer, E. D., Thompson, J. D., & Kauzlarich, S. M. (2015). Structure and Magnetic Properties of Ce3(Ni/Al/Ga)11—A New Phase with the La3Al11 Structure Type. Crystals, 5(1), 1-8. https://doi.org/10.3390/cryst5010001