Interfacial Engineering of Hydrophobic Montmorillonite for High-Energy-Capability Polypropylene Nanocomposite Dielectrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
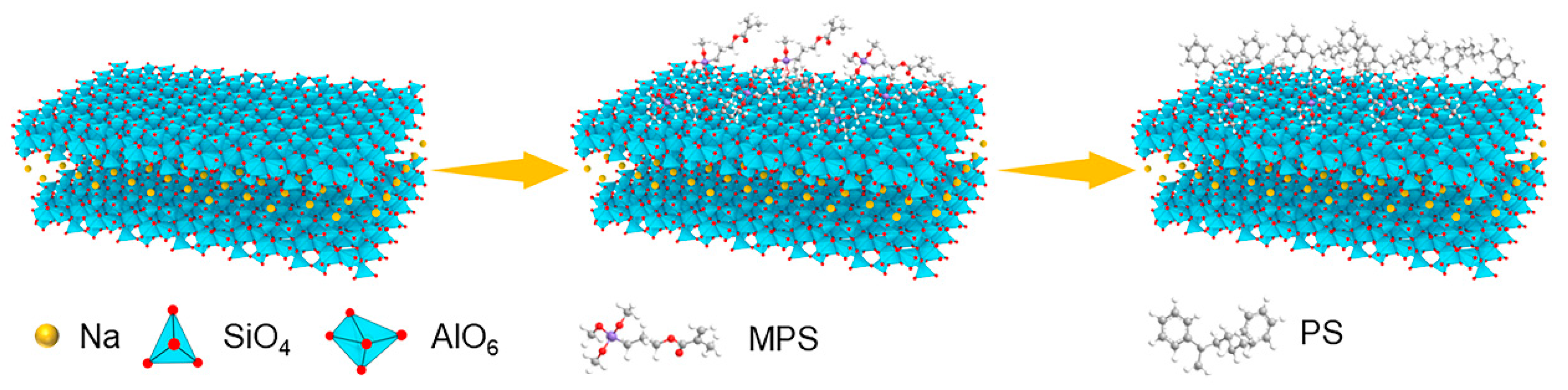
2.2. Synthesis of MPS-Capped MMT (MCM) Nanosheets
2.3. Synthesis of PCM Nanosheets
2.4. Preparation of PP Nanocomposites
2.5. Characterization
2.6. Phase-Field Simulation
3. Results
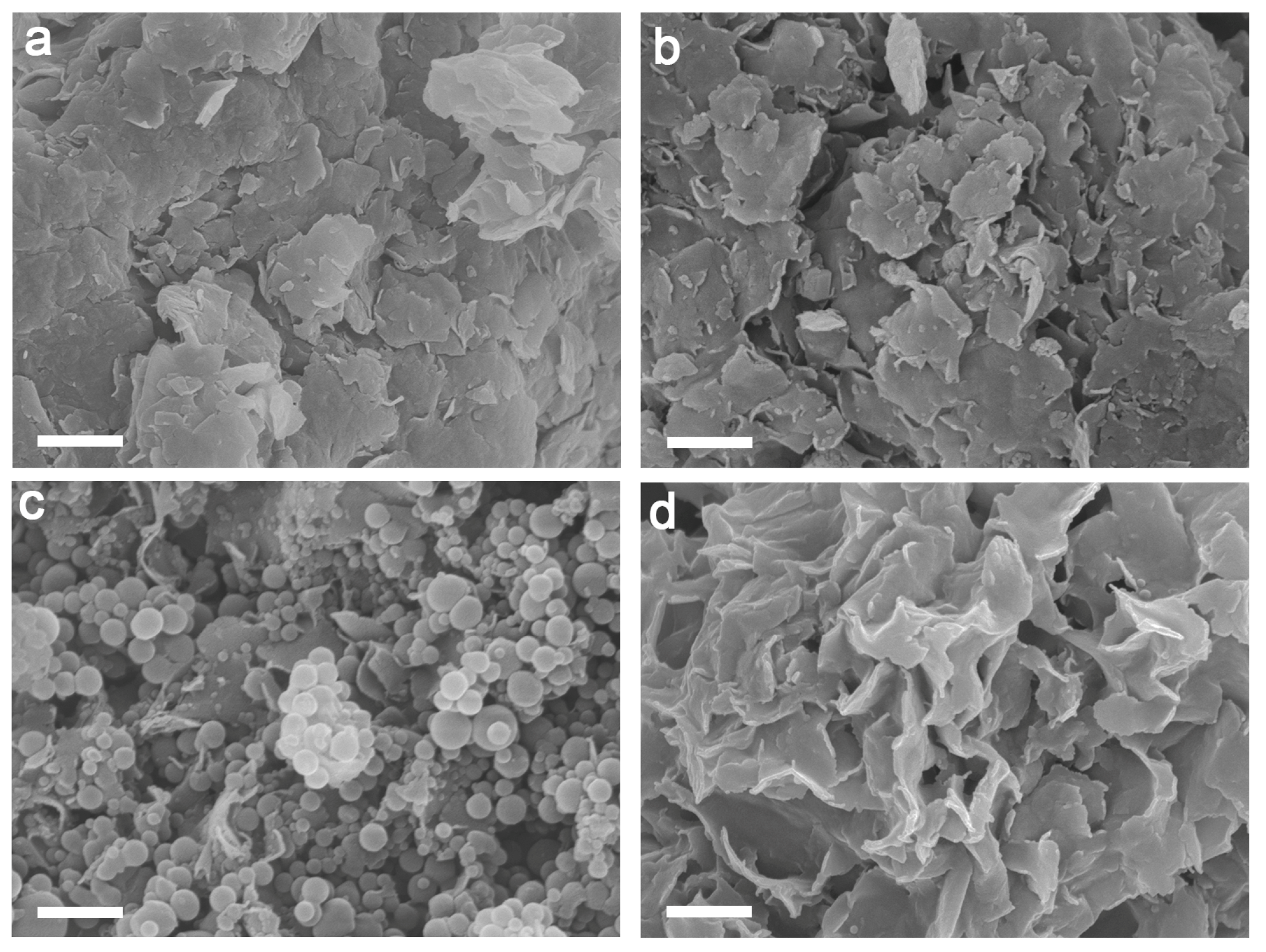
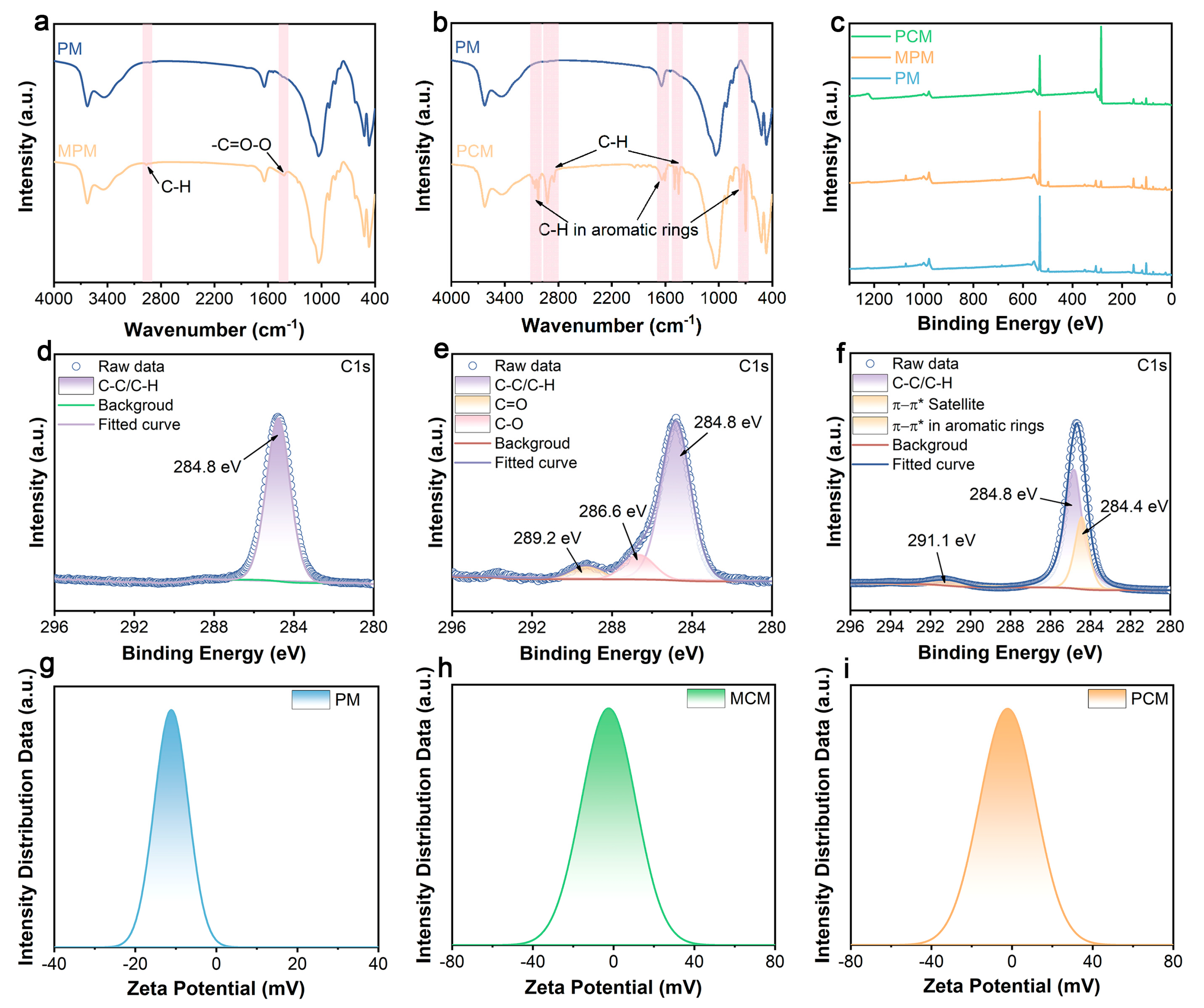
3.1. Characterization of Microstructure and Surface Composition of MMT
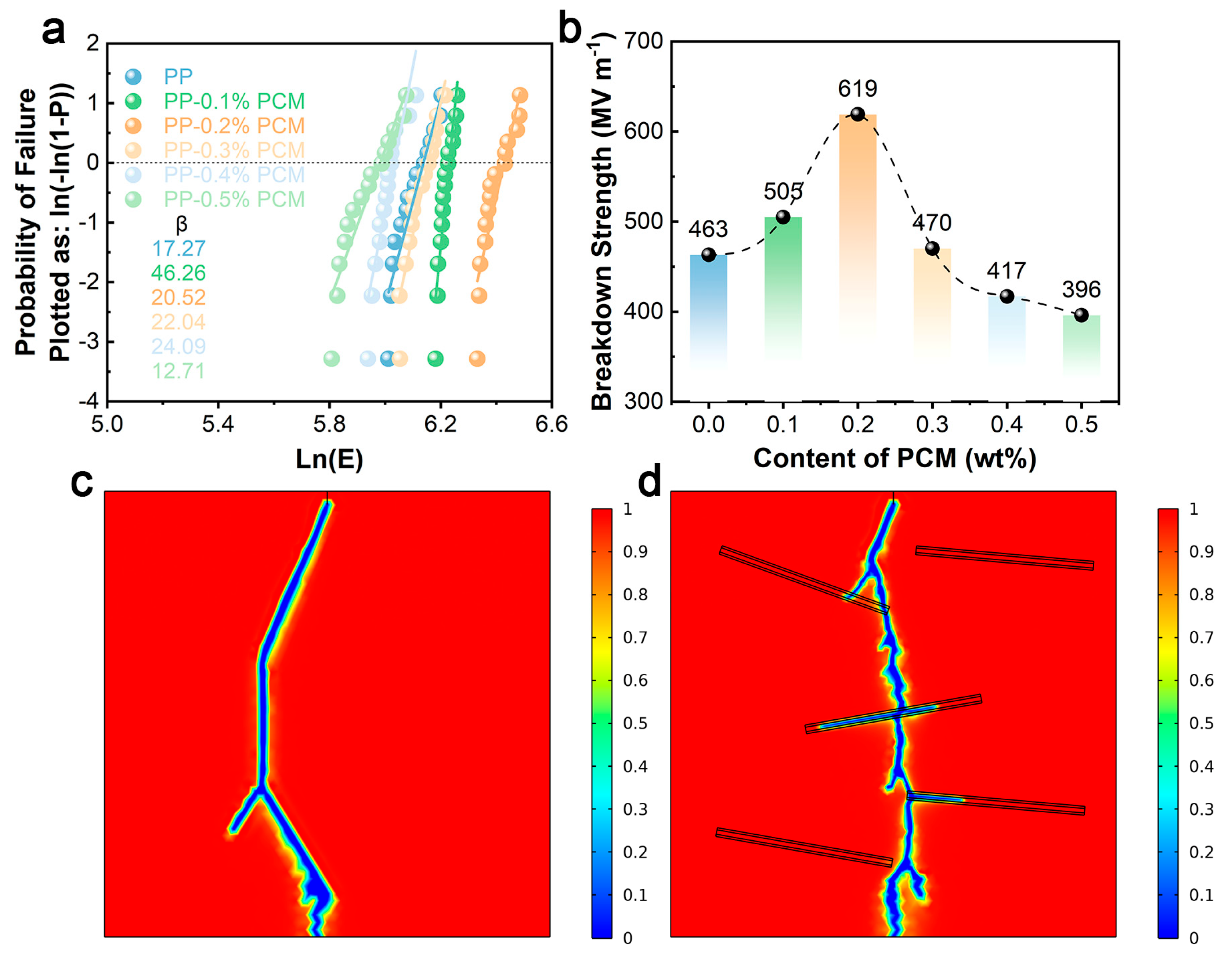
3.2. Dielectric Energy Storage Properties of PP Nanocomposites
4. Discussions
| Sample | εr (1000 Hz) | Eb (MV m−1) | Ue (J cm−3) | Reference |
|---|---|---|---|---|
| BN/BT | 2.92 | 469 | 2.82 | [41] |
| PP-g-MAH/BN/BT | 4.68 | 324 | 2.45 | [42] |
| PP-g-MAH/BN | 2.27 | 437 | 4.11 | [43] |
| MMT | 2.38 | 370 | 1.70 | [44] |
| PP-g-MAH/MMT | 3.35 | 520 | 5.20 | [45] |
| PP-g-MAH/MMT | 3.75 | 530 | 5.21 | [29] |
| PCM | 2.47 | 619 | 4.23 | This work |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, L.; Shi, X.; Zhang, X.; Yang, Z.; Li, W.; Ma, Y.; Liu, Y.; Liu, L.; Cheng, Y.; Wei, L.; et al. Partitioning polar-slush strategy in relaxors leads to large energy-storage capability. Science 2024, 385, 204–209. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, H.U.; Iagodkine, E.; Haque, A.; Chen, L.-Q.; Jackson, T.N.; et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2015, 523, 576–579. [Google Scholar] [CrossRef]
- Zhang, M.; Lan, S.; Yang, B.B.; Pan, H.; Liu, Y.Q.; Zhang, Q.H.; Qi, J.L.; Chen, D.; Su, H.; Yi, D.; et al. Ultrahigh energy storage in high-entropy ceramic capacitors with polymorphic relaxor phase. Science 2024, 384, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, H.; Wang, J.; Shi, W.; Zhang, Q.; Xing, H.; Ren, W.; Sun, B.; Guo, M.; Xu, E.; et al. Roll-to-roll fabricated polymer composites filled with subnanosheets exhibiting high energy density and cyclic stability at 200 °C. Nat. Energy 2024, 9, 143–153. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Luo, B.; Wang, C.; Cai, Z.; Zhu, C.; Zhang, B.; Kang, X.; Zhou, D.; Liu, J.; et al. Nonpolar sub-10 nm TiO2 nanocrystal for high energy density polypropylene nanocomposites. Nano Energy 2024, 121, 109237. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Guo, Y.; Zhao, W.; Guo, T.; Xu, D.; Wang, Z.; He, G.; Zhai, J.; Zhou, Y.; et al. High energy storage density achieved in polymer composites by hierarchical interface engineering design. Chem. Eng. J. 2025, 505, 159343. [Google Scholar] [CrossRef]
- Xie, J.; Liu, H.; Zhao, X.; Sun, S.; Zhang, M. Achieving higher dielectric and energy storage performances of methyl methacrylate-co-glycidyl methacrylate films through the nanocomposite strategy. ACS Appl. Polym. Mater. 2023, 5, 8396–8405. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, H.; Zhang, N.; Zhang, C.; Yin, L.; Dang, Z.M.; Bai, J. Ultrahigh energy storage density in poly(vinylidene fluoride)-based composite dielectrics via constructing the electric potential well. Adv. Energy Mater. 2023, 13, 2203587. [Google Scholar] [CrossRef]
- Wang, F.; Luo, H.; Chen, H.; Zhai, D.; Jiang, X.; Liu, Y.; Zhang, D. Surface-confined winding assembly of SiO2 on the surface of BaTiO3 leading to enhanced performance of dielectric nanocomposites. Adv. Funct. Mater. 2024, 34, 2410862. [Google Scholar] [CrossRef]
- Li, S.; Cai, Z.; Zheng, G.; Cao, C.; Zhu, C.; Zhang, B.; Luo, H.; Feng, P. High-energy-density polypropylene nanocomposite dielectrics incorporating nonpolar TiO2 nanorod. ACS Appl. Energy Mater. 2025, 8, 2516–2522. [Google Scholar] [CrossRef]
- Song, S.; Xia, S.; Liu, Y.; Lv, X.; Sun, S. Effect of Na+ MMT-ionic liquid synergy on electroactive, mechanical, dielectric and energy storage properties of transparent PVDF-based nanocomposites. Chem. Eng. J. 2020, 384, 123365. [Google Scholar] [CrossRef]
- Luo, B.; Shen, Z.; Cai, Z.; Tian, E.; Yao, Y.; Li, B.; Kursumovic, A.; MacManus-Driscoll, J.L.; Li, L.; Chen, L.Q.; et al. Superhierarchical inorganic/organic nanocomposites exhibiting simultaneous ultrahigh dielectric energy density and high efficiency. Adv. Funct. Mater. 2020, 31, 2007994. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Cai, Z.; Zheng, G.; Cao, C.; Zhu, C.; Zhang, B.; Luo, H.; Feng, P. Enhancement of energy storage performance in polymer dielectrics via monodisperse ZrO2 nanoparticles as nanofiller. Small 2025, 21, e2500743. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J.; Xue, J.; Cai, F.; Chen, F.; Fu, Q. Towards suppressing loss tangent: Effect of polydopamine coating layers on dielectric properties of core–shell barium titanate filled polyvinylidene fluoride composites. Compos. Sci. Technol. 2015, 118, 198–206. [Google Scholar] [CrossRef]
- Yu, K.; Niu, Y.; Zhou, Y.; Bai, Y.; Wang, H.; Randall, C. Nanocomposites of surface-modified BaTiO3 nanoparticles filled ferroelectric polymer with enhanced energy density. J. Am. Ceram. Soc. 2013, 96, 2519–2524. [Google Scholar] [CrossRef]
- Seitz, F. On the theory of electron multiplication in crystals. Phys. Rev. 1949, 76, 1376–1393. [Google Scholar] [CrossRef]
- Bao, Z.; Hou, C.; Shen, Z.; Sun, H.; Zhang, G.; Luo, Z.; Dai, Z.; Wang, C.; Chen, X.; Li, L.; et al. Negatively charged nanosheets significantly enhance the energy-storage capability of polymer-based nanocomposites. Adv. Mater. 2020, 32, 1907227. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-C. A Review on conduction mechanisms in dielectric films. Adv. Mater. Sci. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Wang, C.; Ding, Q.; Jiang, J.; Mai, K. A novel montmorillonite with β-nucleating surface for enhancing β-crystallization of isotactic polypropylene. Compos. Part A Appl. Sci. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, H.; Jiang, P.; Wu, J.; Zhu, X.; Huang, X. Two-dimensional high-k nanosheets for dielectric polymer nanocomposites with ultrahigh discharged energy density. J. Phys. Chem. C 2018, 122, 18282–18293. [Google Scholar] [CrossRef]
- Wang, P.; Guo, Y.; Zhou, D.; Li, D.; Pang, L.; Liu, W.; Su, J.; Shi, Z.; Sun, S. High-temperature flexible nanocomposites with ultra-high energy storage density by nanostructured MgO fillers. Adv. Funct. Mater. 2022, 32, 2204155. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Li, J.; Zhong, S.L.; Feng, Y.; Yue, D.; Sheng, D.; Chen, H.; Hao, X.; Dang, Z.M. Atomic-level matching metal-ion organic hybrid interface to enhance energy storage of polymer-based composite dielectrics. Adv. Mater. 2024, 36, 2402239. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B.; Zhu, C.; Cai, Z.; Feng, P. Influences of modified montmorillonite on the high-temperature energy storage performance of polyetherimide dielectric composites. J. Chin. Ceram. Soc. 2025, 53, 1962–1970. [Google Scholar]
- Xie, Z.; Wu, K.; Liu, D.; Zhang, Q.; Fu, Q. One-step alkyl-modification on boron nitride nanosheets for polypropylene nanocomposites with enhanced thermal conductivity and ultra-low dielectric loss. Compos. Sci. Technol. 2021, 208, 108756. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Geier, M.L.; McMorrow, J.J.; Jariwala, D.; Beck, M.E.; Huang, W.; Marks, T.J.; Hersam, M.C. Layer-by-layer assembled 2D montmorillonite dielectrics for solution-processed electronics. Adv. Mater. 2015, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; LaChance, A.M.; Smith, A.T.; Cheng, H.; Liu, Q.; Sun, L. Strategic design of clay-based multifunctional materials: From natural minerals to nanostructured membranes. Adv. Funct. Mater. 2019, 29, 1807611. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, Y.; Feng, Y.; Geng, W.; Liu, Q.; Guo, W.; Jiang, L. Electrokinetic energy conversion in self-assembled 2D nanofluidic channels with janus nanobuilding blocks. Adv. Mater. 2019, 29, 1700177. [Google Scholar] [CrossRef]
- Xie, J.; Liu, H.; Zhao, X.; Hu, J.; Liu, Y.; Wang, Y.; Sun, S.; Song, S. The influence of organic montmorillonite on the breakdown strength and energy density of poly(vinylidene fluoride)-based nanocomposites. J. Appl. Polym. Sci. 2021, 139, 51945. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Xie, J.; Liu, Y.; Sun, S. Influence of organic Na+-MMT on the dielectric and energy storage properties of maleic anhydride-functionalized polypropylene nanocomposites. J. Polym. Res. 2022, 29, 182. [Google Scholar] [CrossRef]
- Liu, D.; Wu, L.; Wu, K.; Xu, S.; Sui, G.; Jing, M.; Zhao, J.; Wei, Y.; Fu, Q. Largely enhanced energy density of polypropylene based nanocomposites via synergistic hybrid fillers and high shear extrusion assisted dispersion. Compos. Part A Appl. Sci. 2019, 119, 134–144. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Q.; Chen, M.; Yang, P.; Xu, Y.; Wu, H.; Yu, J.; He, L.; Zhang, X.-H.; Zhang, Q. Dispersing hydrophilic nanoparticles in nonaqueous solvents with superior long-term stability. RSC Adv. 2017, 7, 25535–25541. [Google Scholar] [CrossRef]
- Rong, Y.; Chen, H.-Z.; Wu, G.; Wang, M. Preparation and characterization of titanium dioxide nanoparticle/polystyrene composites via radical polymerization. Mater. Chem. Phys. 2005, 91, 370–374. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Zhang, T.; Yin, Y. Superparamagnetic composite colloids with anisotropic structures. J. Am. Chem. Soc. 2007, 129, 8974–8975. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, J.; Zhang, K.; Zhang, S.; Lee, Y.; Li, T. Coating the Right Polymer: Achieving ideal metal–organic framework particle dispersibility in polymer matrixes using a coordinative crosslinking surface modification method. Angew. Chem. Int. Ed. 2021, 60, 14138–14145. [Google Scholar] [CrossRef] [PubMed]
- Tokarský, J.; Kulhánková, L.; Stýskala, V.; Mamulová Kutláková, K.; Neuwirthová, L.; Matějka, V.; Čapková, P. High electrical anisotropy in hydrochloric acid doped polyaniline/phyllosilicate nanocomposites: Effect of phyllosilicate matrix, synthesis pathway and pressure. Appl. Clay Sci. 2013, 80–81, 126–132. [Google Scholar] [CrossRef]
- Dai, Z.; Bao, Z.; Ding, S.; Liu, C.; Sun, H.; Wang, H.; Zhou, X.; Wang, Y.; Yin, Y.; Li, X. Scalable polyimide-poly(amic acid) copolymer based nanocomposites for high-temperature capacitive energy storage. Adv. Mater. 2021, 34, 2101976. [Google Scholar] [CrossRef]
- Yu, X.; Yang, R.; Zhang, W.; Yang, X.; Ma, C.; Sun, K.; Shen, G.; Lv, F.; Fan, S. Interface engineering of polymer composite films for high-temperature capacitive energy storage. Chem. Eng. J. 2024, 496, 154056. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, X.; Li, L. Phase-Field Modeling of Electromechanical Breakdown in Multilayer Ceramic Capacitors. Adv. Theory Simul. 2018, 2, 1800179. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, X.; Luo, B.; Hong, W.; Wu, L.; Li, L. Dielectric response and breakdown behavior of polymer-ceramic nanocomposites: The effect of nanoparticle distribution. Compos. Sci. Technol. 2017, 145, 105–113. [Google Scholar] [CrossRef]
- Liu, X.-J.; Cheng, M.; Zhang, Y.; Xing, Y.; Dang, Z.-M.; Zha, J.-W. High-temperature polymer dielectric films with excellent energy storage performance utilizing inorganic outerlayers. Compos. Sci. Technol. 2024, 245, 110305. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, D.; Wu, K.; Fu, Q. Improved dielectric and energy storage properties of polypropylene by adding hybrid fillers and high-speed extrusion. Polymer 2021, 214, 123348. [Google Scholar] [CrossRef]
- Uyor, U.; Popoola, A.; Popoola, O.; Aigbodion, V. Thermal, mechanical and dielectric properties of functionalized sandwich BN-BaTiO3-BN/polypropylene nanocomposites. J. Alloy Compd. 2022, 894, 162405. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Z.; Yan, S.; Shi, L.; Hao, C.; Lei, Q. Dielectric film with high energy density based on polypropylene/maleic anhydride-grafted polypropylene/boron nitride nanosheet ternary system. Mater. Res. Bull. 2022, 155, 111978. [Google Scholar] [CrossRef]
- Tomer, V.; Polizos, G.; Randall, C.; Manias, E. Polyethylene nanocomposite dielectrics: Implications of nanofiller orientation on high field properties and energy storage. J. Appl. Phys. 2011, 109, 074113. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Xie, J.; Liu, Y.; Sun, S. Effect of organic Na+-montmorillonite on the dielectric and energy storage properties of polypropylene nanocomposites with polypropylene-graft-maleic anhydride as compatibilizer. J. Appl. Polym. Sci. 2022, 139, e52047. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zheng, G.; Cao, C.; Zhu, C.; Zhang, B.; Cai, Z.; Feng, P. Interfacial Engineering of Hydrophobic Montmorillonite for High-Energy-Capability Polypropylene Nanocomposite Dielectrics. Crystals 2025, 15, 786. https://doi.org/10.3390/cryst15090786
Li S, Zheng G, Cao C, Zhu C, Zhang B, Cai Z, Feng P. Interfacial Engineering of Hydrophobic Montmorillonite for High-Energy-Capability Polypropylene Nanocomposite Dielectrics. Crystals. 2025; 15(9):786. https://doi.org/10.3390/cryst15090786
Chicago/Turabian StyleLi, Shiheng, Guangsen Zheng, Chu Cao, Chaoqiong Zhu, Baojing Zhang, Ziming Cai, and Peizhong Feng. 2025. "Interfacial Engineering of Hydrophobic Montmorillonite for High-Energy-Capability Polypropylene Nanocomposite Dielectrics" Crystals 15, no. 9: 786. https://doi.org/10.3390/cryst15090786
APA StyleLi, S., Zheng, G., Cao, C., Zhu, C., Zhang, B., Cai, Z., & Feng, P. (2025). Interfacial Engineering of Hydrophobic Montmorillonite for High-Energy-Capability Polypropylene Nanocomposite Dielectrics. Crystals, 15(9), 786. https://doi.org/10.3390/cryst15090786









