1. Introduction
Crystallization is widely used in several industries as an efficient means of separating and purifying chemical compounds [
1]. However, the formation of 3D defects, especially fluid inclusions (FIs), remains a critical issue. Indeed, FIs in crystals raise several important issues, such as impurity concentration, induced chemical degradation [
2,
3], structural evolution of the host crystal, and sensitivity of energetic materials, to name the biggest concerns [
4]. To date, controlling parameters such as temperature range, cooling rate, solvent(s), seeding, and impurities in the mother solutions does not guarantee the production of inclusion-free crystals. These inclusions, containing liquid and/or gas, frequently appear in single crystals formed from solutions or from the melt [
5,
6]. Several studies report that the main causes of FIs are kinetic factors, such as excessively rapid crystal growth and impurity adsorption. It has also been highlighted that hydrodynamics plays a fundamental role in controlling these defects [
7,
8,
9,
10]. Moreover, several research groups have pointed out the role of dissolved gas in the formation of FIs [
11,
12,
13]. This study is an extension of a systematic investigation on this topic, concerning dicumyl peroxide (DCP), because this compound is likely to create IFs, as described in [
14].
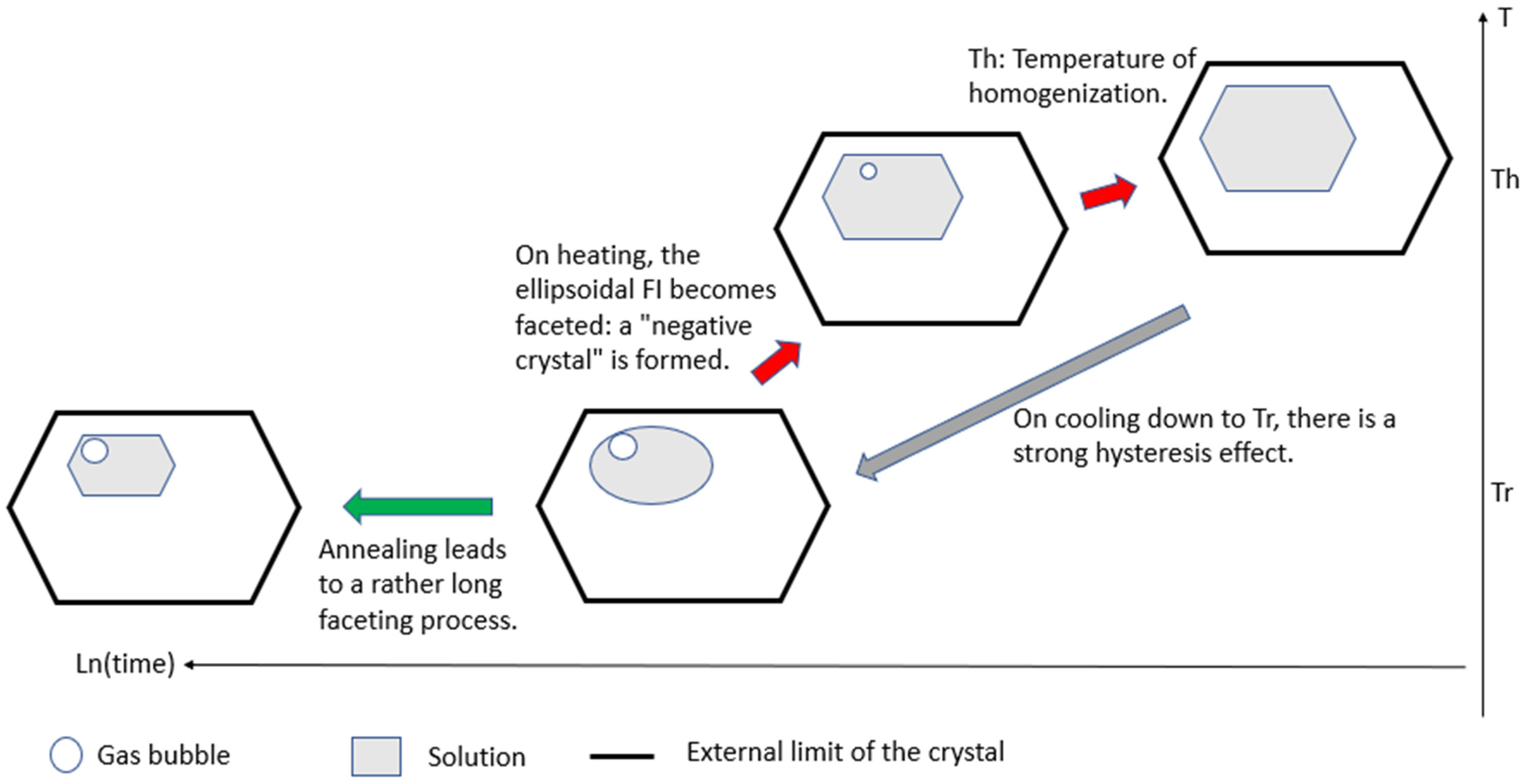
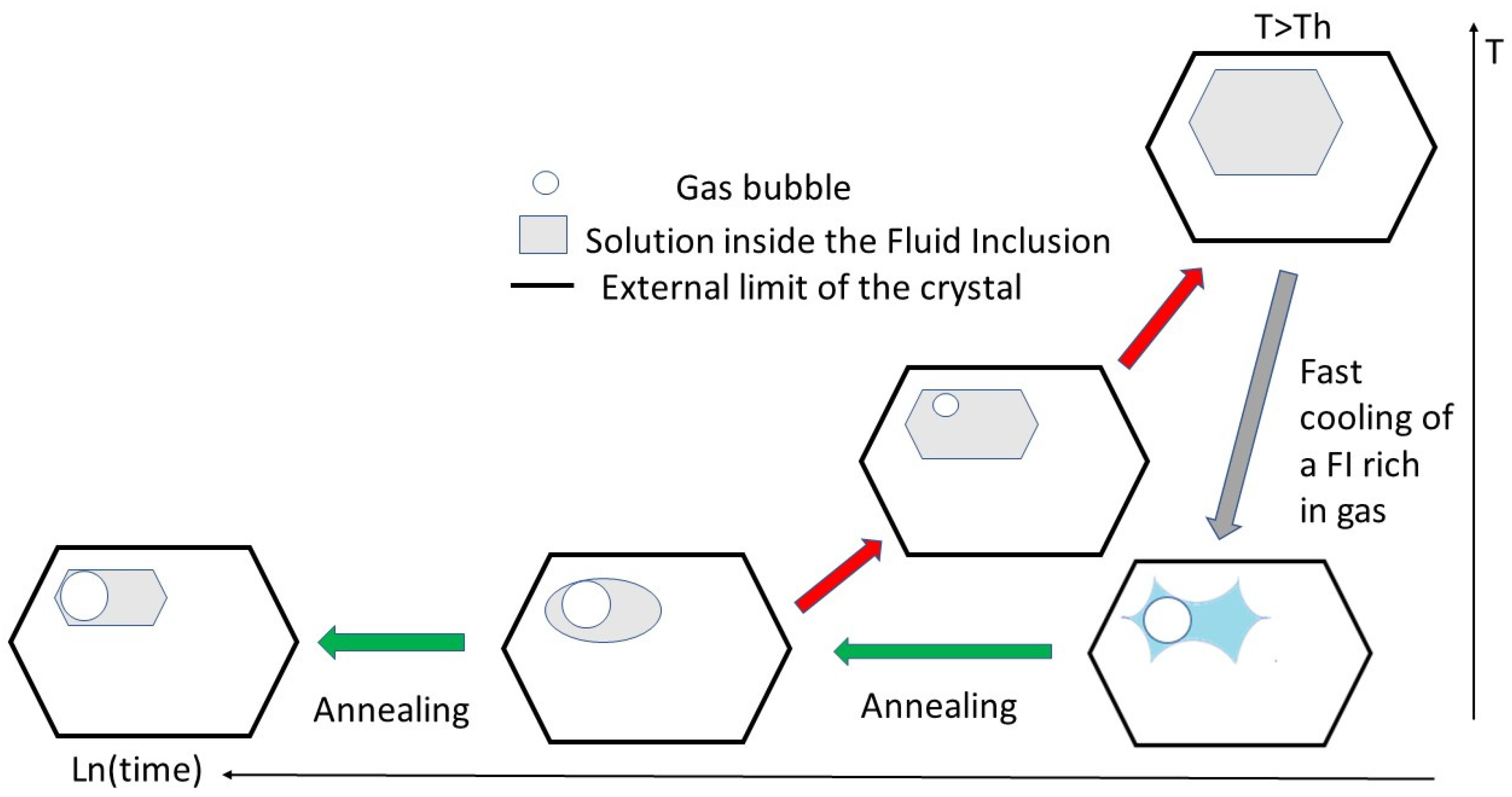
On heating, assuming a direct relationship between solubility and temperature, FIs increase in volume by dissolving the surrounding crystal and simultaneously reshape to form faceted cavities, i.e., negative crystals (
Figure 1). Thus, the situation is the inverse of the usual case, where the crystal grows outward into its nutrient solution. Normally, if a crystal is fed isotropically, the pattern of the FIs reflects the crystal symmetry [
15]. After sufficient annealing, an equilibrium is reached, indicating that the solution is saturated.
On cooling at a medium cooling rate to Tr (temperature of relaxation;
Figure 1), FIs shrink but not in a homothetic way. Indeed, from a faceted cavity (excluding elongated FIs), a progressive transformation results in a rounded pocket of solution. This arises from the competition of various faces trying to grow inward into a space that is shrinking over time. Moreover, the concentration of impurities increases over time if those impurities are not incorporated in a stable or metastable solid solution. If a sufficiently low temperature is reached, a gas bubble nucleates spontaneously. The nucleation of the gas bubble results from the volume deficit created by crystallization during cooling. The solid phase crystallizing from the solution at the rim of the inclusion has a higher density than the same amount of material dissolved. Moreover, this inward crystallization is exothermic, which increases the temperature of the inner wall of the cavity. During this step, the system is out of equilibrium, and convection is the main mechanism for equalizing the temperature throughout the FIs. As gases have a retrograde solubility, this local heating effect on the surface promotes the heterogeneous nucleation of the gas bubble. This gas bubble has been named the retreat bubble (or shrinkage bubble). The presence of a retreat bubble changes the concentration of the solution, potentially interfering with subsequent crystal growth.
If a higher cooling rate is applied, nucleation of several gas bubbles can occur almost simultaneously, but only one usually survives. Usually, the largest is the one that persists because of its lower interfacial energy (cf. Laplace–Young equation) [
10]. Several observations have shown that there are two ways for the number of gas bubbles to reduce to a single one: (i) the bubbles can merge by contact, and (ii) the bubbles can keep their distances, with the smaller ones shrinking until they vanish completely as the largest one enlarges. The temperature of nucleation (Tn) is stochastic, but the trend is that Tn decreases as the cooling rate increases.
When reheating occurs, FIs enlarge and the gas bubbles shrink; on further heating, the gas bubbles disappear completely. The temperature at which the retreat bubble vanishes is called the temperature of homogenization (Th). For a given FI, Th is quite reproducible provided the highest temperature does not cause irreversible changes in the cavity. Th fluctuates slightly from one FI to another in the same single crystal [
10]; nevertheless, these fluctuations are usually small. If heating is performed in a temperature gradient, the FIs move toward the highest temperature [
16].
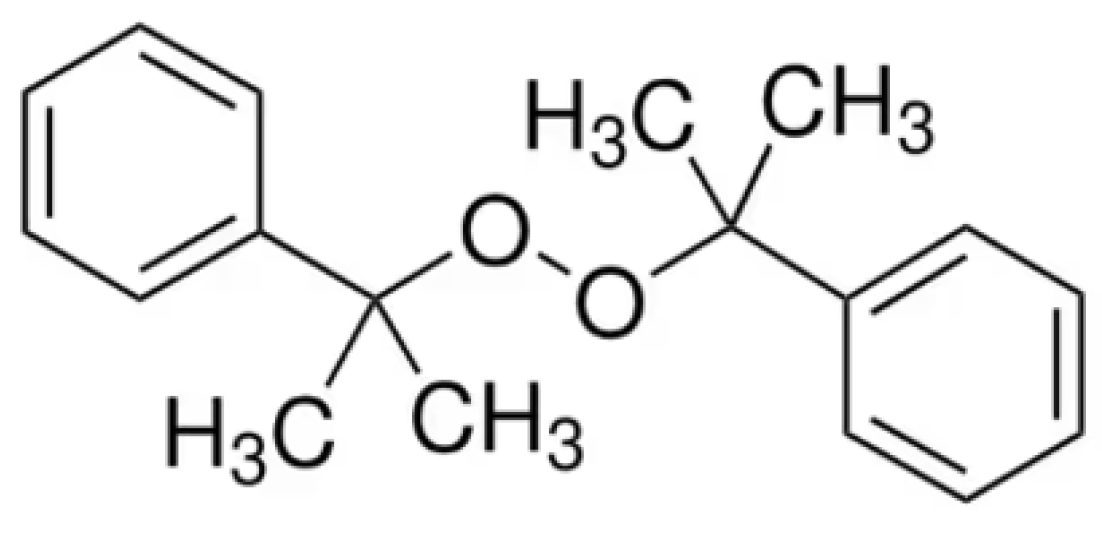
In this work, we first present a new FI behavior in dicumyl peroxide (DCP). This organic peroxide is commonly used as a radical initiator in polymerization processes and as a curing agent in various thermosetting resins [
17]. It has a chemical structure featuring two cumyl groups linked by a peroxide bridge (
Figure 2), making it a powerful agent for promoting radical reactions at elevated temperatures.
The thermal decomposition or UV exposure of dicumyl peroxide (DCP) generates free radicals, which can initiate cross-linking and polymerization [
18]. DCP crystals grown in different solvents exhibit various numbers of FIs and distinct crystal morphologies, but there is no change in the crystalline structure.
This compound has already been identified as an easy FI former [
14]; its crystallographic data can be obtained from the REFCODE: IFUYEO–Space Group: Pbca; Z’ = 0.5 [
19]. This paper first extends that previous study, then describes a high-melting-point mineral with striking analogies.
2. Materials and Methods
2.1. Products
Dicumyl peroxide [C6H5C(CH3)2]2O2 (98% pure) was supplied by Thermo Scientific and used without further purification or recrystallization. Azeotropic ethanol, used as the main solvent, was obtained from VWR. (CO2)g was obtained by sublimation of dry ice supplied by Air Liquide.
The olivine crystals studied here were sampled from basanitic tephras in the Strombolian pyroclastic fallout of the “Coupe de Jaujac” volcano (hereafter referred to as the Jaujac volcano). This volcano is a Strombolian cone whose crater was occupied by a lava lake from which a fluid basanitic flow escaped. The Jaujac volcano is part of the younger phase of the Bas-Vivarais (Ardèche) volcanic field in the southeast of the French Massif Central [
20]. The
40Ar/
39Ar age obtained from the Jaujac’s lava flow is 27.4 ± 9.4 kyears [
21].
2.2. Monitoring and External Factors
A Lauda Eco Silver RE 415 cryo-thermostat (Lauda GmbH -- A-1200 Wien Austria) was used to heat and control cooling profiles during crystal growth. Carbon dioxide (CO2) was used in its solid form and sublimated in a pressure gauge (Top Industrie, Vaux-le-Pénil, France) with a SITEC burst disc (SITEC-Sieber Engineering AG Maur, Zurich, Switzerland).
Dissolution and crystal growth were observed using a Nikon microscope equipped with a Leica Flexcam C3 camera and Leica Application Suite X (version 5.02.24429) software (Leica Camera AG, Wetzlar, Hesse, Germany). A Linkham THMS 600 (Linkam Scientific Instruments, Salfords, RH1 5DZ UK) programmable hot stage, controlled with Linksys32 software, was used to regulate the sample temperature. The temperature cycles were as follows: heating at 2 to 5 °C/min, and cooling rates varying from 60 °C/min to 5 °C/min. Various photos were extracted from the videos.
Olivine crystals were hand-picked from gently crushed black scoria (around 4 cm in diameter) under a binocular microscope. The crystals were mounted on a glass slide with thermal glue, and polished to expose their glass inclusions [
22]. The olivine crystals were examined using a petrographic microscope (Nikon Eclipse)(Nikon Corp. Tokyo, Japan) and a scanning electron microscope (JEOL 6510, JEOL Ltd., Tokyo, Japan). The latter microscope is equipped with a Bruker AXS flash detector, “Quantax,” (Bruker AXS GmbH, Karksruhe, Germany) which was used to determine the chemical composition of various phases by energy-dispersive spectroscopy.
2.3. Crystallization Protocols
The solutions—approximately 4 mL in volume—were stirred until complete dissolution at 40 °C, then slowly cooled to 5 °C at a rate of 0.5 °C/min. Subsequently, the temperature was lowered to 0 °C at 1 °C/min; this final temperature was maintained for 15 h. No seeding was used for the presented results. Carbon dioxide (CO2) bubbling was applied before adding the solute to the azeotropic ethanol and again after the solute had dissolved.
3. Results
3.1. Crystal Growth Behavior Under High Cooling Rates
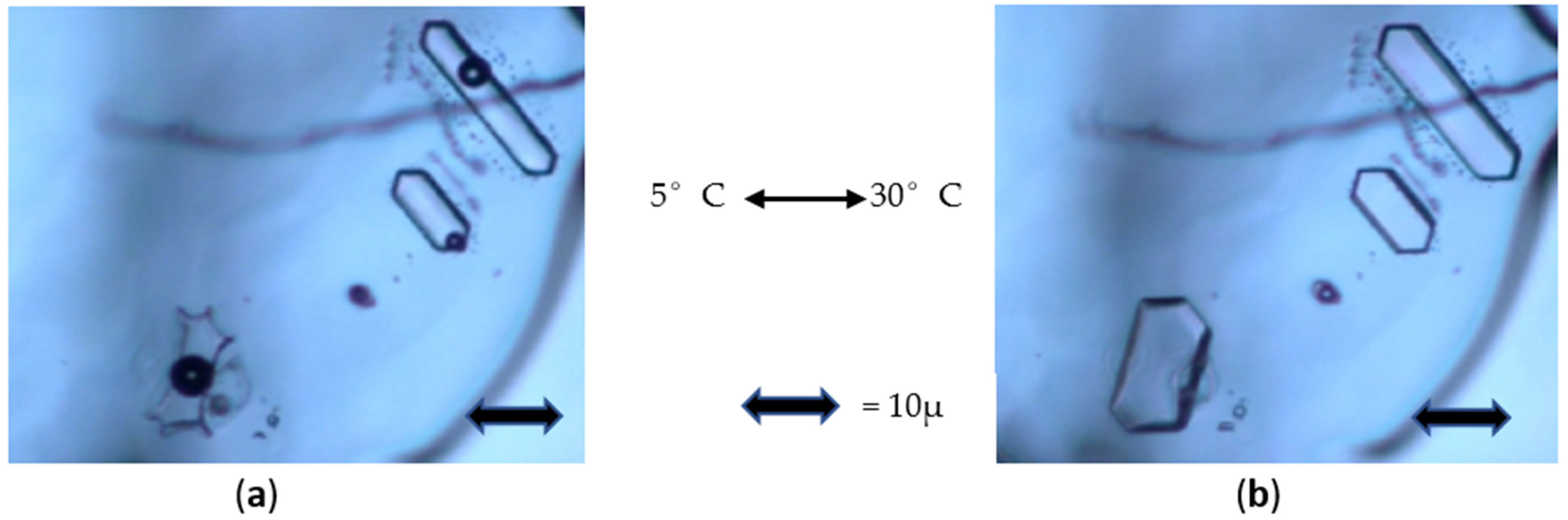
Supplementary Video S1 (accelerated 10×) shows two consecutive heating (5 °C/min) and cooling (−20 °C/min) cycles between 5 °C and 30 °C applied to different FIs in the same single crystal.
Figure 3 shows two snapshots extracted from this video: at 5 °C and at 30 °C.
At 5 °C (
Figure 3a, left), one can see an FI with concave surfaces, hereafter referred to as a “holly-leaf” shape, containing a large gas bubble. On heating (
Figure 3b), the gas bubble disappears; the cavity enlarges and becomes well-faceted at 30 °C, forming a negative crystal. In the upper right of
Figure 3a, two parallel elongated FIs are convex with blunted edges (more visible at the lower extremity of the top elongated FI) and contain a gas bubble of relatively usual volume. On heating (
Figure 3b), the FIs enlarge, the edges sharpen, and the gas bubbles disappear. These phenomena are perfectly reproducible. The temperature of homogenization of the holly-leaf-shaped FI is greater than that of the two other FIs. On cooling, gas bubble nucleation occurs with hysteresis.
We noticed that in the same single crystal, a few tens of microns apart, two very contrasting behaviors can co-exist:
- (i)
The new behavior appears in a limited number of FIs, essentially those presenting a large gas bubble at low temperature.
- (ii)
It requires a fast cooling rate.
Therefore, we enriched the mother solution in CO2, which has relatively good solubility in a water–ethanol mixture compared to other gases (N2, N2O, O2, O3, Ar, CH4, etc.). As a result, the overall number of FIs increased and the fraction of holly-leaf-shaped FIs rose significantly, supporting the hypothesis of the role of gas.
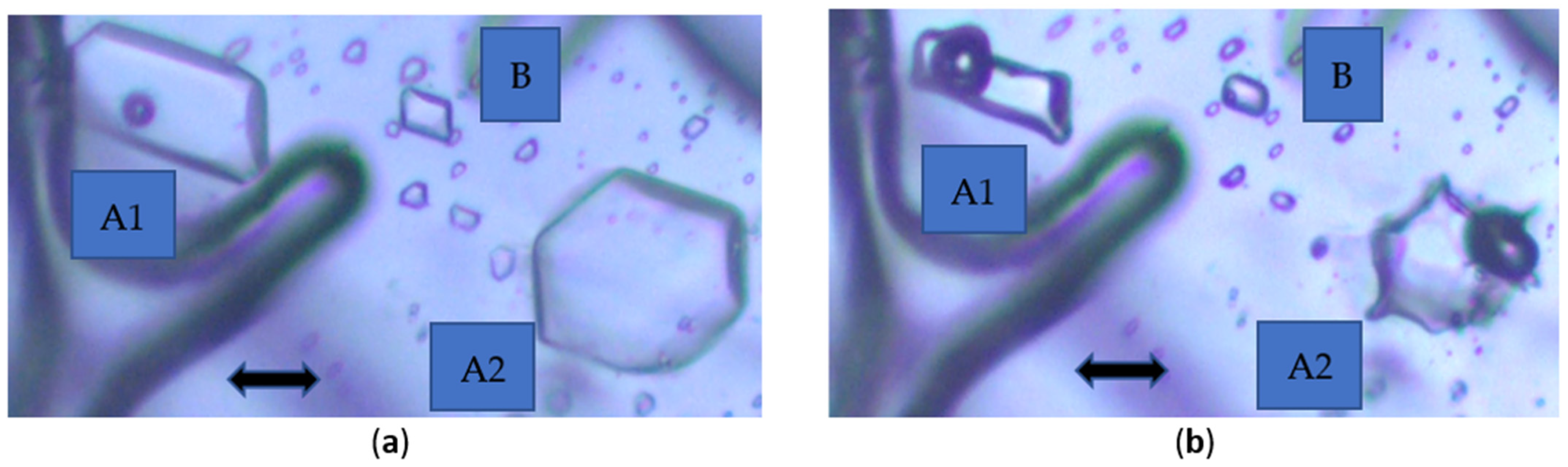
As shown in
Figure 4a,b, two distinct behaviors of DCP FIs in azeotropic ethanol enriched with CO
2 can be observed under fast cooling rates:
(A1) The inclusion exhibits few concave surfaces and contains a relatively large gas bubble compared to the volume of the cavity at low temperature (
Figure 4b). This FI is the only one to retain a gas bubble at 33 °C, illustrating the variability in the temperature of homogenization as a function of the gas content (
Figure 4a).
(A2) The inclusion develops into a holly-leaf shape with a large gas bubble (
Figure 4b).
(B) The inclusion reverts to a rounded shape with a small gas bubble (
Figure 4b).
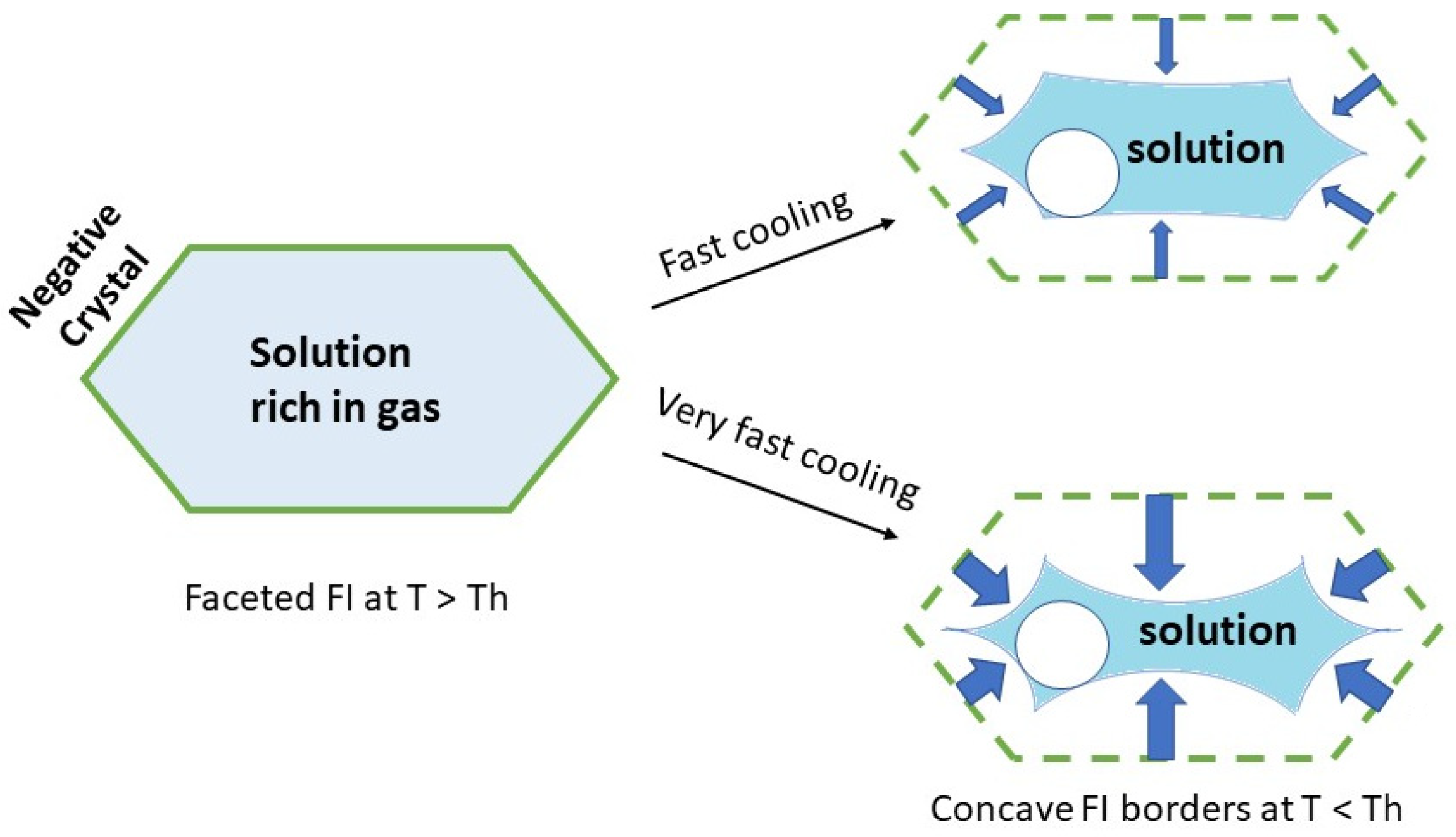
Figure 5 schematically illustrates the behavior of gas-rich FIs under fast cooling. At high temperature, the cavity is polyhedral, forming a negative crystal similar to that observed for the “usual” behavior. However, upon cooling, a sharp acceleration in crystal growth occurs immediately after gas bubble nucleation. This rapid inward crystal growth produces a holly-leaf morphology.
3.2. Relaxation of the Holly-Leaf FIs
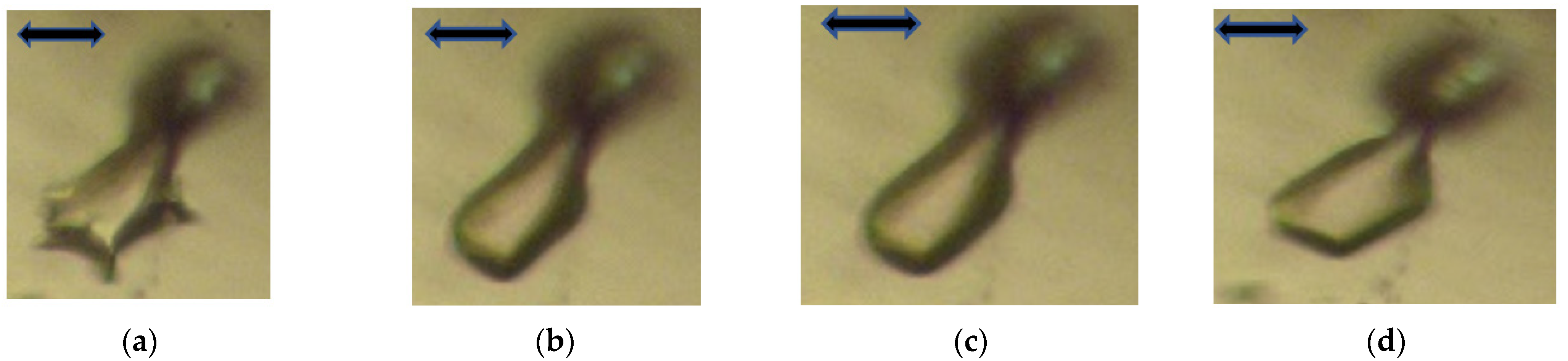
As depicted in
Figure 6a–d, on annealing the cavity reshapes into a convex vacuole first and, after more time, once again forms a faceted FI. This was not really expected, since the inversion of curvature implies that at one point there is a flat interface between the cavity and the rest of the crystal.
3.3. Impact of Cooling Rate on the Behavior of Fluid Inclusions in DCP Crystals
Figure 7 schematizes the effect of cooling rate on the holly-leaf-shaped FIs: the faster the cooling rate, the greater the curvature of the concave surfaces.
3.4. On the Existence of Pneumatic Chambers
Usually, when two FIs merge, the following sequence occurs:
- (i)
Formation of a neck joining the two former FIs.
- (ii)
The two cavities tend to resorb the neck and form a single convex cavity (i.e., the relaxation process).
If the quantity of gas dissolved in the merging cavities is relatively high, on cooling the gas bubble can occupy a large fraction of the smaller cavity, blocking the relaxation process between the two cavities. When the large gas bubble nucleates, the equivalent volume of solution is rapidly expelled from the small cavity to the large one through the neck joining them. The sustainability of these dual FIs depends on the mobility of the crystallized material around the joined cavities.
In
Figure 4b, cavity A2, which was a faceted FI at high temperature (
Figure 4a), shows a distortion of the cavity where the gas bubble is located. This occurs because the nucleation of the gas bubble takes place on the inner surface of the FI. In some cases, the gas bubble adheres to the wall, and as the FI shrinks, deformation of the FI appears. In extreme cases, a pneumatic chamber can be created. See also
Figure 6a,d; the pneumatic chamber is slightly out of focus.
4. Discussion: Interpretation of the Formation of Holly-Leaf-Shaped Fluid Inclusions
We assume that at high temperature there is an equant faceted FI containing a solution rich in gas. This FI undergoes fast cooling. As soon as the gas bubble nucleates, there is a sharp increase in the concentration of the solute. This is consistent with a significant acceleration of crystal growth as the solute concentration dramatically and suddenly increases.
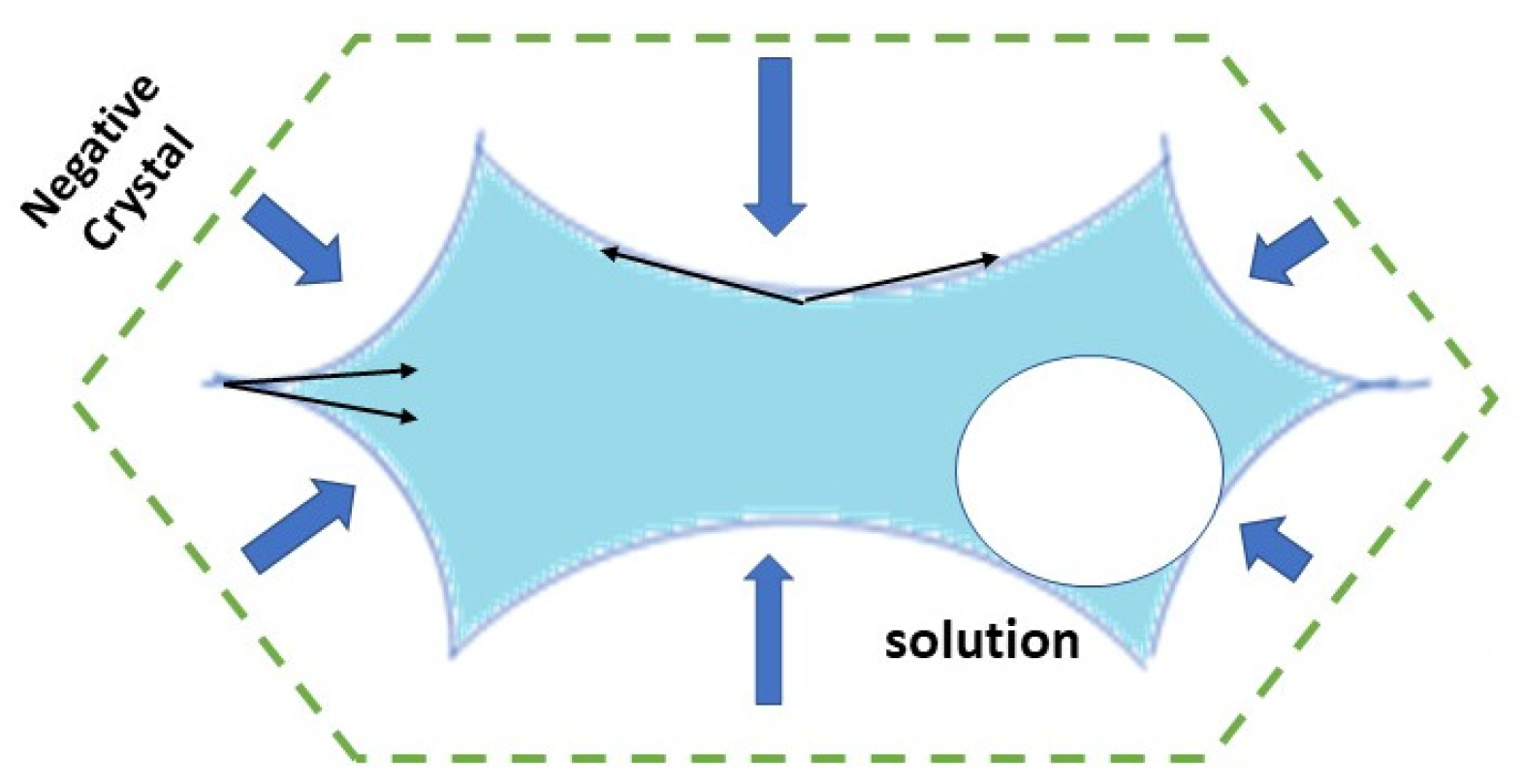
This phenomenon can be reproduced many times if there is no excessive heating, which could cause plastic deformation of the FI. From the initial negative crystal, crystal growth proceeds inward. In this vacuole, there is no stirring, and crystal growth is fed by diffusion only. The spikes of the holly leaf point toward the corners of the former negative crystal because the solid angle is more acute than in the middle of every face of the initial negative crystal, which corresponds to 2π sr (
Figure 9). In other words, a holly-leaf-shaped cavity appears because fast crystal growth depletes solute concentration faster than diffusion can deliver the solute to the corners of the negative crystal. This interpretation is supported by the effect of increasing the cooling rate, which increases the concave curvature of the holly-leaf-shaped FI, further restricting the acute solid angle. Consistently, increasing the CO
2 concentration—which produces larger gas bubbles—also leads to more FIs with greater supersaturation and thus higher concave curvatures.
5. Similar Behaviors Observed with Inclusions in Olivine
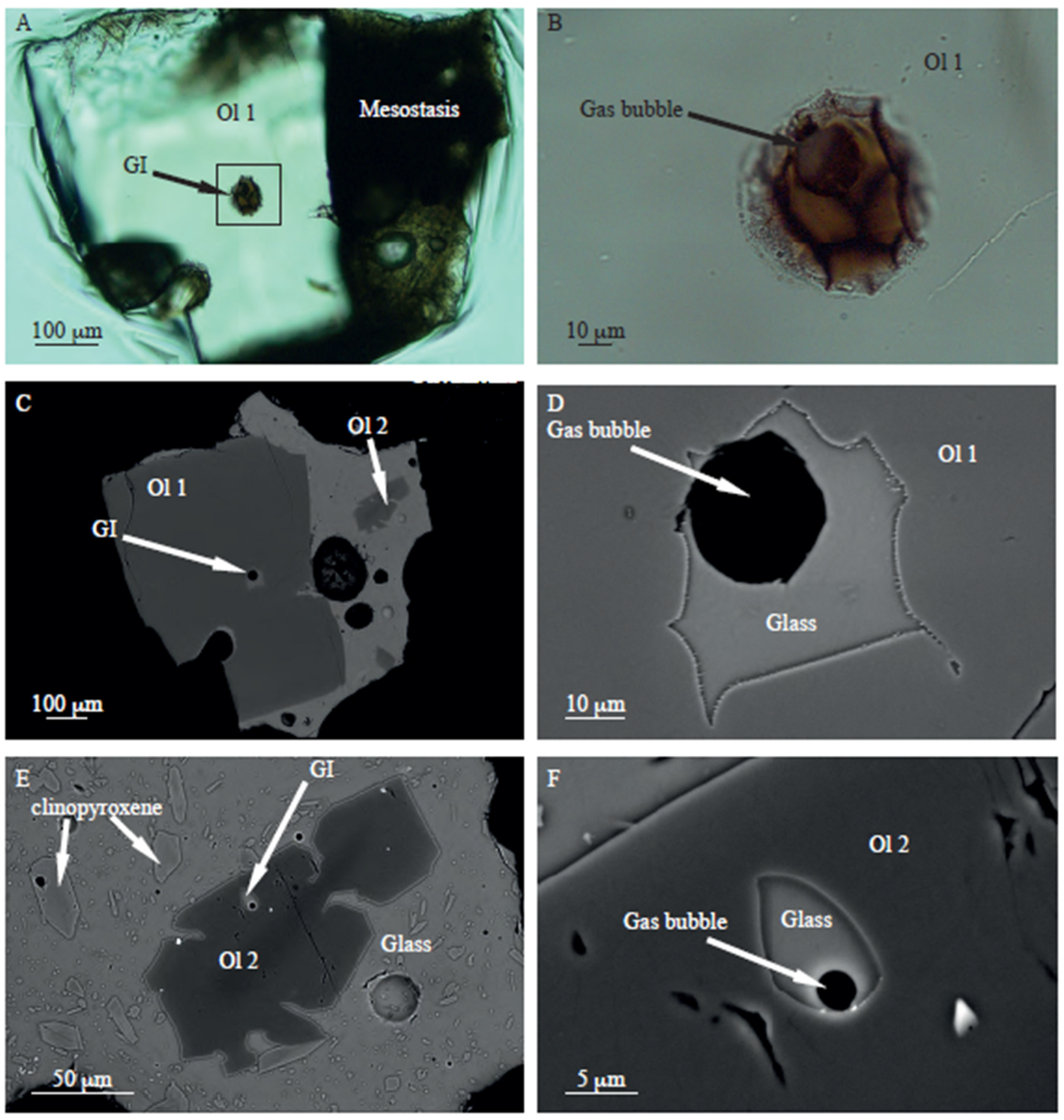
Interestingly, similar holly-leaf-shaped inclusions are observed in olivine crystals from volcanic rocks sampled at the Jaujac volcano in the French Massif Central, Ardèche, France (
Figure 10). In addition, two types of inclusions are seen in olivine crystals: holly-leaf-shaped inclusions and inclusions with a usual ovoid shape. Similarly, holly-leaf-shaped inclusions contain gas bubbles which are always larger, in proportion to the FI volume, than those in usual inclusions.
While both types of inclusion can be found in the same large olivine crystal, small olivine crystals appear to contain only usual inclusions. This suggests that holly-leaf inclusions were trapped early in the magmatic process. This observation aligns with a study of magmatic inclusions from the Thueyts volcano, located just 5 km from the Jaujac volcano. This study was the first to demonstrate the existence of these distinctive inclusions with concave surfaces [
23].
Furthermore, the study revealed through high-pressure, high-temperature homogenization experiments that the magmas trapped within these inclusions were high in volatile content, particularly CO
2. As this species is less soluble in low-pressure magmas, this explains why only inclusions trapped at high pressure exhibit the holly-leaf shape, while those trapped later (shown as smaller crystals in
Figure 10) show more usual ovoid shapes. This variation in inclusion shape, linked to the presence or absence of volatiles, is consistent with the fact that both types of inclusion underwent the same rapid terminal cooling during the eruption.
Furthermore, this hypothesis is consistent with DCP crystals obtained in the presence of CO2 exhibiting an increase in holly-leaf-shaped inclusions. This analogy suggests the possibility that such concave deflections across different materials and environments may share a common mechanism.
6. Conclusions
When homogeneous fluid inclusions (FIs) in crystals contain a large amount of dissolved gas, the temperature of homogenization is higher than without excess gas. At high temperature, these FIs become faceted, adopting the morphology of single crystals—i.e., negative crystals—like usual FIs. Upon fast cooling, these cavities shrink rapidly as soon as the large gas bubble nucleates. This swift crystal growth is associated with the formation of concave surfaces. The 2D projections of these cavities resemble holly leaves. The spikes of the holly-leaf-shaped FIs point toward the corners of the former negative crystals. Moreover, the faster the cooling rate, the greater the concave curvature of the surfaces of the FIs. The sizes of the gas bubbles exceed those of conventional retreat bubbles.
This reproducible behavior has been observed in detail for dicumyl peroxide (DCP) grown in a water–ethanol mixture (5–95 w/w) specifically enriched in CO2. Moreover, FIs in olivine crystals grown rapidly from pyroclastic CO2-rich lava can also present a holly-leaf shape. In addition, the retreat bubble is also unusually large. These observations, separated by three orders of magnitude in temperature, suggest that this behavior could be relatively general. Future research will determine whether, as expected, this behavior is general to fine chemicals (organic and inorganic).