Comparative Analysis of the Substituent Effects on the Supramolecular Structure of N′-(4-Methyl-2-nitrophenyl)benzohydrazide and N′-(2-Nitro-(4-trifluoromethyl)phenyl)benzohydrazide)
Abstract
1. Introduction
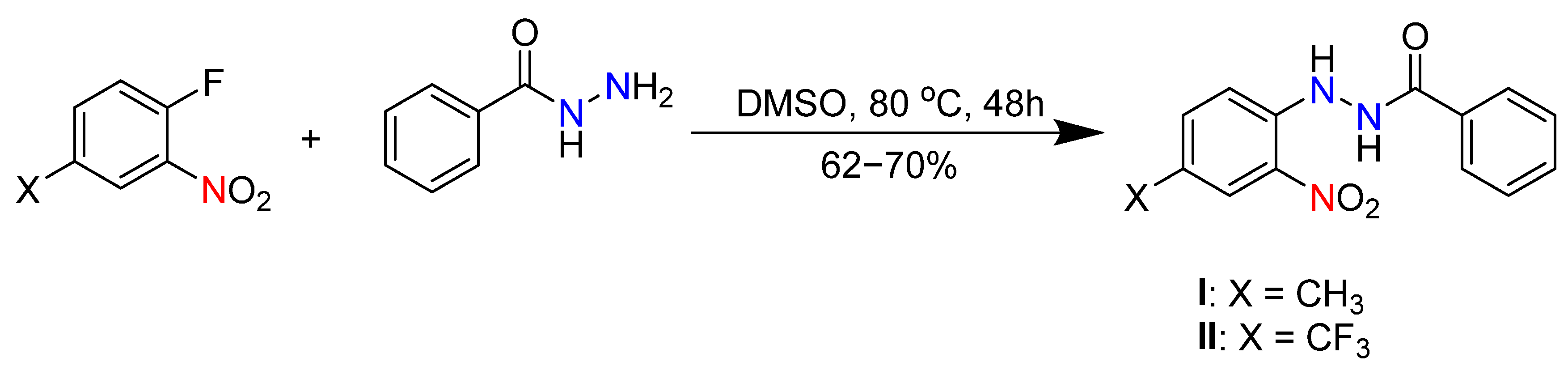
2. Materials and Methods
3. Results and Discussion
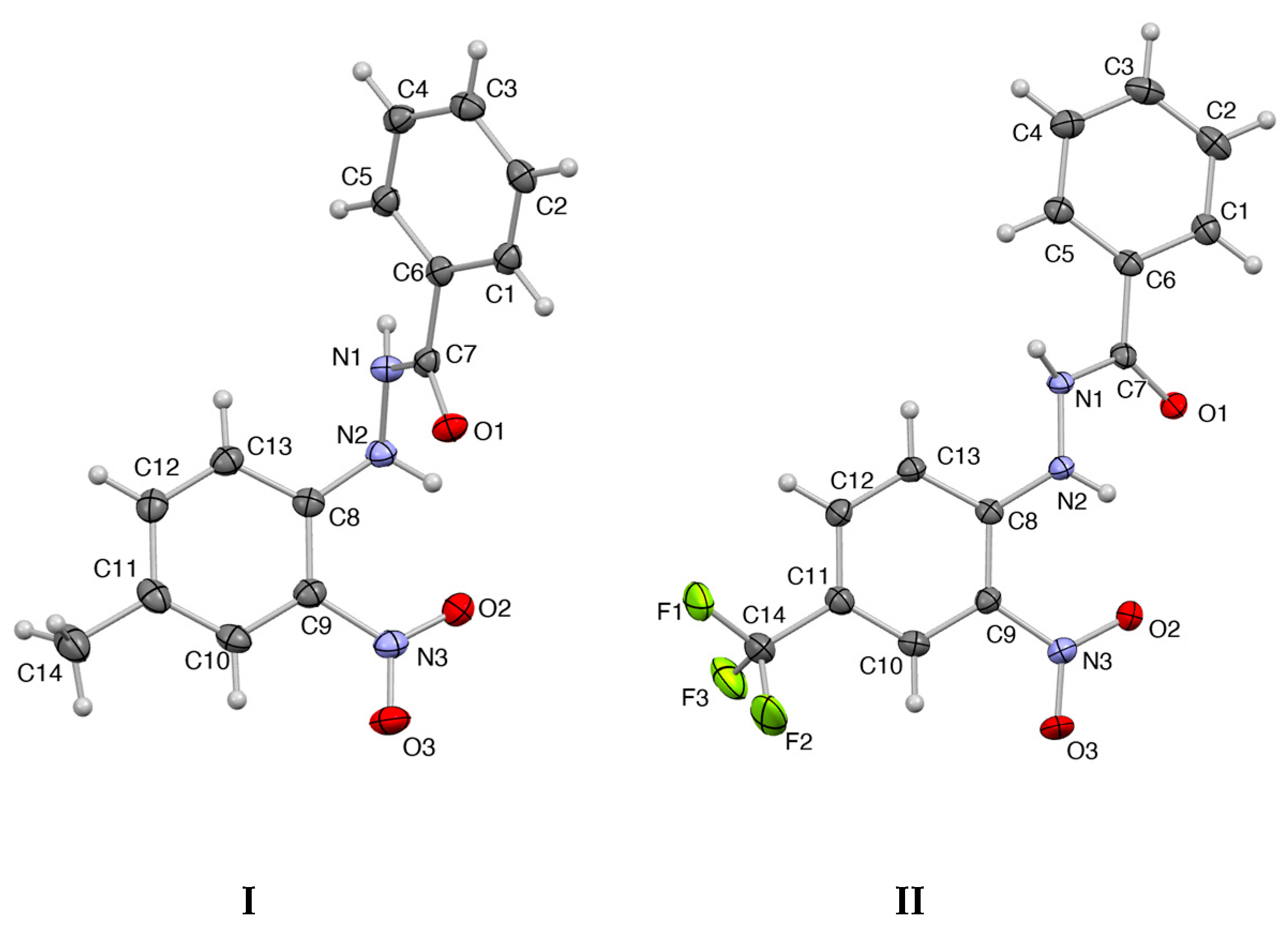
3.1. Geometry Characteristics of the Molecular Structure
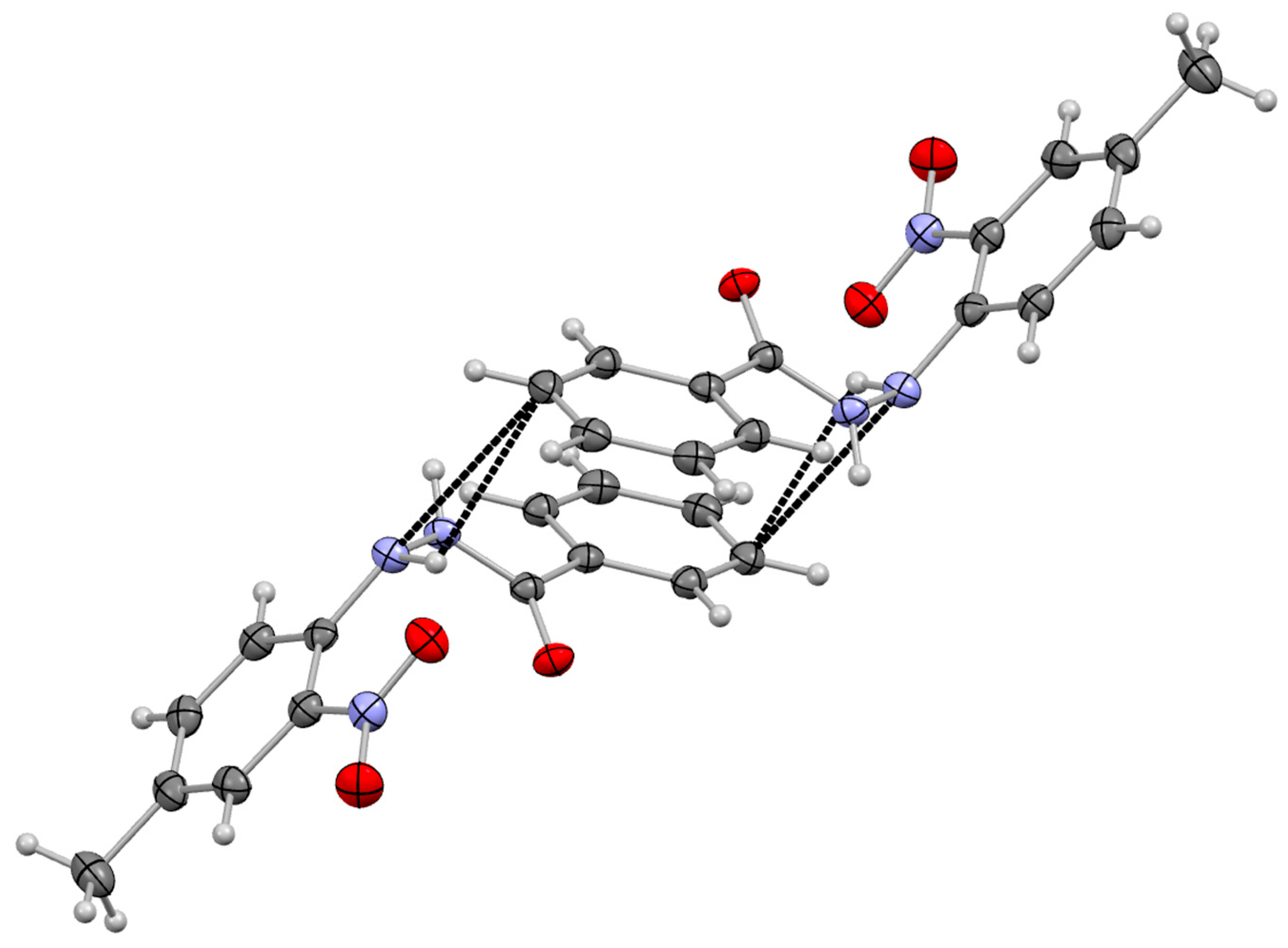
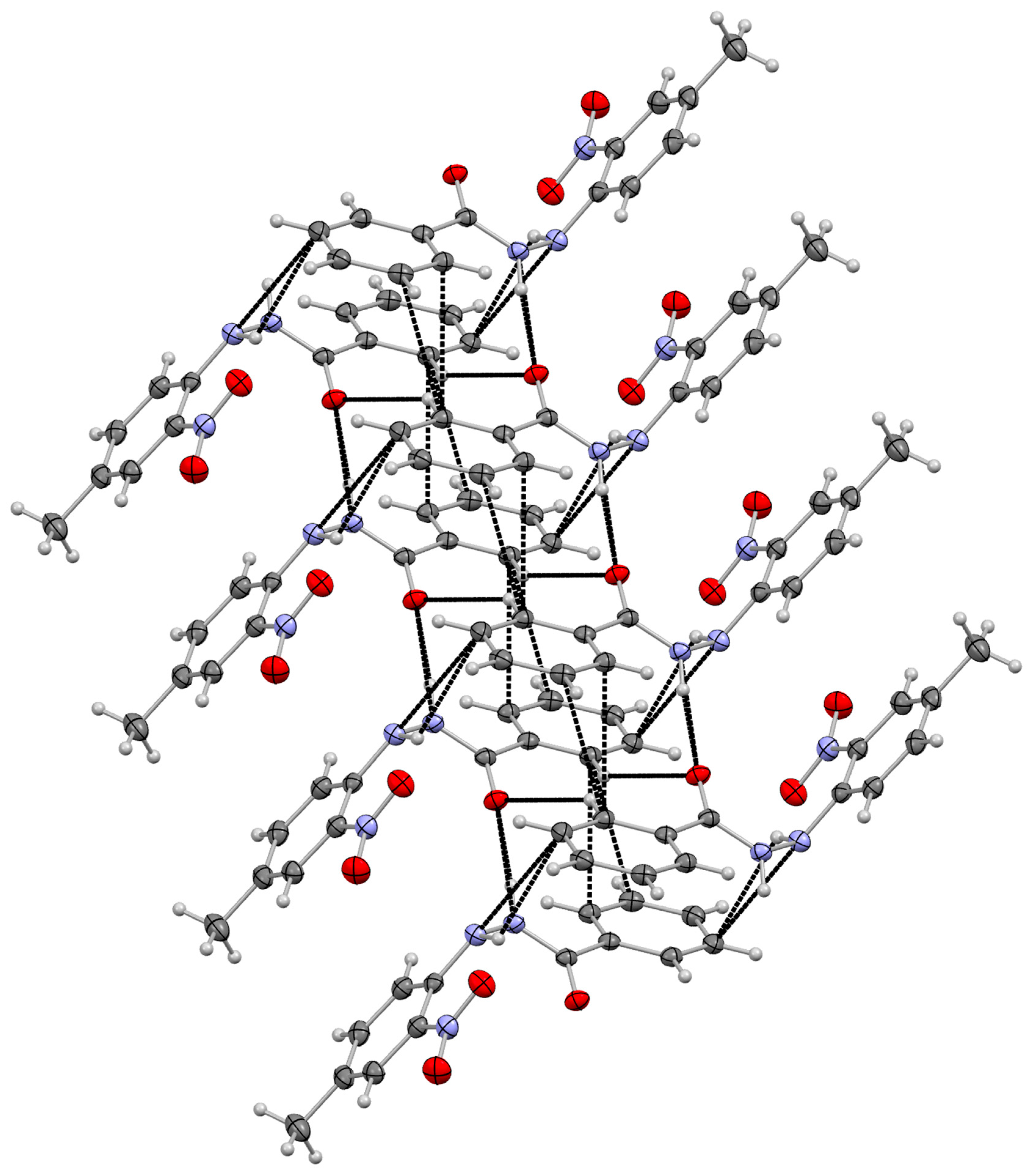
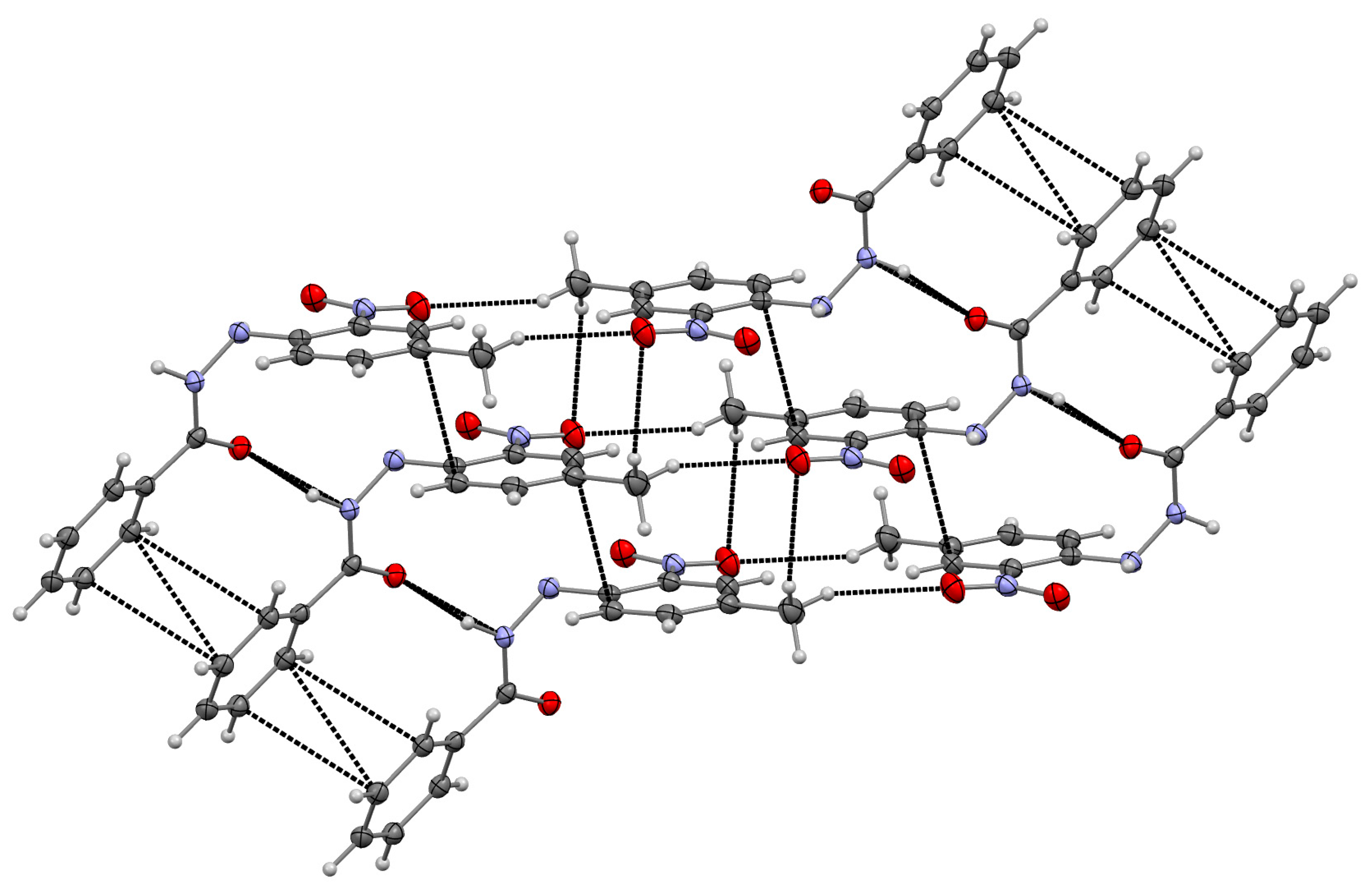
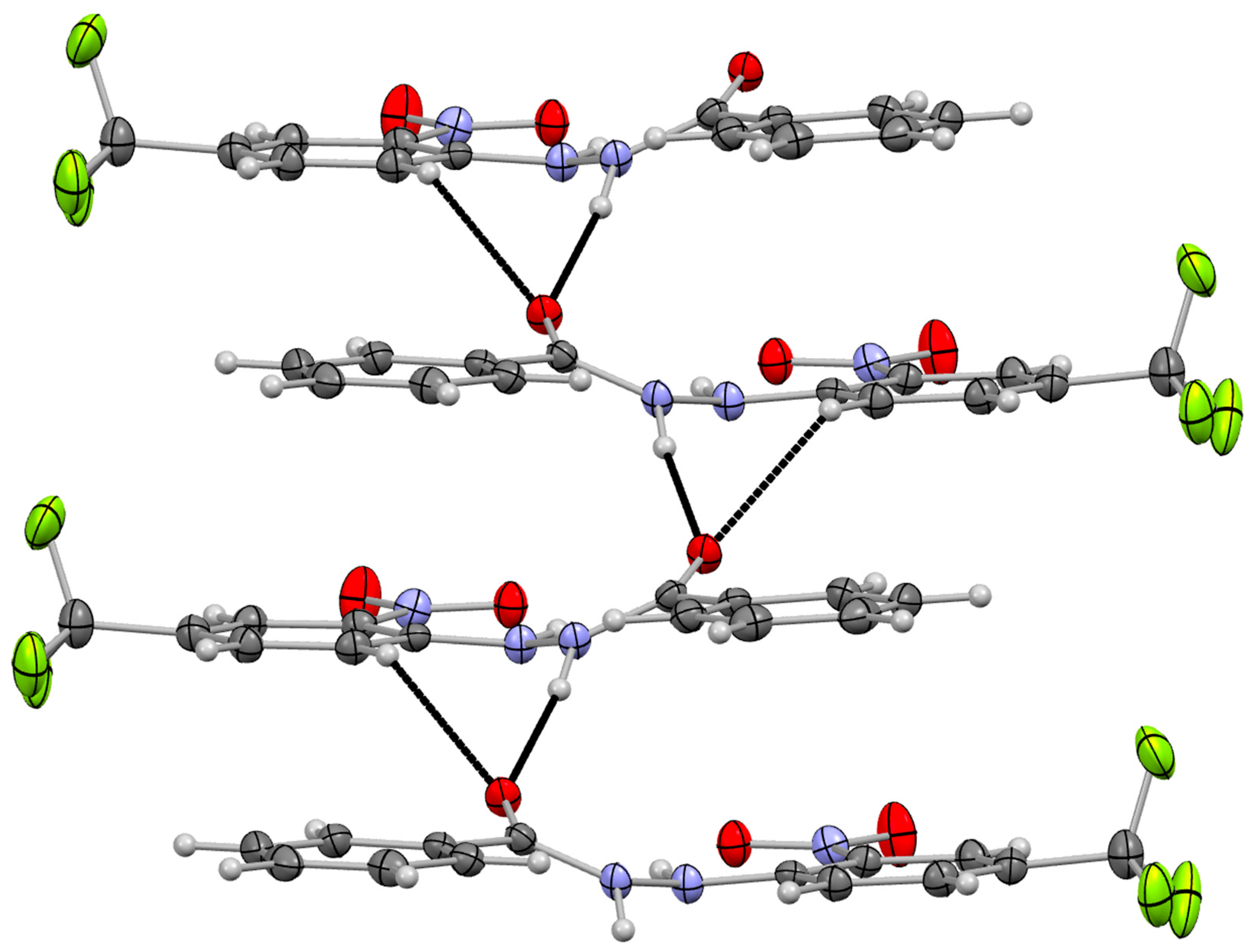
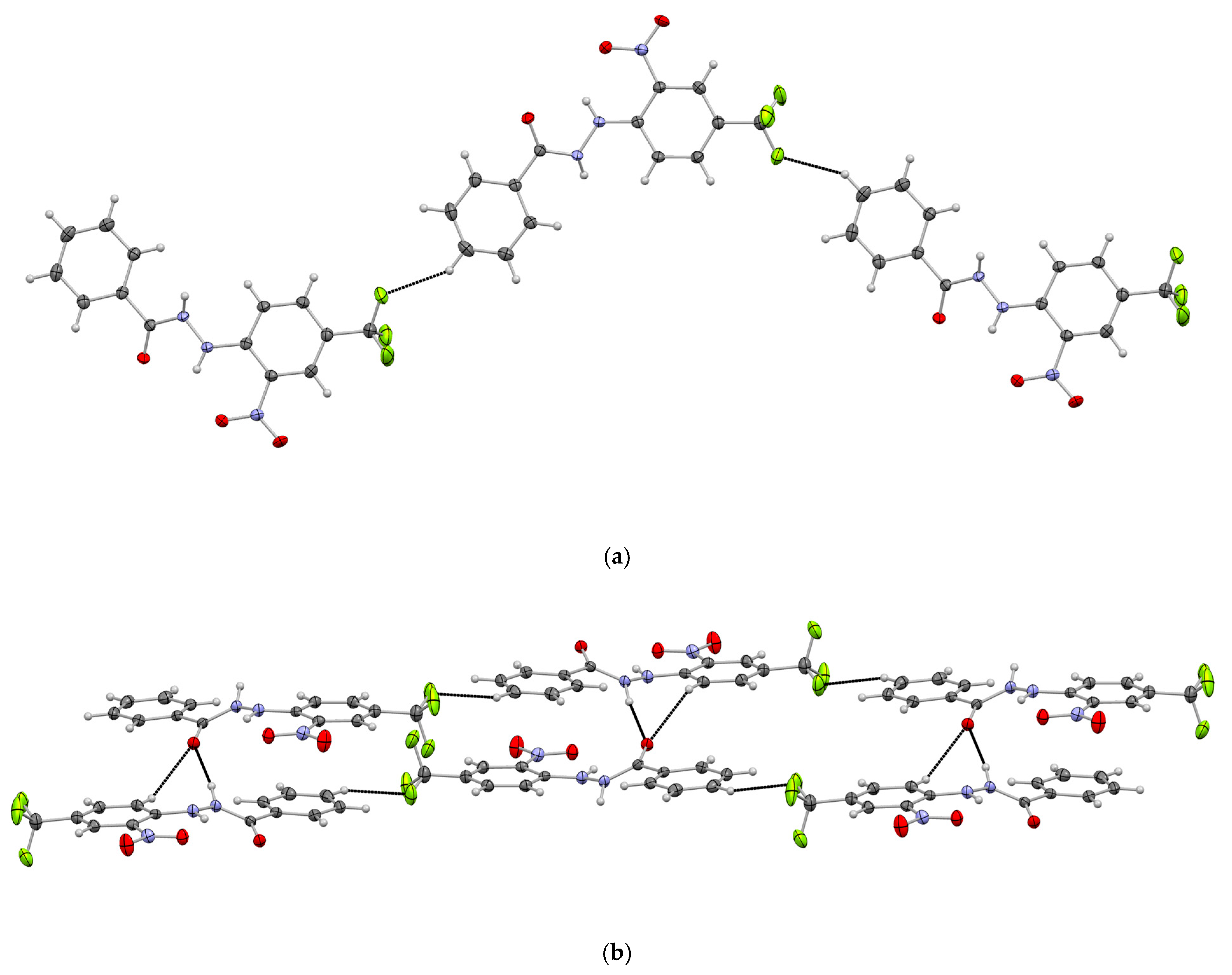
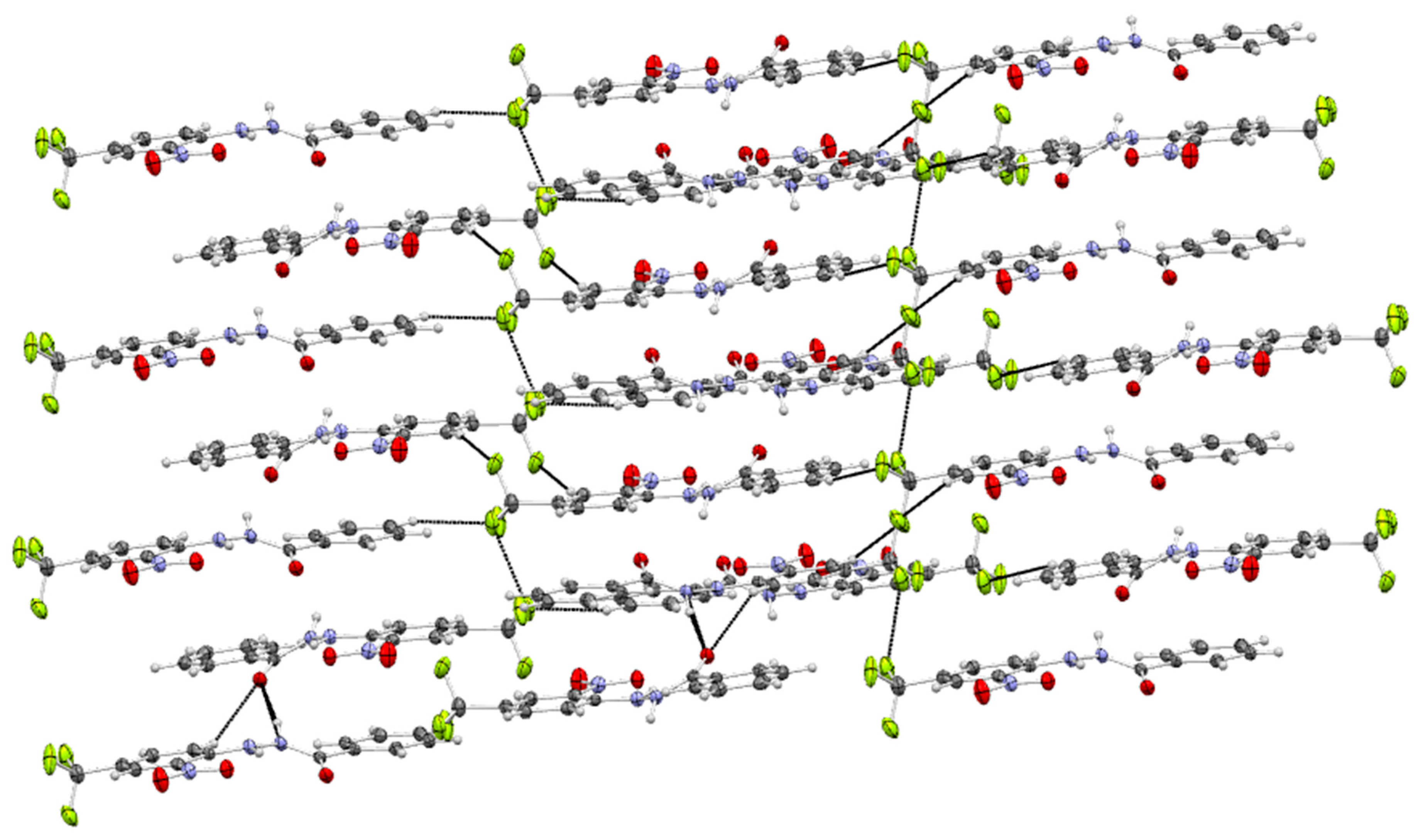
3.2. Supramolecular Arrangement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klabunovskii, E.; Smith, G.V.; Zsigmond, A. Heterogeneous Enantioselective Hydrogenation: Theory and Practice. In Catalysis by Metal Complexes; James, B., Van Leeuwen, P.W.N.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 31, pp. XIV, 305. [Google Scholar]
- Thiyagarajan, S.; Gunanathan, C. Direct Catalytic Symmetrical, Unsymmetrical N,N-Dialkylation and Cyclization of Acylhydrazides Using Alcohols. Org. Lett. 2020, 22, 6617–6622. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A review in biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Ragavendran, J.; Sriram, D.; Patel, S.; Reddy, I.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef]
- Todeschini, A.R.; Miranda, A.L.; Silva, C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and Evaluation of Analgesic, Anti-Inflammatory and Antiplatelet Properties of New 2-Pyridylarylhydrazone Derivatives. Eur. J. Med. Chem. 1988, 33, 189–199. [Google Scholar] [CrossRef]
- Masunari, A.; Tavares, L.C. A new class of nifuroxazide analogues: Synthesis of 5-nitrothiophene derivatives with antimicrobial activity against multidrug-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2007, 15, 4229–4236. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N. Synthesis, characterization and biological study of novel heterocyclic compounds. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef] [PubMed]
- Ergenc, N.; Gunay, N.S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Gursoy, E.; Guzeldemirci-Ulusoy, N. Synthesis and primarycytotoxicity evaluation of new imidazo[2, 1-b]thiazolederivatives. Eur. J. Med. Chem. 2007, 42, 320–326. [Google Scholar] [CrossRef]
- Liu, R.; Li, Z.; Liu, S.; Zheng, J.; Zhu, P.; Cheng, B.; Geng, H. Synthesis, Structure-Activity Relationship, and Mechanism of a Series of Diarylhydrazide Compounds as Potential Antifungal Agents. J. Agric. Food Chem. 2023, 71, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.J.M.; Norcott, P.L.; Coote, M. Recent advances in the chemistry of benzo[e][1,2,4]triazinyl radicals. Org. Biomol. Chem. 2020, 18, 8255–8277. [Google Scholar] [CrossRef]
- Yu, L.; Long, L.; Zheng, Y. Recent advances of stable Blatter radicals: Synthesis, properties and applications. Mater. Chem. Front. 2020, 4, 3433–3443. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Chi, Y.-H.; Shi, J.-M.; Li, H.-N.; Wei, W.; Cottrill, E.; Pan, N.; Chen, H.; Liang, Y.; Zhang, Y.-Q.; Hou, C. P-P Stacking, spin density and magnetic coupling strength. Dalton Trans. 2013, 42, 15559–15569. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Seth, N.; Brown, S.N. CCDC 1863863: Experimental Crystal Structure Determination. 2018. [Google Scholar]
- Kesternich, V.; Gahona, P.; Perez-Fehrmann, M.; Brito, I.; Lopez-Rodriguez, M. 2-Amino-N′-phenylbenzohydrazide. Acta Cryst. 2012, E68, o1847. [Google Scholar] [CrossRef]
- Wardell, J.L.; Low, J.N.; Glidewell, C. N-(4-Nitrobenzoyl)-N′-phenylhydrazine: A three dimensional hydrogen bonded framework. Acta Cryst. 2007, C63, o334. [Google Scholar] [CrossRef]
- Wardell, S.M.S.V.; de Souza, M.V.N.; Wardell, J.L.; Low, J.N.; Glidewell, C. N-(3,5-Dinitrobenzoyl)-N′-phenylhydrazine: Sheets built from N—H···O and C—H···O hydrogen bonds. Acta Cryst. 2006, E62, o2589. [Google Scholar] [CrossRef]
- Saeed, A.; Arshad, I.; Florke, U. N’-(2,4-Dinitrophenyl)benzohydrazide. Acta Cryst. 2012, E68, o2418. [Google Scholar] [CrossRef]
- Nowick, J.S.; Pairish, M.; Quen Lee, I.; Holmes, D.L.; Ziller, J.W. 1-anilidocarbamoyl-2,4-dimethoxybenzene. J. Am. Chem. Soc. 1997, 119, 5413–5424. [Google Scholar] [CrossRef]
- Saeed, A.; Arshad, I.; Florke, U. Synthesis, Crystal Structure, and DFT Study of N′-(2,4-Dinitrophenyl)-2-Fluorobenzohydrazide. J. Chem. 2013, 2013, 648121. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, L.; Yang, S.; Li, Z.; Wang, P.-Y. Synthesis, Antimicrobial Activity, and Molecular Docking of Benzoic Hydrazide or Amide Derivatives Containing a 1,2,3-Triazole Group as Potential SDH Inhibitors. Chin. J. Chem. 2021, 39, 1319–1330. [Google Scholar] [CrossRef]
- Cortés, E.; Abonía, R.; Cobo, J.; Glidewell, C. Four related esters: Two 4-(aroylhydrazinyl)-3-nitrobenzoates and two 3-aryl-1,2,4-benzotriazine-6-carboxylates. Acta Cryst. 2013, C69, 754–760. [Google Scholar] [CrossRef]
- Guru, M.M.; Punniyamurthy, T. Copper(II)-Catalyzed Aerobic Oxidative Synthesis of Substituted 1,2,3- and 1,2,4-Triazoles from Bisarylhydrazones via C–H Functionalization/C–C/N–N/C–N Bonds Formation. J. Org. Chem. 2012, 77, 5063–5073. [Google Scholar] [CrossRef]
- Shuler, W.G.; Smith, E.A.; Hess, S.M.; McFadden, T.M.C.; Metz, C.R.; VanDerVeer, D.G.; Pennington, W.T.; Mabe, P.J.; Knick, S.L.; Beam, C.F. Preparation and X-Ray Crystal Structure of 3-(4-(Dimethylamino)Phenyl)-2-(Phenylamino)Isoquinolin-1(2H)-One,3-(4-Methoxyphenyl)-2-(Phenylamino)Isoquinolin-1(2H)-One, and 2-Methyl-N′-(4-Methylbenzoyl)-N′-Phenylbenzohydrazide from Polylithiated 2-Methylbenzoic Acid Phenylhydrazide and Methyl 4-Dimethylaminobenzoate, Methyl 4-Methoxybenzoate, or Methyl 4-Methylbenzoate. J. Chem. Crystallogr. 2012, 42, 952–959. [Google Scholar]
- Jha, A.K.; Kumari, R.; Easwar, S. A Hydrazine Insertion Route to N′-Alkyl Benzohydrazides by an Unexpected Carbon–Carbon Bond Cleavage. Org. Lett. 2019, 21, 8191–8195. [Google Scholar] [CrossRef]
- Ke, C.; Cao, Q.; Luo, Y.; Liu, X.; Feng, X. Catalytic asymmetric amination of azlactones with azobenzenes. Chem. Commun. 2022, 58, 5881–5884. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhuo, X.; Wang, Y.; Zou, X.; Zhong, Y.; Guo, W. Photocatalytic cross-dehydrogenative coupling reaction toward the synthesis of N,N-disubstituted hydrazides and their bromides. Org. Chem. Front. 2022, 9, 3012–3021. [Google Scholar] [CrossRef]
- CrysAlisPro, version 1.171.43.130a; Rigaku Oxford Diffraction: Cedar Park, TX, USA, 2024.
- Pflugrath, J.W. The finer things in X-ray diffraction data collection. Acta Cryst. 1999, D55, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Cole, J.C.; Taylor, R. Intermolecular Interactions of Organic Fluorine Seen in Perspective. Cryst. Growth Des. 2022, 22, 1352–1364. [Google Scholar] [CrossRef]
- Reichenbacher, K.; Suss, H.I.; Hullinger, J. Fluorine in crystal engineering—‘the little atom that could’. J. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
| Entry | Compound Name | Reference |
|---|---|---|
| 1 | 1-Phenyl-2-benzoylhydrazine | [16] |
| 2 | 2-Amino-N′-phenylbenzohydrazide | [17] |
| 3 | N -(4-Nitrobenzoyl)-N′-phenyl hydrazine | [18] |
| 4 | N-(3,5-Dinitrobenzoyl)-N′-phenylhydrazine | [19] |
| 5 | N′-(2,4-Dinitrophenyl)benzohydrazide | [20] |
| 6 | 1-Anilidocarbamoyl-2,4-dimethoxybenzene | [21] |
| 7 | Methyl-5-(N-benzoylhydrazino)-2-methoxybenzoate | [21] |
| 8 | Methyl-3-(N-benzoylhydrazino)-4-methoxybenzoate | [21] |
| 9 | N′-(2,4-Dinitrophenyl)-2-fluorobenzohydrazide | [22] |
| 10 | 4-{[4-(2-Chlorophenyl)-1H-1,2,3-triazol-1-yl]methyl}-N′-[2-(trifluoromethyl)phenyl]benzohydrazide | [23] |
| 11 | Methyl-4-(2-(4-chlorobenzoyl)hydrazino)-3-nitrobenzoate | [24] |
| 12 | 4-Chloro-N′-((4-chlorophenyl)(imino)methyl)-N′-phenylbenzohydrazide | [25] |
| 13 | N′-(3-Methylbut-2-en-yl)-N′-phenylbenzohydrazide | [26] |
| 14 | Methyl-3-[2-(4-methylbenzene-1-carbonyl)-1-phenylhydrazinyl]propanoate | [27] |
| 15 | N′-(4-benzyl-5-oxo-2-phenyl-4,5-dihydro-1,3-oxazol-4-yl)-N′-(4-fluorophenyl)-2-methoxybenzohydrazide | [28] |
| 16 | N′-(4-bromophenyl)-N′-methylbenzohydrazide | [29] |
| Bond | Compound I | Compound II | Angle | Compound I | Compound II |
|---|---|---|---|---|---|
| C1-C2 | 1.387(2) | 1.387(2) | C1-C2-C3 | 120.1(1) | 120.1(1) |
| C2-C3 | 1.389(2) | 1.388(2) | C2-C3-C4 | 120.2(1) | 119.9(1) |
| C3-C4 | 1.382(2) | 1.389(2) | C3-C4-C5 | 120.1(1) | 120.6(1) |
| C4-C5 | 1.386(2) | 1.389(2) | C4-C5-C6 | 119.5(1) | 119.8(1) |
| C5-C6 | 1.397(2) | 1.387(2) | C5-C6-C1 | 119.9(1) | 119.8(1) |
| C6-C1 | 1.393(2) | 1.395(2) | C6-C1-C2 | 120.1(1) | 120.0(1) |
| C8-C9 | 1.408(2) | 1.418(2) | C8-C9-C10 | 121.6(1) | 121.6(1) |
| C9-C10 | 1.398(2) | 1.392(2) | C9-C10-C11 | 121.2(1) | 120.1(1) |
| C10-C11 | 1.380(2) | 1.375(2) | C10-C11-C12 | 117.4(2) | 119.5(1) |
| C11-C12 | 1.402(2) | 1.403(2) | C11-C12-C13 | 121.7(1) | 120.8(1) |
| C12-C13 | 1.375(2) | 1.367(2) | C8-C12-C13 | 121.8(1) | 121.5(1) |
| C8-C13 | 1.401(2) | 1.415(2) | C8-C9-C13 | 116.2(1) | 116.5(1) |
| C7-N1 | 1.355(2) | 1.350(2) | N1-N2-H2 | 111.0(1) | 115.0(1) |
| C8-N2 | 1.397(2) | 1.352(2) | H2-N2-C8 | 113.0(1) | 121.0(1) |
| C9-N3 | 1.458(2) | 1.444(2) | C8-N2-N1 | 114.5(1) | 121.6(1) |
| N1-N2 | 1.410(1) | 1.391(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinides, C.P.; Raza, S.; Bazzi, F.; Sharara, N.; Marincean, S. Comparative Analysis of the Substituent Effects on the Supramolecular Structure of N′-(4-Methyl-2-nitrophenyl)benzohydrazide and N′-(2-Nitro-(4-trifluoromethyl)phenyl)benzohydrazide). Crystals 2025, 15, 732. https://doi.org/10.3390/cryst15080732
Constantinides CP, Raza S, Bazzi F, Sharara N, Marincean S. Comparative Analysis of the Substituent Effects on the Supramolecular Structure of N′-(4-Methyl-2-nitrophenyl)benzohydrazide and N′-(2-Nitro-(4-trifluoromethyl)phenyl)benzohydrazide). Crystals. 2025; 15(8):732. https://doi.org/10.3390/cryst15080732
Chicago/Turabian StyleConstantinides, Christos P., Syed Raza, Fadwat Bazzi, Nisreen Sharara, and Simona Marincean. 2025. "Comparative Analysis of the Substituent Effects on the Supramolecular Structure of N′-(4-Methyl-2-nitrophenyl)benzohydrazide and N′-(2-Nitro-(4-trifluoromethyl)phenyl)benzohydrazide)" Crystals 15, no. 8: 732. https://doi.org/10.3390/cryst15080732
APA StyleConstantinides, C. P., Raza, S., Bazzi, F., Sharara, N., & Marincean, S. (2025). Comparative Analysis of the Substituent Effects on the Supramolecular Structure of N′-(4-Methyl-2-nitrophenyl)benzohydrazide and N′-(2-Nitro-(4-trifluoromethyl)phenyl)benzohydrazide). Crystals, 15(8), 732. https://doi.org/10.3390/cryst15080732







