Abstract
The absolute configuration and structure of aegelinol, a pyranocoumarin isolated from Ferulago asparagifolia Boiss (Apiaceae), has been determined by crystallography. Crystal structure of the inclusion complex of aegelinol in β-cyclodextrin was determined (a = 15.404(1) Å, b = 15.281(1) Å, c = 17.890(1) Å, α = 99.662(1), β = 113.4230(1), γ = 102.481(1)°, P1; R1 = 6.71%) and allowed unambiguous determination of the absolute configuration of the stereogenic center of aegelinol. The pyranocoumarin guest is included within the cylindrical cavity formed by dimeric β-cyclodextrin molecules with a head-to-head arrangement. Crystal structure of aegelinol alone was also determined (a = 6.8921(3) Å, b = 11.4302(9) Å, c = 44.964(3) Å, P212121; R1 = 4.44%) and allowed precise determination of its geometry. Aegelinol crystallizes with three molecules in the asymmetric unit held together by H-bonds and π-stacking interactions.
1. Introduction
Aegelinol (1) is a pyranocoumarin that was isolated from Ferulago asparagifolia Boiss (Apiaceae), a species growing in Turkey [1]. Aegelinol has also been reported as a minor lactonic constituent of Aegle marmelos [2]. Three abundant pyranocoumarins (Figure 1) (grandivittin (2), agasyllin (3) and aegelinol benzoate (4)) were also isolated from the roots of Ferulago campestris collected in Sicily and both aegelinol (1), their hydrolysis product, and agasyllin (3) showed a significant antibacterial effect against Gram-negative and Gram-positive bacteria [3].
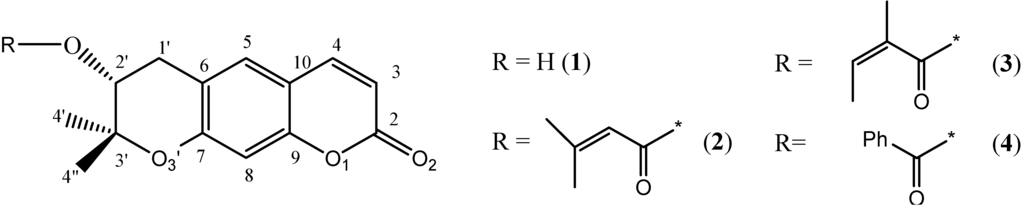
Figure 1.
The chemical structure and numbering scheme of pyranocoumarins.
The absolute configuration of 1 could not be determined because NMR spectral data of α and β epimers do not significantly differ. Optical rotation measurements, however, suggest an α configuration for the compound [1]. Compounds 2-4 are ester derivatives of aegelinol (1), whose absolute configuration at C-2’ was also not clear. Abyshev et al. [4] reported an (S) C-2’ absolute configuration, although they cited the degradative determination of the absolute configuration performed by Lemmich [5]. On the other hand, Erdelmeyer et al. [6] indicated an (R) C-2’ configuration. In both cases, the reported optical rotation of the compound was negative. Hydrolysis of the three esters gives the same compound (1) with a negative optical rotation. Consequently, Basile et al. [3] decided to determinate the absolute stereochemistry by means of Mosher’s and Horeau’s (indirect) methods and assigned from their NMR data an (R) C-2’ absolute configuration for 1 and its esters 2-4.
Rigorous differentiation of enantiomers by the X-ray diffraction method is now possible through the use of the Flack parameter [7,8]. However, compounds composed only of light atoms (e.g., C, H, O) give rise to standard uncertainties on the Flack parameter which are too large for reliable absolute configuration determination [9].
In many cases, it may thus be necessary to perform a derivatization of the compound to get a crystalline product. If a chiral reagent is used for this purpose, a second stereogenic center of known absolute configuration is introduced and the X-ray structure obtained is then unambiguous and gives the absolute configuration of the first compound. This approach is classical when salts can be formed, which is not the case for molecules such as aegelinol.
To circumvent this limit, we decided to co-crystallize aegelinol (1) with β-cyclodextrin. In principle, formation of such an inclusion complex with a host of known chirality (β-cyclodextrin consists of seven optically active D-glucose units) should allow direct determination of the absolute configuration of the guest (aegelinol). Indeed, in principle, cyclodextrins form diastereoisomers by including optically active guests within their cavity and should be excellent reagents for the resolution of racemic compounds (and subsequent determination of their absolute configuration). In practice, possibly due to their round and symmetrical structure, only low optical resolution is achieved [10]. As a matter of fact, only a limited number of crystal structures of inclusion complexes of cyclodextrins with chiral guests are available in the Cambridge Structure DataBank (CSD), among which permethylated α-cyclodextrin binding S- and R-mandelic acid (CECMAY10 and CECMEC10, respectively) [11] or R- and S-isomers of flurbiprofen and S-ibuprofen (COYXAP10, COYXET20 [12] and RONWOG [13] respectively) included within the cavity of permethylated β-cyclodextrin. Selected examples of crystal structures of cyclodextrin’s inclusion complexes with different chiral molecules are presented in Table S1 (supplementary material) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
In the present work, we report on our efforts to determine by X-ray crystallography the absolute configuration of aegelinol and show that crystallization of an inclusion complex of aegelinol in β-cyclodextrin solved the problem.
2. Results and Discussion
Crystals of aegelinol (1) were obtained at room temperature by slow evaporation from a solution in ethanol. The structure belongs to the orthorhombic P212121 space group with cell parameters a = 6.8921(3) Å, b = 11.4302(9) Å, c = 44.964(3) Å. The structure was solved by direct methods and refined to a final R1 = 4.44%. Main statistics on data quality and refinement are given in Table 1.

Table 1.
Main statistics on data quality and refinement for the crystal structures of aegelinol (1) and its inclusion complex in β-cyclodextrin (1-BCD).
| Crystallographic data | Aegelinol | Β-CD-aegelinol |
|---|---|---|
| Chemical formula | C14H14O4 | 2C42H70O35. 2C14H14O4.23.5H2O |
| Temperature (K) | 150.05(10) | 150(2) |
| Wavelength (Å) | Mo Kα (0.71073) | Cu Kα (1.54179) |
| Crystal system | Orthorhombic | Triclinic |
| Space group | P212121 | P1 |
| a (Å) | 6.8921(3) | 15.404(1) |
| b (Å) | 11.4302(9) | 15.281(1) |
| c (Å) | 44.964(3) | 17.890(1) |
| α (°) | 90.00 | 99.662(1) |
| β (°) | 90.00 | 113.423(1) |
| γ (°) | 90.00 | 102.481(1) |
| Volume (Å3) | 3542.2(4) | 3618.4(5) |
| Z | 12 | 1 |
| Calculated density (g.cm−3) | 1.385 | 1.466 |
| F(0 0 0) | 1560 | 1704 |
| Absorption coefficient (mm−1) | 0.102 | 1.148 |
| Crystal description | Long Rod | Plate |
| Crystal size (mm) | 0.2 × 0.2 × 0.4 | 0.13 × 0.32 × 0.37 |
| Theta range for data collection (°) | 3.25- 25.02 | 2.81–69.59 |
| Limiting indices | −8 ≤ h ≤ 6, −11 ≤ k ≤ 13, −46 ≤ l ≤ 53 | −18 ≤ h ≤ 14, −18 ≤ k ≤ 18, −21 ≤ l ≤ 21 |
| Reflections collected/unique | 10327/5883 | 36967/15938 |
| R (int) | 0.0246 | 0.0355 |
| Data/restraints/parameters | 5883/0/491 | 15938/88/2082 |
| Goodness-of-fit (S) on F2 | 1.097 | 1.060 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Final R indices [I > 2sigma(I)] | 4.44% | 6.71% |
| Final R indices (all data) | 5.22% | 7.1% |
| Largest difference peak and hole (e/Å3) | 0.218 and −0.229 | 1.249 and −0.419 |
| Flack × parameter | 0.4(9) | 0.5(2) |
The structure reveals an infinite three-dimensional network stabilized mainly by ideal intramolecular hydrogen bonds in addition to very strong π-stacking interactions along the a direction. Aegelinol crystallizes with three molecules in the asymmetric unit held together by H-bonds and π-stacking interactions (Figure 2). The three molecules are identical with negligible differences in the three mutual RMSDs: A&B = 0.0894, A&C = 0.0642 and B&C = 0.105 Å. This is further confirmed by similar geometrical features (Table 2 and supplementary data).
The coumarin moiety is planar with maximum deviations from the least-squares planes of 0.0247, 0.0396 and 0.0181 Å for the three molecules A, B and C, respectively. The dihedral angles between these planes in crystal packing are AˆA=2.65(3)°, AˆB = 13.39(3)°, BˆC = 2.99(4)° and AˆC =10.76(2)°. The bond lengths and bond angles observed in this structure are comparable with those reported for other coumarin derivatives in CSD (e.g., GEHWEW [33] and MOFJIA [34]).
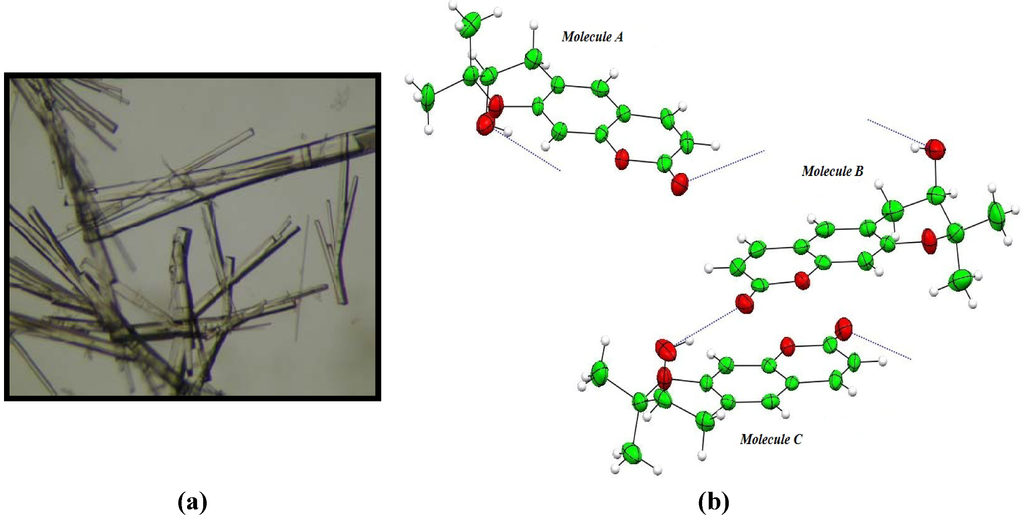
Figure 2.
(a) Morphology of crystals of aegelinol (1) obtained by slow evaporation from a solution in ethanol; (b) Conformation of the three independent molecules in the asymmetric unit showing thermal motion (ORTEP, 30% probability).

Table 2.
Selected structural features (bond lengths (Å), valence and torsion angles (°)) of the crystal structures of aegelinol (1, three molecules in asymmetric unit (A, B, C)) and its inclusion complex in β-cyclodextrin (1-BCD two molecules in asymmetric unit (A, B))
| Geometrical aspects | 1 A | 1 B | 1 C | 1-BCD A | 1-BCD B |
|---|---|---|---|---|---|
| C6-C1` | 1.503(3) | 1.501(3) | 1.501(3) | 1.49(2) | 1.52(2) |
| C1`-C2` | 1.518(4) | 1.523(4) | 1.520(4) | 1.45(2) | 1.49(3) |
| C2`-O2` | 1.431(3) | 1.422(3) | 1.429(3) | 1.43(2) | 1.43(2) |
| C2`- C3` | 1.522(4) | 1.527(4) | 1.521(4) | 1.52(2) | 1.45(3) |
| C3`-O3` | 1.463(3) | 1.470(3) | 1.467(3) | 1.46(2) | 1.50(2) |
| C7-O3` | 1.363(3) | 1.361(3) | 1.34(2) | 1.35(1) | 1.36(2) |
| C7-C6-C1` | 120.0(2) | 120.0(2) | 120.1(2) | 121(1) | 120(1) |
| C6-C1`-C2` | 110.4(2) | 111.6(2) | 110.2(2) | 110(1) | 113(2) |
| O2`-C2`-C1` | 111.4(2) | 111.8(2) | 111.3(2) | 110(1) | 114(2) |
| C1`-C2`-C3` | 110.3(2) | 110.7(2) | 110.6(2) | 114(1) | 112(2) |
| O3`-C3`-C2` | 109.7 (2) | 109.7(2) | 110.6(2) | 106(1) | 114(1) |
| O3`-C7-C6 | 122.9(2) | 123.0(2) | 123.2(2) | 122(1) | 125(1) |
| C7-O3`-C3` | 118.6 (2) | 118.4 (2) | 119.0(2) | 118(1) | 114(1) |
| C5-C6-C1`-C2` | −156.9(2) | −159.3(2) | −154.0(2) | −165(1) | 170(2) |
| C7-C6-C1`-C2` | 21.4(3) | 20.5(3) | 24.7(3) | 14(1) | −10(3) |
| C6-C1`-C2`-O2` | 70.3(3) | 79.0(3) | 71.9(3) | −172(1) | −78(3) |
| C6-C1`-C2`-C3` | −50.4(3) | −47.1(3) | −50.8(3) | −46(1) | 37(3) |
| O2`-C2`-C3`-O3` | −62.3(3) | −67.7(3) | −65.7(3) | −173(1) | 68(2) |
| C1`-C2`-C3`-O3` | 60.0(3) | 58.2(3) | 57.7(3) | 62(1) | −55(2) |
| O2`-C2`-C3`-C4` | 177.6(2) | 172.2(2) | 173.7(2) | −60(1), 69(2) | −176(1), −53(2) |
| C1`-C2`-C3`-C4` | −60.0(3) | −62.0(3) | −62.9(3) | 175(1), −56(2) | 62(2), −175(2) |
| C1`-C6-C7-O3` | −0.4(4) | −4.0(4) | −4.3(4) | 1(1) | 0(2) |
| C8-C7-O3`-C3` | −172.5(2) | −166.6(2) | −172.4(2) | −162.8(9) | 162(1) |
| C6-C7- O3`-C3` | 10.1(3) | 15.8(3) | 11.0(4) | 18(2) | −16(2) |
| C2`-C3`-O3`-C7 | −39.6(3) | −42.6(3) | −37.6(3) | −47(1) | 44(2) |
| C4`-C3`-O3`-C7 | 82.3(3) | 79.1(3) | 84.9(3) | −165(1), 75(1) | −77(2), 172(2) |
The packing of molecules in the unit cell gives some indication of the types of interaction that such a molecule would show in biological systems. Two types of interaction are found. First, the molecules are partially flat and pack in planes, where molecules A and its symmetrical equivalences are stacked one above the other in the opposite direction. B and C molecules have exactly the same arrangement as A molecules. The distances between these planes and the centroid of aromatic ring in the next layer are A-A = 3.481Å and B-C = 3.391Å. This arrangement allows optimal π-π interaction (Figure 3 (see also supplementary material Figure S2)). Second, the carbonyl group interacts with hydroxyl groups in the next layer’s molecule with OH…O distances ranging from 2.816(2) to 2.903(3) Å, as shown in Figure 2, Table 3.
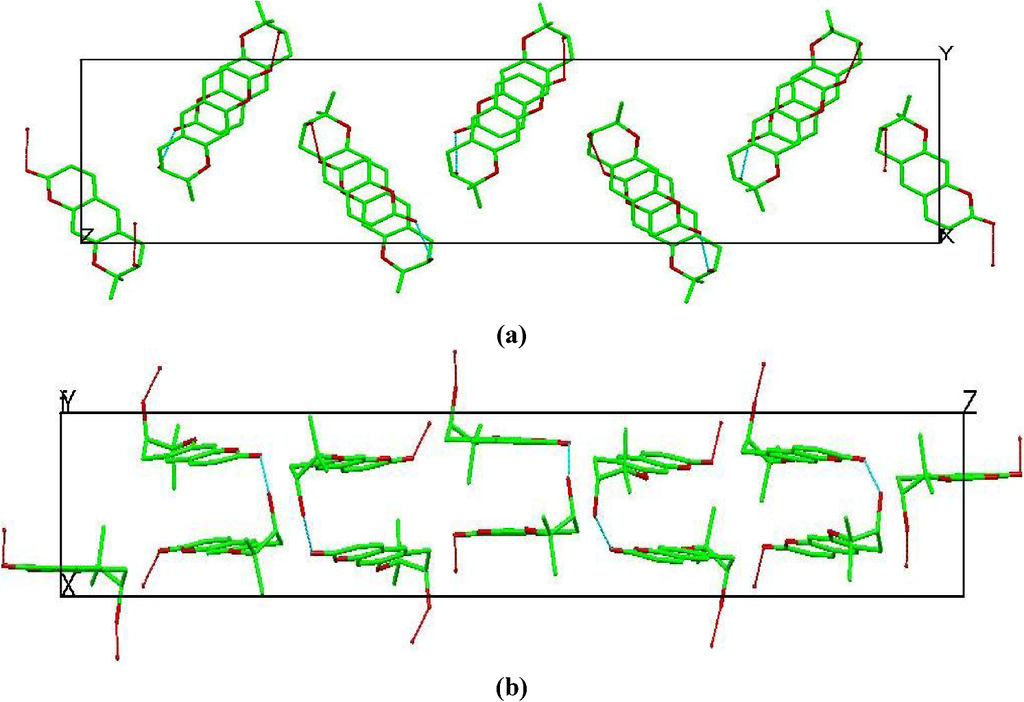
Figure 3.
Crystal packing of aegelinol. (a) Stacking along the a axis; (b) Stacking along the b axis.

Table 3.
Selected hydrogen bonds.
| Aegelinol (1) | |||||
|---|---|---|---|---|---|
| D | A | d (D-H) (Å) | d (H...A) (Å) | d (D...A) (Å) | <(DHA) (°) |
| O(2`A) | O(2A) i | 0.84 | 2.08 | 2.902(3) | 164.5 |
| O(2`B) | O(2C) ii | 0.84 | 2.01 | 2.816(2) | 159.4 |
| O(2`C) | O(2B) | 0.84 | 2.03 | 2.851(3) | 165.8 |
| BCD-1 complex ( 1-BCD) | |||||
| O2`A1 | O(13W) | 0.84 | 2.122 | 2.73(1) | 129.16 |
| O2`B1 | O(61A) | 0.84 | 2.524 | 3.278(3) | 150.01 |
| O2`A2 | O(17`W) | 0.84 | 2.04 | 2.49(2) | 145.6 |
| O2`B2 | O(63B) iii | 0.84 | 2.065 | 2.83 (2) | 151.37 |
| O(65B) | O2`A2 iv | 0.84 | 2.05 | 2.76(2) | 144.5 |
Symmetry transformations used to generate equivalent atoms: i [x-1/2, -y+3/2, -z+2]; ii [x+1, y, z]; iii [x+1, y, z+1]; iv [x, y, z+1] (See supplementary data for molecular numbering Figure S1 and Figure S3).
Statistics of the Flack parameters (x = 0.4(9)) confirm that the absence of suitable heavy atoms (anomalous scatterers) in the molecule is a limitation for reliable absolute configuration of such molecules by X-ray diffraction [9].
Crystal structure of the inclusion complex of aegelinol in β-cyclodextrin (1-BCD) was also determined (a = 15.404(1) Å, b = 15.281(1) Å, c = 17.890(1) Å, α = 99.662(1)°, β = 113.423(1)°, γ = 102.481(1)°, P1; R1 = 6.71%) and allowed unambiguous determination of the absolute configuration of the stereogenic center of aegelinol. Crystals were grown from a solution in water/ethanol (50:50). Two morphologies of crystals are observed: the long and yellow crystals correspond to aegelinol alone, and parallelepiped and colorless ones are related to the inclusion complex with β-cyclodextrin. Primary statistics on data quality and refinement are given in Table 1. Figure 4 presents the structure of the inclusion complex. From this structure, the absolute configuration of C2’ of aegelinol can unambiguously be assigned and corresponds to the (R) enantiomer.
Interestingly, disorder of the included aegelinol molecule within the cyclodextrin cavity was modeled in two sites, nearly similar, with 60:40 occupancy. The coumarin moieties are planar with maximum deviations from the least-squares planes of 0.0034 and 0.0038 Å for the two molecules A and B, respectively. The dihedral angles between these planes, inside the cavity, is 22.2(4)° in the first site and 3.0(3)° in the second site.
The solution of the crystal structure revealed a 2:2 stoichiometry. The pyranocoumarin guest is included within the cylindrical cavity formed by dimeric β-cyclodextrin molecules with a head-to-head arrangement. Crystalline complexes of hydrogen-bonded β-cyclodextrin dimers complexed with organic guest molecules have been found previously to adopt one of four crystal packing motifs: a channel motif (Ch), a checker board motif (CB), an intermediate motif (Im), and, most recently, a tetrad motif (Tt). Packing diagrams are available in different reports on β-cyclodextrin complexes; the reader is referred to these works for both diagrams and discussion of the different structural properties for these packing arrangements [35,36,37]. The CD host molecules are associated through hydrogen bonding between secondary hydroxyl groups as dimers, packing in the previously observed (Im) packing arrangement [35,36,37]. The fact that this arrangement is common to these complexes of β-CD is important for two reasons:
- (1) It implies that crystal packing does not severely limit conformational and translational freedom of guest molecules.
- (2) It provides an environment for the guest molecules that resembles a macromolecular binding pocket by providing a hydrophobic pocket rimmed with a scaffolding of hydrophilic binding sites that can interact directly with guest molecules or via bridging water molecules.
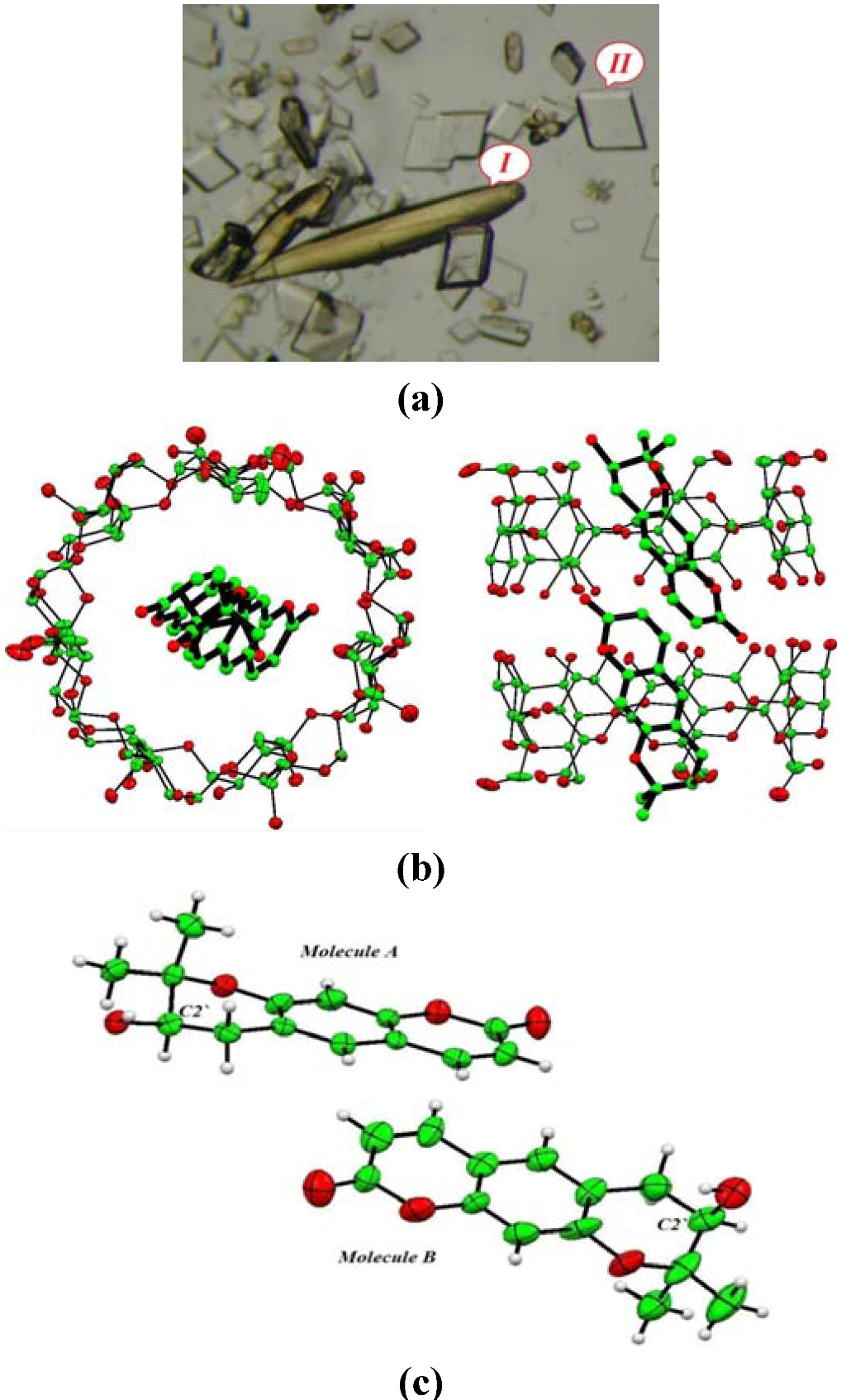
Figure 4.
(a) Morphology of crystals of aegelinol-β-cyclodextrin inclusion complex (1-BCD). Two forms of crystals are observed: (I) corresponds to cell parameters of aegelinol alone, and (II) corresponds to cell parameters of the inclusion complex; (b) Two perpendicular views showing the conformation of the 1-BCD inclusion complex (ORTEP, 30% probability) and confirming the R configuration at C2’. Only one component of the disorder of the guest molecule inside the cyclodextrin cavity is presented and hydrogen atoms and water molecules are removed for clarity; (c) View of the two aegelinol molecules after removing all the rest of the structure to show their configuration.
As a consequence, guest molecules experience considerable variation in their specific interactions with the host molecules and waters of hydration, often resulting in the observation of crystallographic disorder. This semichannel-type packing orients along the c-axis direction (Figure 5).
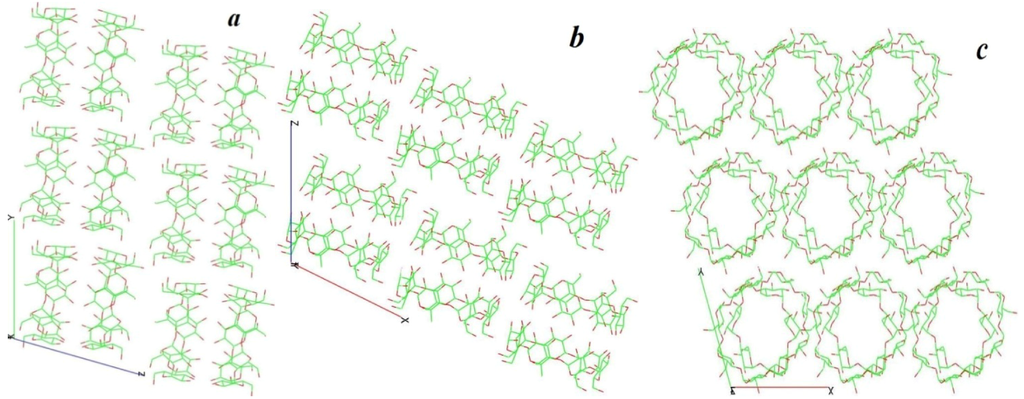
Figure 5.
Crystal packing of β-cyclodextrins in the 1-BCD crystal structure shown along the a, b and c directions. Guest molecules (aegelinol) and waters are omitted for clarity.
One hydrogen bond is observed between the cyclodextrin and the first site and two in the second site of the disordered guest Table 3 (See supplementary material Figure S1 and Figure S3). These hydrogen bonds are between the hydroxyl groups on the guest and on the primary side of cyclodextrin. The two molecules of aegelinol stack together (mainly involving the coumarin moieties) and are stabilized by hydrophobic interactions within the cavity formed by two cyclodextrins (Figure 4). The distances between the planar coumarin and the centroid of the other coumarin are 3.233Å in the first site and 3.215 Å in the second site. Moreover, this arrangement serves a strong π-π interaction (See supplementary material Figure S2). Notable differences are observed at the level of the pyroano ring of the two aegelinol molecules (RMSD = 0.871 Å and Table 2) most likely resulting from less constraints in 1-BCD compared to the structure of 1 alone. Twenty-four ordered water molecules are also observed in the structure. They are situated outside the cavity at the borders of the toroid rims and in interstices between β-CD molecules. Five water molecules are disordered over two sites.
3. Experimental Section
Isolation and purification of aegelinol (1) has been reported elsewhere [1]. Briefly, roots of F. asparagifolia (500 g) were dried, powdered and extracted successively with light petroleum, EtOAc and MeOH at room temperature. Extracts were then evaporated and lyophilized. The EtOAc extract (4.0 g) was chromatographed over silica gel columns. Six compounds were isolated from the EtOAc extract, one of which corresponding to aegelinol.
Crystals of aegelinol were prepared by dissolving a 1–2 mg amount of aegelinol in a minimal amount of ethanol. The mixture was then stored at room temperature. Colorless long crystals suitable for X-ray data collection were obtained by slow evaporation after one week.
Crystals of the β-CD in complex with aegelinol were prepared by dissolving a few milligrams of β-CD in water (2 mL) at 65 °C and aegelinol in ethanol (2 mL) at 65 °C, in stoichiometric (2:1) ratio with respect to β-CD. Both solutions were mixed and stirred at 65 °C for 6 hours. Then the mixture was stored at room temperature. Two forms of crystals (colorless plates and pale yellow needle) suitable for X-ray data collection were obtained by slow evaporation after one week.
Single crystals of aegelinol and of the β-CD-aegelinol complex were mounted on the head of a 4-circle kappa goniometer. X-ray diffraction experiment was carried out at low temperature (150 K) on an Oxford Gemini R Ultra using Mo Kα (0.71073 Å) (aegelinol) or Cu Kα (1.54179 Å) radiation (inclusion complex). Data reduction was carried out with the CrysAlisPro program [38]. Crystal data collection and refinement details are listed in Table 1.
The structure of aegelinol was determined by direct methods and refined using full-matrix least-squares based on F2 with the program SHELX [39], based on 491 parameters and 0 restraints. The refinement converged to R1 = 4.44% for I > 2σ(I) and 5.22% for all data. Hydrogen atoms were calculated at ideal positions and refined using a riding model.
The structure of the β-CD-aegelinol complex was solved by atomic coordination replacement methods using a β-CD complex structure from CSD [BOSZOZ] [40] with the same cell parameters after making suitable transformation and deleting the guest from the structure. Extra electronic density in the cavity corresponded to aegelinol. This structure was refined using full-matrix least-squares on F2 with the program SHELX [39], based on 2083 parameters and 89 restraints. The refinement converged to R1 = 6.71% for I > 2σ(I) data and 7.10% for all data. Most hydrogen atoms were calculated at ideal positions and refined using a riding model and the rest of the hydrogen atoms were located on a different Fourier map. Ordered water molecules were located in a difference Fourier map. All non-hydrogen atoms were anisotropically refined. Secondary hydroxyl groups were refined with two alternate conformations. Disorder of the included aegelinol molecules within the cyclodextrin cavity, five disordered water molecules and two primary hydroxyl groups in β-CD were modeled in two sites. The second sites were located from difference Fourier maps.
Coordinates have been deposited at the Cambridge Structure DataBank (CCDC codes 884098 and 884099) and are available as supplementary material to this article.
4. Conclusions
Crystals of aegelinol (1) alone were obtained and led to determination of its crystal structure. Statistics of the Flack parameters confirm that the absence of suitable heavy atoms (no strong anomalous scatterers) in the molecule is a limitation for reliable absolute configuration of such molecules by X-ray diffraction. Derivatization of the molecule by formation of a chiral salt was not an alternative as the molecule does not contain any basic or acidic function. As an alternative, we were able to grow crystals of the inclusion complex of aegelinol in β-cyclodextrin and refined the crystal structure of the complex, which allowed unambiguous determination of the absolute configuration of the stereogenic center of aegelinol. The (R) configuration of C2’ obtained by this crystallographic study confirms previous conclusions based on indirect NMR data.
Acknowledgments
Authors thank the Belgian Fonds National de la Recherche Scientifique (FNRS), les Facultés Universitaires Notre Dame de la Paix (FUNDP) and Ministry of Higher Education in Syrian Arab Republic for financial support, as well as Laurence Goossens for her kind interest in this work.
Conflict of Interest
The authors declare no conflict of interest.
References
- Alkhatib, R.; Hennebelle, T.; Roumy, V.; Sahpaz, S.; Suzgeç, S.; Akalın, E.; Meriçli, A.H.; Bailleul, F. Coumarins, caffeoyl derivatives and a monoterpenoid glycoside from Ferulago Asparagifolia. Biochem. Syst. Ecol. 2009, 37, 230–233. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sen, R.; Ganguly, D. Aegelinol, a minor lactonic constituent of Aegle Marmelos. Phytochemistry 1978, 17, 328–329. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant activities of coumarins from the roots of ferulago campestris (apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Abyshev, A.Z.; Gindin, V.A.; Semenov, E.V.; Agaev, E.M.; Abdulla-Zade, A.A.; Guseinov, A.B. Structure and biological properties of 2H-1-benzopyran-2-one (coumarin) derivatives. Pharm. Chem. J. 2006, 40, 607–610. [Google Scholar]
- Lemmich, J.; Nielsen, B.E. Stereochemistry of natural coumarins containing the 3-hydroxy-2,2-dimethylchroman system. Tetrahedron Lett. 1969, 1, 3–4. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarins derivatives from Eryngium Campestre. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Flack, H.D. On Enantiomorph-Polarity Estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar]
- Bernardinelli, G.; Flack, H.D. Least-squares absolute-structure refinement. Practical experience and ancillary calculations. Acta Cryst. 1985, A41, 500–511. [Google Scholar]
- Flack, H.D.; Bernardinelli, G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Cryst. 2000, 33, 1143–1148. [Google Scholar]
- Harata, K.; Uekama, K.; Otagiri, M.; Hirayama, F. Crystal structures of cyclodextrin complexes with chiral molecules. J. Incl. Phenom. 1984, 2, 583–594. [Google Scholar] [CrossRef]
- Harata, K.; Uekama, K.; Otagiri, M.; Hirayama, F. The structure of the cyclodextrin complex. XIX. Crystal structures of hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin complexes with (S)- and (R)-mandelic acid. chiral recognition through the induced-fit conformational change of the macrocyclic ring. Bull. Chem. Soc. Jpn. 1987, 60, 497–502. [Google Scholar] [CrossRef]
- Harata, K.; Uekama, K.; Imai, T.; Hirayama, F.; Otagiri, M. Crystal structures of heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin complexes with (R)- and (S)-Flurbiprofen. J. Incl. Phenom. Molcycl. Chem. 1988, 6, 443–460. [Google Scholar]
- Brown, G.R.; Caira, M.R.; Nassimbeni, L.R.; van Oudtshoorn, B. Inclusion of ibuprofen by heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin: An X-ray diffraction and thermal analysis study. J. Incl. Phenom. Mol. Recogn. Chem. 1996, 26, 281–294. [Google Scholar] [CrossRef]
- Makedonopoulou, S.; Yannakopoulou, K.; Mentzafos, D.; Lamzin, V.; Popov, A.; Mavridis, I.M. Non-covalent interactions in the crystallization of the enantiomers of 1,7-dioxaspiro[5.5]undecane (olive fly sex pheromone) by enantiospecific cyclodextrin hosts, hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. Acta Crystallogr. Sect. B Struct. Sci. 2001, 57, 399–409. [Google Scholar] [CrossRef]
- Yannakopoulou, K.; Mentzafos, D.; IMavridis, M.; Dandika, K. Chiral Recognition of (R)-(−)-1,7-Dioxaspiro-[5.5]undecane by Hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin. Angew. Chem. 1996, 35, 2480–2482. [Google Scholar] [CrossRef]
- Mentzafos, D.; Mavridis, I.M.; Yannakopoulou, K. Structure of the 1:1 Complex of Hexakis(2,3,6-tri-O-methyl) α-Cyclodextrin with (R)-(-)-1,7-Dioxaspiro[5.5]undecane. J. Incl. Phenom. Macrocycl. Chem. 1999, 33, 321–330. [Google Scholar] [CrossRef]
- Alexander, J.M.; Clark, J.L.; Brett, T.J.; Stezowski, J.J. Chiral discrimination in cyclodextrin complexes of amino acid derivatives: β-cyclodextrin N-acetyl-L-phenylalanine and N-acetyl-D-phenylalanine complexes. Proc. Nat. Acad. Sci. USA 2002, 99, 5115–5120. [Google Scholar] [CrossRef]
- Caira, M.R.; de Vries, E.; Nassimbeni, L.R.; Jacewicz, V.W. Inclusion of the antidepressant paroxetine in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2003, 46, 37–42. [Google Scholar] [CrossRef]
- Harata, K. The structure of the cyclodextrin complex. XII. Crystal structure of α-cyclodextrin-1-phenylethanol (1:1) tetrahydrate. Bull. Chem. Soc. Jpn. 1982, 55, 1367–1371. [Google Scholar] [CrossRef]
- Ferron, L.; Guillen, F.; Coste, S.; Coquerel, G.; Plaquevent, J.-C. Is the optical rotation sign/absolute configuration relationship still a problem? Examples taken from unnatural quaternary aminoacids. Chirality 2006, 18, 662–666. [Google Scholar] [CrossRef]
- Clark, J.L.; Stezowski, J.J. Molecular recognition in cyclodextrin complexes of amino acid derivatives. 1. Crystallographic studies of â-cyclodextrin complexes with N-Acetyl-L-phenylalanine methyl ester and N-Acetyl-L-phenylalanine amide pseudopeptides. J. Am. Chem. Soc. 2001, 123, 9880–9888. [Google Scholar] [CrossRef]
- Clark, J.L.; Booth, B.R.; Stezowski, J.J. Molecular recognition in cyclodextrin complexes of amino acid derivatives. 2.1. A new perturbation: The room-temperature crystallographic structure determination for the N-Acetyl-p-methoxy-L-phenylalanine methyl ester/â-cyclodextrin complex. J. Am. Chem. Soc. 2001, 123, 9889–9895. [Google Scholar]
- Clark, J.L.; Peinado, J.; Stezowski, J.J.; Vold, R.L.; Huang, Y.Y.; Hoatson, G.L. Molecular recognition in cyclodextrin complexes of amino acid derivatives: The effects of kinetic energy on the molecular recognition of a pseudopeptide in a nonconstraining host environment as revealed by a temperature-dependent crystallographic study. J. Phys. Chem. B 2006, 110, 26375–26387. [Google Scholar]
- Grandeury, A.; Petit, S.; Gouhier, G.; Agasse, V.; Coquerel, G. Enantioseparation of 1-(p-bromophenyl)ethanol by crystallization of host–guest complexes with permethylated β-cyclodextrin: Crystal structures and mechanisms of chiral recognition. Tetrahedron Asymmetry 2003, 14, 2143–2152. [Google Scholar]
- Amharar, Y.; Grandeury, A.; Sanselme, M.; Petit, S.; Coquerel, G. Crystallization and structural investigation of supramolecular compounds with modified cyclodextrins. Application to chiral discrimination. Ann. Pharm. Fr. 2010, 68, 212–217. [Google Scholar] [CrossRef]
- Grandeury, A.; Renou, L.; Dufour, F.; Petit, S.; Gouhier, G.; Coquerel, G. Chiral resolution by crystallization of host-guest supramolecular complexes: A paradoxal situation with an efficient discrimination despite structural similarities. J. Therm. Anal. Calorim. 2004, 77, 377–390. [Google Scholar] [CrossRef]
- Caira, M.R.; Griffith, V.J.; Nassimbeni, L.R.; van Oudtshoorn, B. X-ray structure and thermal analysis of a 1:1 complex between (S)-naproxen and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. J. Incl. Phenom. Mol. Recogn. Chem. 1995, 20, 277–290. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Chen, L.Y. Crystal structures of inclusion complexes of β-Cyclodextrin with (S)-(+)- and (R)-(-)-fenoprofen. J. Am. Chem. Soc. 1988, 110, 4379–4391. [Google Scholar] [CrossRef]
- Harata, K. Role of hydrogen bond and spacial fitting in the chiral recognition by cyclodextrins. Crystal structures of hexakis(2,3,6-tri-O-methyl)-α-cyclodextrin inclusion complexes with (R)- and (S)-1-phenylethanol. J. Chem. Soc. Perkin Trans. 2 1990, 5, 799–804. [Google Scholar] [CrossRef]
- Malpezzi, L.; Fronza, G.; Fuganti, C.; Mele, A.; Bruckner, S. Crystal architecture and conformational properties of the inclusion complex, neohesperidin dihydrochalcone-cyclomaltoheptaose (β-cyclodextrin), by X-ray diffraction. Carbohydr. Res. 2004, 339, 2117–2125. [Google Scholar] [CrossRef]
- Steiner, T.; Saenger, W. Relief of steric strain by intramolecular C-H···O interactions: Structural evidence for the 1,4-disubstituted cyclohexanes. J. Chem. Soc. Perkin Trans. 2 1998, 2, 371–378. [Google Scholar] [CrossRef]
- Kokkinou, A.; Tsorteki, F.; Karpusas, M.; Papakyriakou, A.; Bethanis, K.; Mentzafos, D. Study of the inclusion of the (R)- and (S)-camphor enantiomers in α-cyclodextrin by X-ray crystallography and molecular dynamics. Carbohydr. Res. 2010, 345, 1034–1040. [Google Scholar] [CrossRef]
- Doriguetto, A.C.; Ellena, J.; Santos, M.H.; Moreira, M.E.C.; Nagem, T.J. Mammeigin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, o350–o352. [Google Scholar] [CrossRef]
- Gonzalez, J.C.; Lobo-Antunes, J.; Perez-Lourido, P.; Santana, L.; Uriarte, E. Synthesis of angular pyrrolocoumarins. Synthesis 2002, 4, 475–478. [Google Scholar]
- Saenger, W.; Atwood, J.L.; Davies, J.E.D.; MacNicol, D.D. Inclusion Compounds; Academic Press: London, UK, 1984; Volume 2, pp. 231–259. [Google Scholar]
- Mentzafos, D.; Mavridis, M.; LeBas, G.; Tsoucaris, G. Structure of the 4-tert-butylbenzyl alcohol-[beta]-cyclodextrin complex. Common features in the geometry of [beta]-cyclodextrin dimeric complexes. Acta Crystallogr. Sect. B 1991, 47, 746–757. [Google Scholar] [CrossRef]
- Brett, T.J.; Alexander, J.M.; Stezowski, J.J. Chemical insight from crystallographic disorder-structural studies of supramolecular photochemical systems. Part 2. The β-cyclodextrin-4,7-dimethylcoumarin inclusion complex: A new β-cyclodextrin dimer packing type, unanticipated photoproduct formation, and an examination of guest influence on β-CD dimer packing. Chem. Soc. Perkin Trans. 2 2000, 6, 1095–1103. [Google Scholar]
- CrysAlisPro, version 1.171.33.55, Oxford Diffraction Ltd.: Oxfordshire, UK, 2010.
- Sheldrich, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Zhao, Y.L.; Benitez, D.; Yoon, I.; Stoddart, J.F. Inclusion behavior of β-cyclodextrin with bipyridine molecules: Factors governing host-guest inclusion geometries. Chem. Asian J. 2009, 4, 446–456. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).