1. Introduction
Titanium alloys have garnered widespread application in the aerospace, aviation, and marine engineering sectors due to their outstanding properties, including high specific strength, excellent ductility, and superior corrosion resistance [
1,
2]. As the application of titanium alloys in these high-performance fields continues, research into their behavior and performance has deepened correspondingly. In particular, the use of titanium alloys in aircraft engines has prompted extensive investigations into their high-temperature performance to meet the growing demands for enhanced operational efficiency.
Among titanium alloys, TA1 and Ti-6Al-4V (TC4) are widely adopted in aerospace applications due to their favorable mechanical and chemical properties compared to conventional structural materials [
1]. TA1, a commercially pure titanium, is characterized by its high strength-to-weight ratio, exceptional corrosion resistance, and thermal stability, making it well-suited for lightweight components operating under elevated temperatures. TC4, one of the most extensively used titanium alloys, offers excellent corrosion resistance in both oxidative and reductive environments, and is commonly employed in spacecraft structures and other critical aerospace components. However, during high-speed atmospheric flight, aerospace vehicles are subjected to extreme temperatures, which can induce high-temperature oxidation reactions that compromise the material’s structural integrity.
High-temperature oxidation refers to the chemical reaction between metals and oxygen in elevated-temperature environments, resulting in the formation of oxide layers. This phenomenon commonly occurs in both industrial and natural high-temperature settings, such as aircraft engines, gas turbines, and industrial furnaces. High-temperature oxidation can markedly degrade the mechanical properties of metals, including strength, hardness, and surface finish, thereby compromising their performance in demanding service conditions [
3]. Given the extensive use of titanium alloys in aerospace applications, a comprehensive understanding of their microstructural evolution under high-temperature oxidation is essential [
4]. In addition, heat treatment plays a critical role in tailoring and optimizing the mechanical performance of titanium alloy sheets. The microstructure of titanium alloys undergoes significant transformation depending on the heat treatment temperature, which directly influences their mechanical behavior. Previous studies on various titanium alloy bars have demonstrated that variations in solution and aging treatment temperatures can significantly affect microstructural characteristics, room-temperature tensile strength, and fracture toughness.
Casadebaigt et al. [
5] investigated the high-temperature oxidation behavior of TC4 titanium alloy subjected to laser and electron beam melting. Their findings indicated that the resulting oxide layer predominantly consisted of rutile-phase TiO
2, with Al
2O
3 (alumina) forming during extended oxidation durations. Furthermore, a high density of pores was observed within the oxide layer and at the metal–oxide interface, with porosity increasing as oxidation progressed. Sun et al. [
6] studied the oxidation behavior of Ti65 titanium alloy at elevated temperatures (650 °C, 750 °C, and 850 °C) and found that the formation of multilayered oxide films enhanced protection of the underlying matrix. The oxidation resistance was shown to improve with an activation energy of 282 ± 1.4 kJ·mol
−1. Optasanu et al. [
7] examined the correlation between high-temperature oxidation and nitrogen uptake in a near-α titanium alloy. Their results revealed a strong linear relationship between total mass gain (measured via gravimetry) and nitrogen mass gain (measured via nuclear reaction analysis, NRA), suggesting that reduced oxidation is accompanied by increased nitrogen involvement in the reaction process. Zhong et al. [
8] investigated the oxidation behavior of TA15 aerospace titanium alloy at various temperatures. Microstructural analysis showed that minimal surface oxidation occurred at 500 °C, resulting in a shallow heat-affected zone. However, at 800 °C, repeated oxidation–spallation cycles led to significant grain growth and a marked reduction in the β-phase fraction.
Although numerous studies have examined the high-temperature behavior of various titanium alloys, limited research has focused specifically on the microstructural evolution and oxidation performance of TA1 and TC4 alloys under elevated temperatures [
9]. To address this gap, the present study investigates the high-temperature oxidation behavior of commercially pure titanium (TA1) and Ti-6Al-4V (TC4) alloys—both widely used in industrial and aerospace applications. The alloys were subjected to oxidation treatments at 450 °C and 750 °C for 50 h. The resulting oxide layers were analyzed to assess oxidation behavior, while the microstructural evolution and elemental distribution across the oxidation gradient were characterized. Detailed compositional analysis was also performed to examine both the oxide layers and the underlying substrate.
3. Results
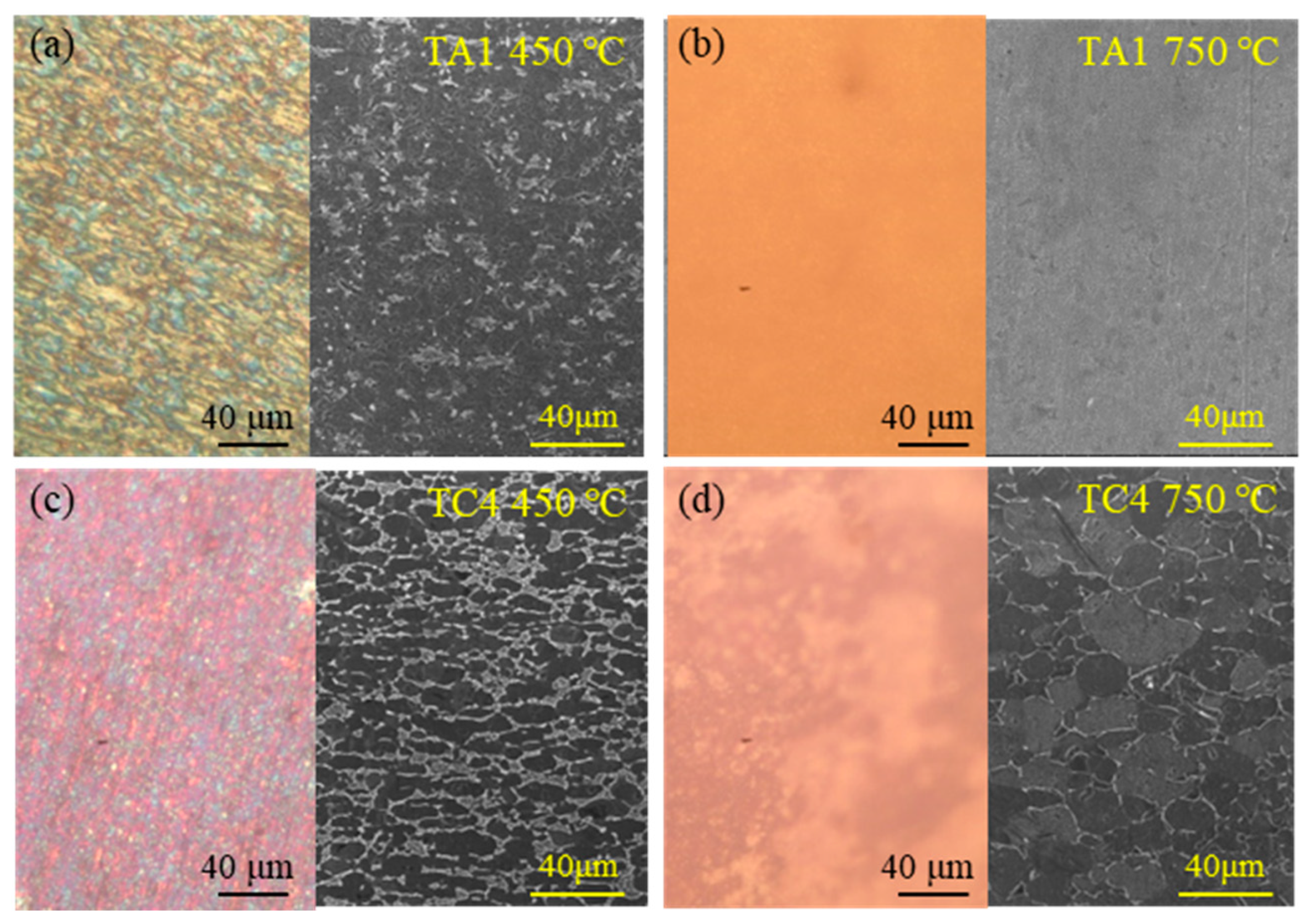
The macroscopic surface morphology of TA1 and TC4 titanium alloys after 50 h of high-temperature oxidation was examined using optical microscopy, as presented in
Figure 2. Following oxidation at 450 °C, the surface of the TA1 alloy developed an oxide film characterized by a mottled yellow–gray and blue–gray appearance. In contrast, the TC4 alloy exhibited a grayish-blue to purplish-blue oxide film under the same conditions. After 50 h of exposure at 750 °C, the oxide layer on the TA1 surface became porous and prone to spallation, presenting a brown coloration. Meanwhile, the TC4 alloy formed a flaky, yellowish-brown oxide film, indicative of more extensive oxidation at elevated temperatures.
According to literature reports, surface oxidation at 450 °C primarily results from the rapid formation of a thin, protective oxide layer when titanium is exposed to an oxygen-rich, high-temperature environment. This initial oxide film acts as a barrier, effectively limiting further oxygen diffusion and suppressing continued oxidation of the underlying material. In contrast, at 750 °C, the elevated temperature promotes the growth of a thicker but more brittle oxide layer, which is prone to spallation due to thermal stresses and oxidation-induced expansion.
Figure 3 presents the microstructural morphology of the oxidized samples after 50 h of high-temperature exposure, captured using optical microscopy and scanning electron microscopy (SEM). As shown in
Figure 3a, the oxide layer formed on TA1 at 450 °C consists primarily of yellow, gray, and blue hues, with a relatively loose structure. In contrast,
Figure 3b reveals that at 750 °C, TA1 develops a highly porous oxide layer. Additionally, after oxidation at 450 °C, the TA1 surface exhibits numerous fine particles, whereas at 750 °C, the microstructure transitions to include some elongated or strip-like features. For TC4, as shown in
Figure 3c, the oxide layer formed at 450 °C appears denser, with dominant gray-blue and purple-blue coloration. At 750 °C (
Figure 3d), TC4 develops a compact yellowish-brown oxide film. Notably, a significant increase in grain size is observed in the TC4 alloy after 50 h of exposure at 750 °C, compared to that at 450 °C.
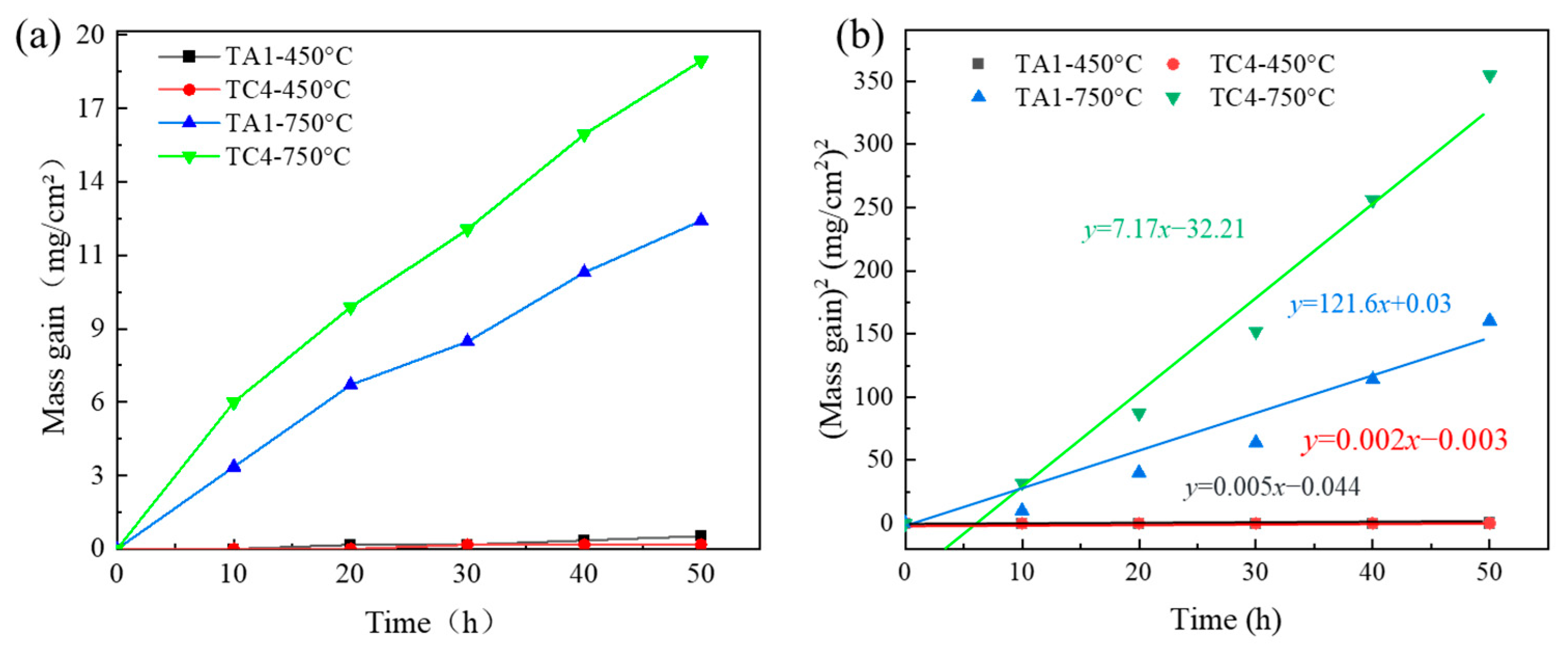
Figure 4a illustrates the corrosion kinetics curves of TA1 and TC4 alloys after 50 h of thermal exposure at 450 °C and 750 °C. At 450 °C, both alloys exhibit negligible mass gain, indicating that oxidation primarily affects the surface with minimal penetration into the bulk material. In contrast, at 750 °C, the mass gain increases significantly over time for both alloys, reflecting the progression of oxidation and the partial detachment of the oxide layer. This detachment exposes the underlying material to the oxidizing environment, promoting further internal oxidation and material degradation. The relatively steady increase in the curve suggests that the oxidation eventually approaches a quasi-stable state under prolonged exposure.
When comparing the two alloys, it is evident that at 750 °C, TC4 exhibits a much higher mass gain than TA1. Although titanium inherently has a strong affinity for oxygen—implying that TA1, as a commercially pure titanium alloy, should oxidize more readily—the opposite trend is observed. This anomaly may be attributed to the brittle nature of the oxide film formed on TC4 at elevated temperatures, which is more susceptible to spallation. Such spallation exposes fresh surfaces to the environment, thereby accelerating oxidation and mass gain.
From the standpoint of thin-film interference theory, although the mass gain data show only a weak direct relationship with optical interference effects, the oxidation behavior can still be interpreted in conjunction with oxide film growth. At 450 °C, the limited diffusion of metal and oxygen atoms results in the formation of a dense and protective oxide film, thus suppressing further oxidation in both TA1 and TC4. At 750 °C, however, TA1 displays a relatively slow but linear increase in mass due to the stability of its TiO2-based oxide film. In contrast, TC4 shows a significantly steeper mass gain curve, suggesting that its alloying elements influence the oxide film’s structure and stability—possibly introducing defects that enhance oxygen permeability. Overall, these results demonstrate that both alloy composition and oxidation temperature significantly influence the oxidation kinetics, consistent with the Arrhenius behavior of thermally activated processes.
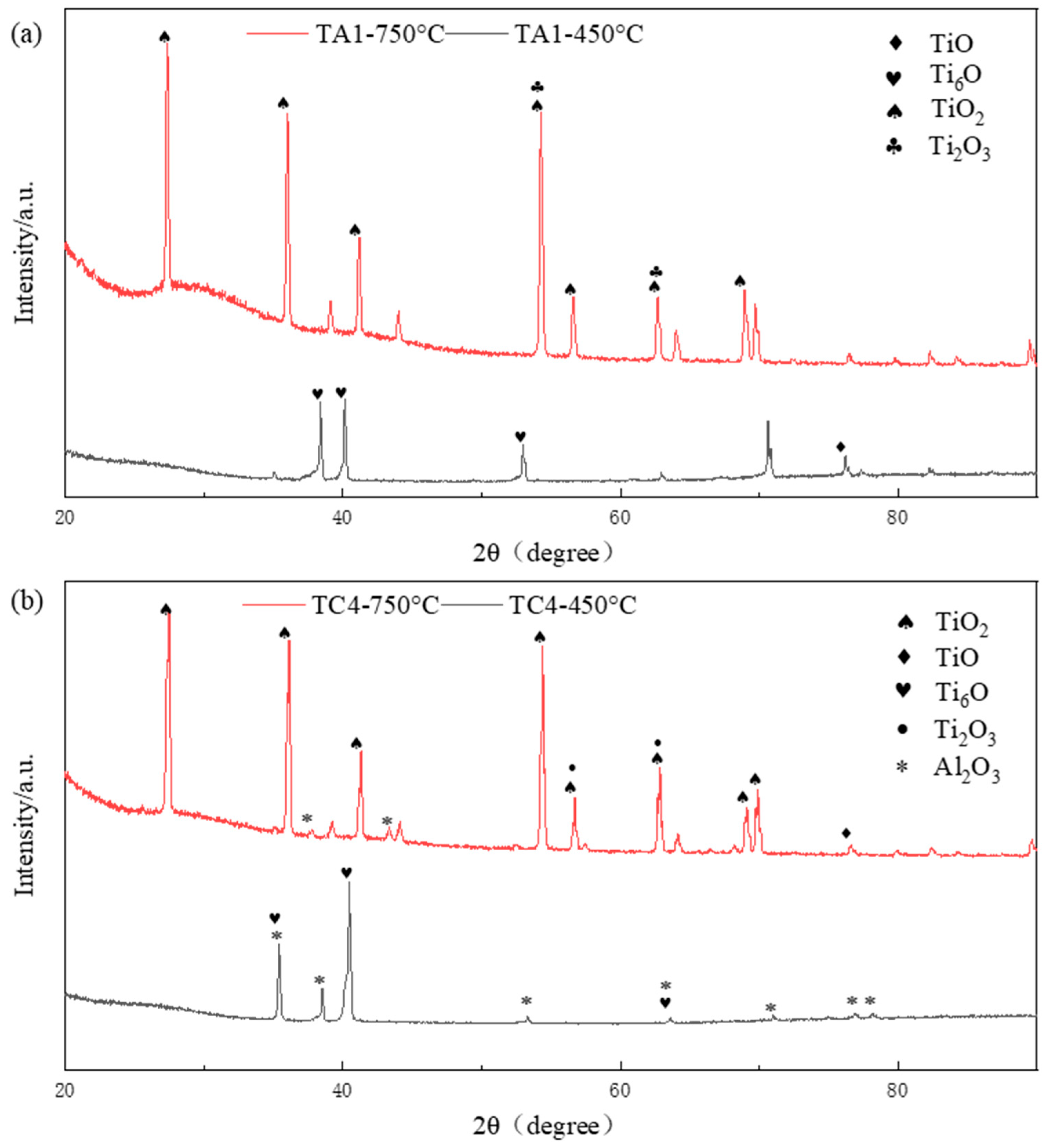
Figure 5 presents the X-ray diffraction (XRD) patterns of the oxide layers formed on TA1 and TC4 alloys after 50 h of oxidation at 450 °C and 750 °C, with a scanning rate of 5°/min. Phase identification reveals that the oxide layer on TA1 at 450 °C is primarily composed of Ti
6O. As the oxidation temperature increases to 750 °C, the dominant phase shifts to TiO
2, indicating a transformation toward more thermodynamically stable oxides. For TC4, the oxide layer formed at 450 °C consists mainly of Ti
6O and Al
2O
3. However, at 750 °C, Al
2O
3 is barely detectable, and TiO
2 becomes the predominant phase. The reduced presence of Al
2O
3 at higher temperatures may be attributed to the significantly increased oxidation rate at 750 °C, which favors the rapid formation of TiO
2 over the slower-forming alumina. In contrast, at 450 °C, the slower oxidation kinetics allow sufficient time for the formation and stabilization of dense Al
2O
3 on the alloy surface. Additionally, both TA1 and TC4 show traces of TiO and Ti
2O
3 at 750 °C. Nonetheless, due to the higher formation rate and thermodynamic stability of TiO
2 at elevated temperatures, it dominates the oxide surface and likely inhibits the further development of less stable oxides such as TiO, Ti
2O
3, and Al
2O
3.
To precisely identify the chemical compounds formed on the surfaces of TA1 and TC4 alloys after oxidation at two different temperatures, X-ray photoelectron spectroscopy (XPS) was performed.
Figure 6 presents the XPS results, focusing on the oxidation states of Ti and O in TA1, and Ti, O, and Al in TC4 [
10]. Due to unavoidable exposure of the samples to ambient air, the C 1s peak at 284.8 eV was used for binding energy calibration. For TA1 oxidized at 750 °C, the Ti
4+ and Ti
3+ states were detected at binding energies of 457.0 eV and 463.6 eV, respectively, corresponding to the formation of TiO
2 (Ti
4+) and Ti
2O
3 (Ti
3+). Among these, TiO
2 was the predominant oxidation product, as indicated by the significantly higher concentration of Ti
4+ relative to Ti
3+. Additionally, the O 1s peaks were observed at 530.3 eV and 531.3 eV, which are characteristic of TiO
2 and Ti
2O
3, respectively, confirming that oxygen on the oxidized surface primarily exists in the form of titanium oxides.
For the TC4 alloy oxidized at 750 °C, the binding energies of Ti
4+ and Ti
3+ were measured at 456.5 eV and 462.3 eV, respectively. Similarly to TA1, the primary oxidation products were identified as TiO
2 and Ti
2O
3 [
11]. In addition to titanium oxides, the presence of Al
3+ was also detected, originating from the oxidation of aluminum in the TC4 alloy to form Al
2O
3. The Al
3+ peaks were observed at binding energies of 68.3 eV and 72.5 eV, consistent with the formation of alumina [
12]. These findings indicate that at 750 °C, both TA1 and TC4 primarily form TiO
2 and Ti
2O
3 on their surfaces, with TiO
2 being the dominant oxide species. However, due to the presence of aluminum in TC4, Al
2O
3 is additionally formed, distinguishing its oxidation behavior from that of commercially pure TA1.
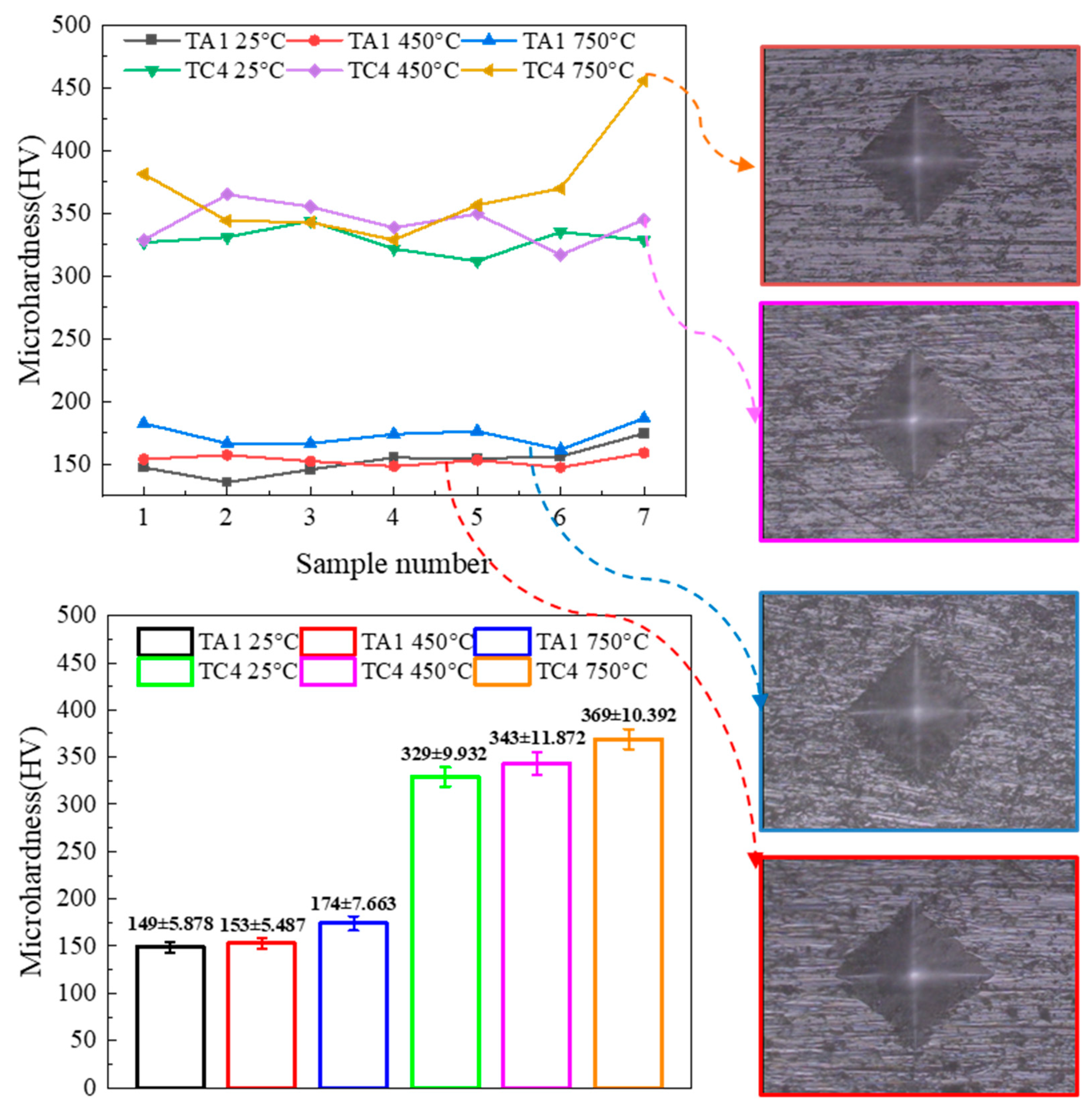
Figure 7 shows the microhardness profiles of TA1 and TC4 after 50 h of high-temperature oxidation at 450 °C and 750 °C. Hardness measurements were taken from the center of each sample toward the right edge, with indentation intervals of approximately 1 mm. Both TA1 and TC4 samples were tested under a load of 300 N. Although the indentation sizes were similar, the results clearly show that TC4 exhibits higher microhardness than TA1 after oxidation, indicating greater resistance to deformation under load.
A more detailed analysis of the microhardness profiles reveals that, overall, the hardness variation across each sample was relatively uniform. However, regions closer to the oxidized surface exhibited slightly elevated hardness values, likely due to the higher concentration of oxidation products in these areas [
13]. After 50 h of oxidation at 450 °C, the TA1 alloy showed a microhardness of approximately 174 ± 7.66 HV, representing a 13.27% increase compared to its baseline hardness at the same temperature (153 ± 5.49 HV). For the TC4 alloy, the microhardness increased from 343 ± 11.87 HV after oxidation at 450 °C to 369 ± 10.39 HV after oxidation at 750 °C, indicating a significant enhancement in surface hardness. This increase in hardness at elevated temperatures is primarily attributed to the formation of thicker and more pronounced oxide layers. Although the oxide scale formed at 750 °C is more prone to spallation, the near-surface region retains a higher oxide content compared to that formed at 450 °C. Given that oxides such as TiO
2 and Al
2O
3 possess inherently high hardness, their accumulation near the surface substantially contributes to the observed increase in microhardness.
Figure 8 presents the microstructural features of TA1 and TC4 alloys after 50 h of thermal exposure at 450 °C and 750 °C, observed both near the surface and deeper into the substrate. For TA1 oxidized at 450 °C, the microstructure predominantly comprises a small quantity of primary α-phase and fine secondary α laths. Due to relatively slow air cooling, α-phase nucleation initiates at grain boundaries and subsequently grows inward along the β-phase boundaries, forming elongated and aligned α structures, commonly referred to as “α colonies.” At 750 °C, although the temperature approaches the α-to-β transformation point (approximately 882.5 °C), the transformation is not fully activated. Consequently, the microstructure remains primarily composed of equiaxed α-phase with a small fraction of intergranular β-phase.
At 450 °C, the TC4 alloy exhibits a predominantly equiaxed microstructure composed mainly of primary α and β phases [
14]. Within the β-phase regions, a considerable amount of secondary α-phase appears. This is attributed to the heating within the α + β phase region, where the β phase decomposes into α phase during cooling. As the oxidation temperature increases to 750 °C and approaches the α → β transformation temperature, the final microstructure displays a reduced residual heat-affected zone compared to that at 450 °C, which allows for more significant grain growth [
13]. Internally, the microstructure evolves from a fine-grained, fragmented state to a coarser structure, characterized by a substantial decrease in β-phase content and the development of larger α grains. Meanwhile, the secondary α phase disappears. This phenomenon is linked to the behavior of the secondary α during air cooling. The secondary α tends to nucleate epitaxially at the interface between primary α and β phases (via the β → α + β transformation), especially at regions enriched with α-stabilizing elements such as Al. Because the α/β phase boundaries are incoherent, they facilitate the nucleation of secondary α at the edges of the primary α-phase. Once nucleated, the α phase grows rapidly along the <11–20> crystallographic direction, forming slender secondary α laths (commonly referred to as α
2 or secondary α
1). As these laths impinge upon one another, longitudinal growth is halted. Subsequent lateral growth proceeds more slowly, eventually forming a crab-like morphology characteristic of coarsened primary α grains.
Figure 9 presents the results of point-by-point elemental analysis conducted on the surface of the TA1 alloy after 50 h of thermal oxidation at 450 °C and 750 °C. In both cases, the oxygen concentration was found to be highest near the oxidized surface. Specifically, for TA1 oxidized at 450 °C, the oxygen content near the surface reached approximately 19.28%, while at 750 °C, it increased to around 21.30%. This comparison indicates that higher oxidation temperatures result in slightly greater oxygen enrichment at the surface. Moreover, a clear gradient is observed in oxygen content: as the measurement points move deeper into the material—away from the oxidized surface—the oxygen concentration decreases. This trend confirms that oxygen diffusion is more pronounced at elevated temperatures and that oxidation primarily occurs near the surface, with limited penetration into the interior. A similar trend is observed for carbon (C) content, which also increases both with rising temperature and proximity to the oxidized surface. This suggests that, alongside oxygen, carbon may also accumulate near the surface during high-temperature exposure, potentially due to atmospheric contamination or decomposition of organic residues during heating.
Figure 10 presents the results of point-by-point elemental analysis conducted on the surface of the TC4 alloy after 50 h of thermal oxidation at 450 °C and 750 °C. Similarly to the observations for TA1, the oxidation of TC4 is more pronounced near the oxidized surface, and the degree of oxidation intensifies with increasing temperature. However, a notable difference is observed when comparing elemental distribution across the α and β phases. At both 450 °C and 750 °C, the oxygen content within the β phase is consistently higher than that in the α phase. This suggests that the β phase is more susceptible to oxygen diffusion and oxidation under high-temperature conditions. The greater affinity of the β phase for oxygen may be attributed to differences in crystal structure or the localized distribution of alloying elements such as vanadium, which may influence oxidation behavior. These findings indicate that, within the TC4 microstructure, the β phase plays a more active role in oxidation processes, especially at elevated temperatures.
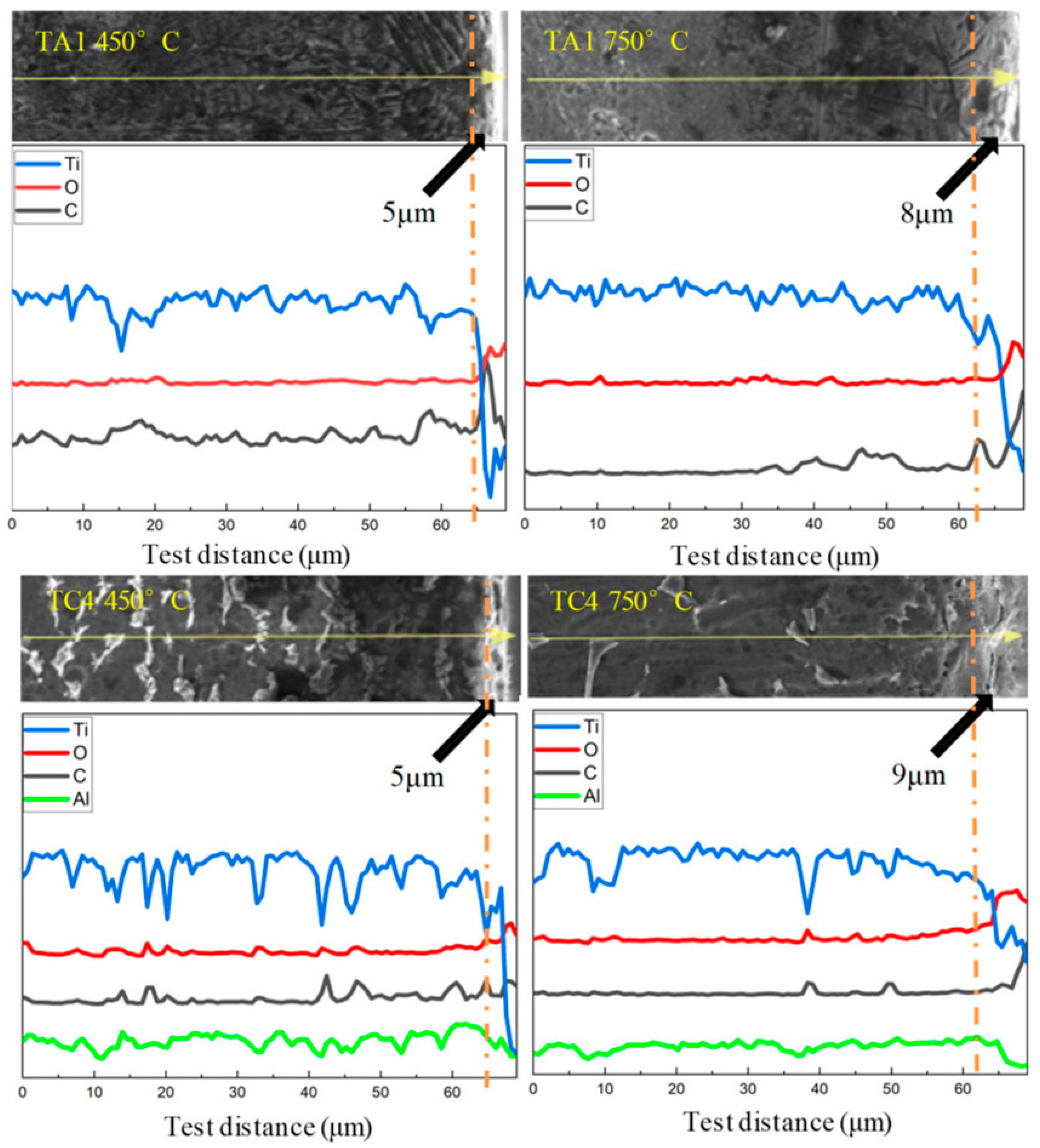
The distinct contrast between the oxidized layer and the underlying substrate observed under high magnification, along with the point-by-point EDS analysis showing a gradual increase in oxygen content toward the surface, suggests that elemental concentration gradients can be effectively used to estimate oxide layer thickness. As illustrated in
Figure 11, the oxide layer on TA1 reaches approximately 5 μm in thickness after 50 h of oxidation at 450 °C. When the oxidation temperature is increased to 750 °C, the oxide layer grows to about 8 μm. This relatively moderate increase in thickness may be attributed to the formation of a dense and adherent oxide film at 750 °C, which acts as a diffusion barrier and limits further oxygen ingress. The compact nature of the oxide layer suppresses rapid oxidation kinetics, thereby slowing down the rate of oxide layer growth despite the elevated temperature.
For the TC4 alloy, the oxide layer thickness after 50 h of oxidation at 450 °C is also approximately 5 μm. However, the corresponding oxygen content is slightly lower than that observed in TA1 under the same conditions, indicating that TC4 exhibits slightly superior oxidation and high-temperature resistance at this temperature. At 750 °C, the oxide layer thickness on TC4 increases to approximately 9 μm, which is notably greater than the 8 μm observed for TA1 at the same temperature. This suggests that the oxidation process is more severe in TC4 at elevated temperatures. Based on surface morphology observations after 50 h of oxidation at 750 °C, the oxide film on TC4 appears more brittle and prone to spallation. The detachment of the outer oxide layer exposes fresh underlying material to the environment, promoting continued oxidation. This repetitive cycle of oxide formation and spallation contributes to the increased oxide layer thickness and higher oxygen uptake in the alloy.
4. Discussion
4.1. Discussion on Oxidation Color
The formation of oxidation colors in titanium alloys is closely related to the composition and distribution of titanium oxide compounds within the oxide layer, as shown in
Figure 3. During oxidation, titanium reacts with oxygen to form various oxides, including TiO (yellow), TiO
2 (white), and Ti
2O
3 (blue). As the oxidation temperature increases, the relative proportions of these oxides change, thereby altering the overall color of the oxide film. At ambient temperatures, a thin and compact oxide layer forms rapidly on the titanium surface, which serves as a protective barrier, limiting further oxygen diffusion and oxidation. However, at elevated temperatures, oxygen penetrates more deeply into the oxide film and continues to react with the titanium substrate, forming a mixture of oxides such as TiO, TiO
2, Ti
2O
3, and Ti
3O
5. As temperature rises, the oxidation rate accelerates and the oxide layer thickens. Concurrently, oxygen diffuses into the titanium matrix, forming a high-oxygen concentration zone beneath the oxide film.
This oxygen-enriched layer is characterized by increased hardness and significantly reduced plasticity. Studies have shown that numerous microcracks, oriented perpendicular to the surface, tend to develop in this brittle high-oxygen region. During mechanical loading, these microcracks serve as stress concentrators, propagating into the matrix as deformation progresses. Once a critical strain is reached, the cracks coalesce and lead to premature fracture. This mechanism explains the deterioration of mechanical properties in titanium alloys following high-temperature oxidation treatment.
Peng et al. [
15] reported that the surface appearance of titanium alloys varies significantly with oxidation temperature. When the exposure temperature is below 750 °C, the alloy surface remains bright; however, within the temperature range of 750–1000 °C, the surface becomes visibly dull. Specifically, at temperatures below 250 °C, the surface appears silvery-white, while at 250 °C, it shifts to a light yellow hue. As the oxidation temperature increases from 300 °C to 500 °C, the surface darkens progressively, transitioning from light yellow to golden yellow. At around 500 °C, a faint blue coloration may be observed, particularly at the edges and corners. With further heating to 600 °C, the surface color changes to light green. When the oxidation temperature exceeds 750 °C, the oxide layer typically appears brown, which corresponds to the formation of a thicker outer layer dominated by TiO
2 [
16].
4.2. Oxidation Behavior
The differences in oxidation behavior and microstructural evolution of the two titanium alloys under high-temperature conditions are illustrated in
Figure 12. By integrating the XRD results (
Figure 5) with XPS analysis (
Figure 6) of titanium oxidation states, the presence of both Ti
3+ and Ti
4+ species on the oxidized surfaces was confirmed, indicating the formation of Ti
2O
3 and TiO
2 phases. However, due to the predominance of TiO
2, the characteristic peaks associated with TiO are relatively weak, and the peak corresponding to Ti
2O
3—typically associated with a blue coloration—is not clearly observed.
This phenomenon can be attributed to the thermal instability of Ti
2O
3 and TiO at elevated temperatures. Both oxides tend to decompose or further oxidize under high-temperature conditions, which reduces their detectability in surface analyses. The decomposition or oxidation reactions can be described by the following equations [
17,
18]:
As illustrated in
Figure 12, when the oxidation temperature reaches 750 °C, both TA1 and TC4 alloys undergo severe oxidation, resulting in a distinct brown surface coloration. At this elevated temperature, the initially thin and compact oxide layer transforms into a thicker, more porous, and less adherent structure. This change facilitates increased oxygen diffusion through the oxide scale, allowing greater oxygen uptake by the underlying titanium substrate.
The accumulation of oxygen near the surface enhances hardness but significantly reduces toughness. This results in the formation of a hard yet brittle oxygen-enriched layer, which is widely recognized as the primary cause of the observed deterioration in mechanical performance after high-temperature oxidation. Furthermore, thermal stresses arising from the mismatch in the coefficients of thermal expansion between the oxide layer and the base metal can induce localized delamination. As the oxidation progresses, these stresses may lead to partial or complete detachment of the oxide scale, further exposing the substrate to the high-temperature oxidizing environment and accelerating degradation [
19].
Both TA1 and TC4 alloys exhibit negligible mass gain at 450 °C, as this temperature is significantly below the typical service temperature range for these titanium alloys and therefore induces minimal oxidation. In contrast, at 750 °C, the mass gain increases substantially for both alloys. This pronounced increase can be attributed to two primary factors: oxidation-induced growth of the oxide layer and partial detachment of the scale, which exposes fresh surfaces to the oxidizing environment, thereby promoting further internal oxidation and contributing to additional mass gain. Experimental observations suggest that the oxide film on TC4 is more susceptible to spallation than that on TA1. This difference is likely due to the presence of alumina (Al
2O
3) in TC4, which, despite its initial protective nature, may create mechanical instability in the oxide scale under thermal stress. In contrast, the oxide layer formed on TA1 consists primarily of titanium oxides, which tend to be more adherent and stable. Consequently, once the external oxide layer on TC4 delaminates, rapid re-oxidation of the underlying material occurs, accelerating mass gain. The growth of the oxide scale in both alloys is governed by diffusion mechanisms. According to Fick’s first law, the oxidation kinetics follow a parabolic rate law, indicating that the majority of oxide layer formation is controlled by inward diffusion of oxygen through the existing scale. This diffusion-driven process becomes more pronounced at elevated temperatures, further contributing to the observed differences in oxidation behavior between the two alloys [
20].
At relatively low temperatures, the oxidation resistance of the TA1 alloy is primarily attributed to the formation of a dense and adherent oxide layer on its surface. This layer serves as a moderate barrier to oxygen ingress from the surrounding environment, thereby significantly slowing the oxidation process. Within the temperature range of 400 °C to 500 °C, the oxide film on TA1 grows slowly and retains a compact structure, which provides effective protection against further oxidation. In contrast, the TC4 titanium alloy exhibits enhanced oxidation resistance not only due to the formation of a similarly dense oxide layer, but also due to the presence of aluminum in its composition. Aluminum contributes to the formation of alumina (Al2O3), which is chemically stable and further densifies the oxide layer, enhancing its protective capability. Additionally, although vanadium is primarily added to improve the mechanical strength and toughness of TC4, it may also play a role in retarding oxidation reactions at elevated temperatures by altering the oxide layer’s composition and structure.
However, after 50 h of exposure at 750 °C, TA1 and TC4 exhibit significant differences in both oxidation behavior and microstructural evolution. In the case of TA1, although surface oxidation occurs, the oxide film remains largely intact and adherent, with no extensive spallation observed. Nonetheless, numerous surface cracks are evident, indicating the buildup of internal stresses during prolonged oxidation. In addition, the microstructure beneath the surface transforms noticeably—from fine, equiaxed grains to elongated structures—suggesting that oxygen has diffused into the alloy and induced grain rearrangement or preferential orientation growth.
By contrast, TC4 undergoes more severe degradation under the same conditions. Its oxide layer exhibits large-scale delamination, exposing the underlying substrate and initiating a cyclic “spallation–reoxidation” mechanism. This leads to continued oxidation and deeper material penetration. The presence of aluminum and vanadium in TC4 contributes to the formation of their respective oxides, which exhibit a greater mismatch in thermal expansion with the matrix. This mismatch exacerbates instability at the oxide–metal interface and accelerates oxide layer detachment. Internally, TC4 shows pronounced grain coarsening—from fine grains to significantly larger ones—which is known to degrade strength, fatigue life, and creep resistance. The extent of grain growth indicates poor microstructural stability of TC4 under long-term high-temperature service.
In high-temperature environments, the oxidation of titanium alloys not only leads to the formation of surface oxide films but also promotes oxygen diffusion into the substrate, resulting in the so-called “oxidized hardened layer” or “alpha-case.” This layer is typically brittle, with significantly higher hardness than the substrate but extremely poor plasticity and toughness. Under external loading or thermal cycling, the alpha-case is prone to cracking or spallation, thereby serving as an initiation site for fatigue cracks and fracture. Moreover, the distinct mechanical property gradient between the brittle layer and the substrate causes stress concentration at the interface, further accelerating crack propagation. Temperature plays a critical role in this process. At moderate conditions such as 450 °C, oxidation progresses relatively slowly, and the alpha-case layer remains thin, thus its effect on bulk mechanical properties is less pronounced. However, at higher temperatures such as 750 °C, the oxidation rate increases significantly, leading to a much thicker and more brittle alpha-case, which severely deteriorates fracture toughness and fatigue life. In particular, under cyclic loading, the surface embrittlement effect at 750 °C has a much stronger influence on crack initiation life compared to 450 °C. Therefore, the brittle layer induced by high-temperature oxidation not only degrades the overall mechanical performance of titanium alloys but also limits their application in demanding environments such as aerospace. Developing effective approaches, such as protective coatings or alloy design, to suppress the formation of this brittle layer is thus critical to improving the high-temperature service reliability of titanium alloys.
In summary, although TC4 initially demonstrates better oxidation resistance due to the formation of alumina and vanadium oxides, its long-term structural stability under elevated temperatures is inferior to that of TA1. The simpler composition of TA1 contributes to more stable oxide film adhesion and improved microstructural integrity during prolonged thermal exposure. These findings have important implications for the selection of titanium alloys in aerospace and energy applications requiring reliable performance in high-temperature environments.