The Molecular Structure of 1,2:5,6-Di-O-isopropylidene-3-O-toluenesulfonyl-α-D-glucofuranose
Abstract
:1. Introduction
2. Results and Discussion
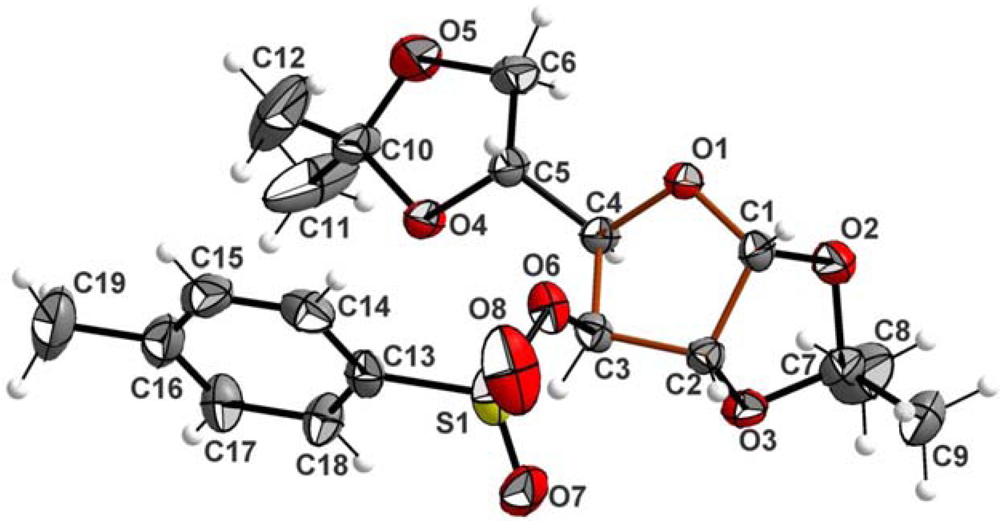
| Crystal data | Refinement | ||
|---|---|---|---|
| Formula | C19H26O8S | Refinement method | Full-matrix least- |
| Formula weight | 414.48 g·mol−1 | squares on F2 | |
| Temperature | 173(2) K | Data/restraints/parameters | 6469/0/254 |
| Wavelength | 0.71073 Å | Measured reflections | 18550 |
| Crystal system | orthorhombic | Goodness-of-fit on F2 | 1.032 |
| Space group | P212121 | Final R indices | R1 = 0.0509 |
| Unit cell dimensions | a = 9.7945(7) Å | [I > 2σ(I)] | wR2 = 0.1238 |
| b = 10.1945(7) Å | R indices (all data) | R1 = 0.0660 | |
| c = 21.306(1) Å | wR2 = 0.1357 | ||
| Volume | 2127.4(2) Å3 | Largest diff. peak/hole | 0.417/−0.375 eÅ−3 |
| Z | 4 | ||
| Density (calcd.) | 1.294 g·cm−3 | ||
| Absorption coefficient | 0.193 mm−1 | ||
| F(000) | 880 | ||
| Crystal size | 0.70 × 0.22 × 0.11 mm3 | ||
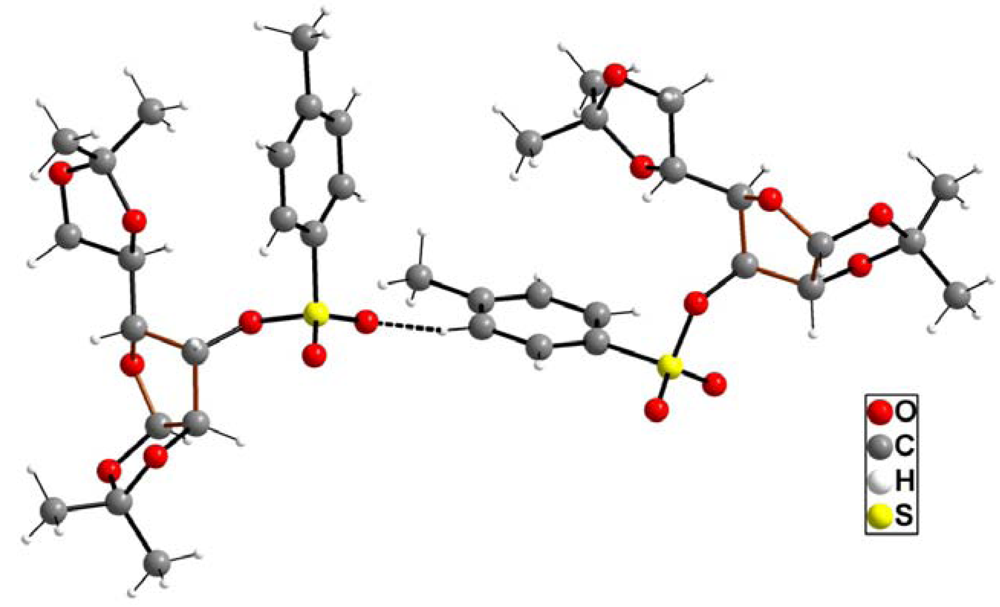
| atoms | distance | atoms | Distance |
|---|---|---|---|
| O1–C1 | 1.439(2) | S1–O7 | 1.431(2) |
| O1–C4 | 1.407(2) | S1–O8 | 1.418(2) |
| C1–C2 | 1.518(2) | S1–C1 | 1.748(2) |
| C2–C3 | 1.526(2) | C5–C6 | 1.508(3) |
| C3–C4 | 1.538(2) | C5–O2 | 1.420(2) |
| C1–C5 | 1.506(3) | C6–O3 | 1.394(3) |
| C2–O4 | 1.451(2) | C7–O2 | 1.437(2) |
| C3–O5 | 1.415(2) | C7–O3 | 1.406(3) |
| C4–O6 | 1.406(2) | C17–O5 | 1.427(3) |
| S1–O4 | 1.588(2) | C17–O6 | 1.431(3) |
3. Experimental Section
3.1. General
3.2. Synthesis of Compound 3
3.3. Data Collection and Refinement
4. Conclusions
References and Notes
- Lindhorst, T.K. Essentials of Carbohydrate Chemistry and Biochemistry, 2nd ed; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Einhorn, C.; Luche, J.-L. Ready preparation of sugar acetals under ultrasonic irradiation. Carbohydrate Res. 1986, 155, 258–261. [Google Scholar] [CrossRef]
- Freudenberg, K.; Ivers, O. Synthesen gemischt-acylierter Halogen-zucker. Ber. Deut. Chem. Gesellsch. (A and B Series) 1922, 55, 929–941. [Google Scholar] [CrossRef]
- Boons, G.-J.; Hale, K. Organic Synthesis with Carbohydrates, 1st ed; Sheffield Academic Press: Sheffield, UK, 2000. [Google Scholar]
- Horton, D.; Roski, J.P.; Norris, P. Cycloaddition of Cyclopentadiene to 3-Deoxy-1,2:5,6-di-O-isopropylidene-α-d-erythro-hex-3-enofuranose. Synthesis and Representative Chemistry of 1,6-Anhydro-2,3-dideoxy-β-d-glycero-hex-2-enopyran-4-ulose (“Isolevoglucosenone”). J. Org. Chem. 1996, 61, 3783–3793. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS/L-97, Programs for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mamat, C.; Peppel, T.; Köckerling, M. The Molecular Structure of 1,2:5,6-Di-O-isopropylidene-3-O-toluenesulfonyl-α-D-glucofuranose. Crystals 2012, 2, 105-109. https://doi.org/10.3390/cryst2010105
Mamat C, Peppel T, Köckerling M. The Molecular Structure of 1,2:5,6-Di-O-isopropylidene-3-O-toluenesulfonyl-α-D-glucofuranose. Crystals. 2012; 2(1):105-109. https://doi.org/10.3390/cryst2010105
Chicago/Turabian StyleMamat, Constantin, Tim Peppel, and Martin Köckerling. 2012. "The Molecular Structure of 1,2:5,6-Di-O-isopropylidene-3-O-toluenesulfonyl-α-D-glucofuranose" Crystals 2, no. 1: 105-109. https://doi.org/10.3390/cryst2010105
APA StyleMamat, C., Peppel, T., & Köckerling, M. (2012). The Molecular Structure of 1,2:5,6-Di-O-isopropylidene-3-O-toluenesulfonyl-α-D-glucofuranose. Crystals, 2(1), 105-109. https://doi.org/10.3390/cryst2010105







