Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Synthesis
- 4-(4-Decyloxyphenylazo)pyridine, A12. Orange solid. 61% Yield, m.p. 73 °C. 1H NMR (400 MHz, CDCl3) δ 8.77 (d, J = 6.0 Hz, 2H, Ar-H), 7.95 (d, J = 8.4 Hz, 2H, Ar-H), 7.71 (d, J = 6.0 Hz, 2H, Ar-H), 7.03 (d, J = 8.4 Hz, 2H, Ar-H), 4.06 (t, J = 6.4 Hz, 2H, Ar-OCH2), 1.85–1.76 (m, 2H, Ar-OCH2CH2), 1.46–1.27 (m, 18H, CH2), 0.90 (t, J = 6.8 Hz, 3H, -CH3).
- 4-(3-Fluoro-4-octyloxyphenylazo)pyridine, AF38. Orange crystals. 30% Yield, m.p. 58–59 °C. 1H NMR (500 MHz, CDCl3) δ 8.81–8.76 (m, 2H, Ar-H), 7.82 (ddd, J = 8.6, 2.3, 1.3 Hz, 1H, Ar-H), 7.72 (dd, J = 11.9, 2.3 Hz, 1H, Ar-H), 7.69–7.64 (m, 2H, Ar-H), 7.09 (t, J = 8.5 Hz, 1H, Ar-H), 4.14 (t, J = 6.6 Hz, 2H, Ar-OCH2), 1.92–1.83 (m, 2H, Ar-OCH2CH2), 1.55–1.29 (m, 10H, CH2), 1.43–1.33 (m, 3H, -CH3).
- 4-(2-Fluoro-4-octyloxyphenylazo)pyridine, AF28. Orange crystals. 37% Yield, m.p. 56–58 °C. 1H NMR (500 MHz, CDCl3-d) δ 8.88–8.72 (m, 2H, Ar-H), 7.84–7.77 (m, 1H, Ar-H), 7.72–7.66 (m, 2H, Ar-H), 6.80–6.74 (m, 2H, Ar-H), 4.04 (t, J = 6.6 Hz, 2H, O-CH2), 1.89–1.78 (m, 2H, Ar-OCH2), 1.52–1.42 (m, 2H, CH2, Ar-OCH2CH2), 1.42–1.23 (m, 8H, CH2), 0.96–0.85 (t, J = 6.9 Hz, 3H, -CH3).
- 4-(2,3-Difluoro-4-octyloxyphenylazo)pyridine, AF238. Orange crystals. 36.4% yield. m.p. 54–55 °C. 1H NMR (500 MHz, CDCl3) δppm: 8.93–8.66 (m, 2H, Ar-H), 7.77–7.64 (m, 2H, Ar-H), 7.59 (ddd, J = 9.6, 7.5, 2.3 Hz, 1H, Ar-H), 6.82 (ddd, J = 9.4, 7.4, 2.0 Hz, 1H, Ar-H), 4.15 (t, J = 6.6 Hz, 2H, Ar-OCH2), 1.92–1.82 (m, 2H, Ar-OCH2CH2), 1.55–1.44 (m, 2H, CH2), 1.43–1.22 (m, 8H, CH2), 0.96–0.80 (t, J = 6.8 Hz, 3H, -CH3).
2.3. Preparation of Supramolecular Complexes (An/B, AF3n/B, AF28/B, AF238/B)
3. Results
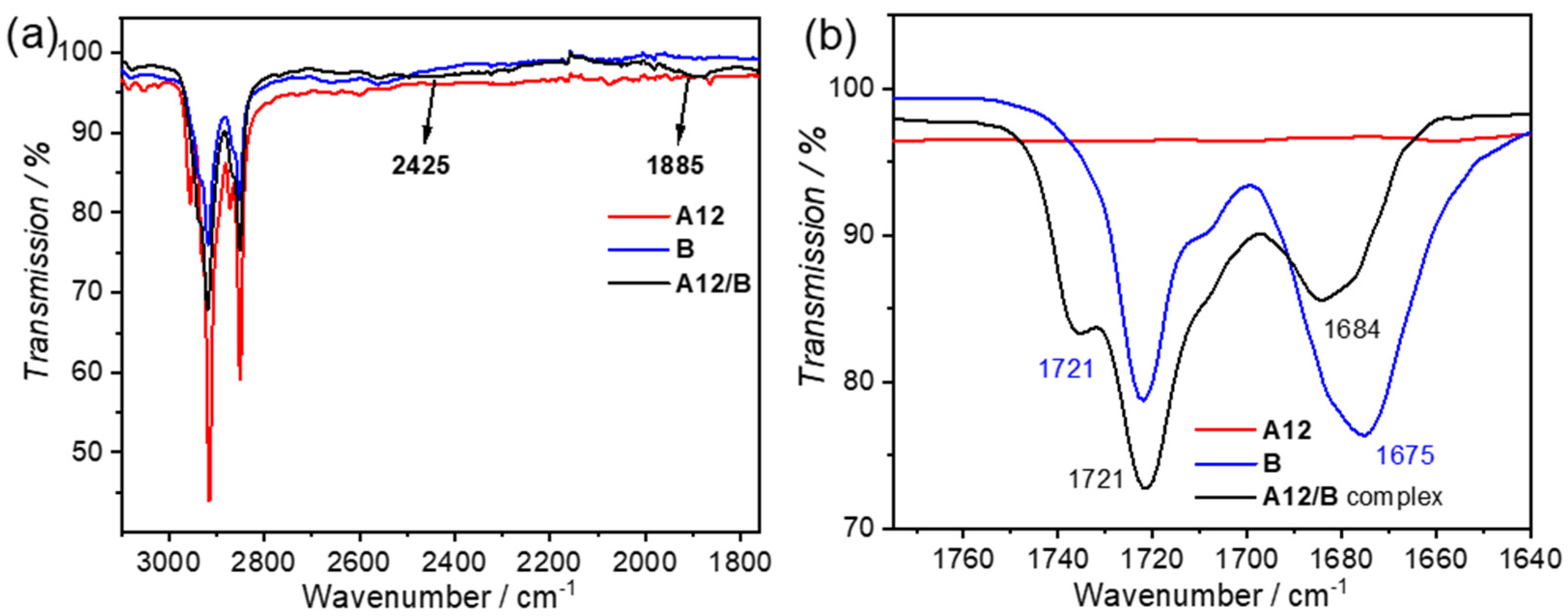
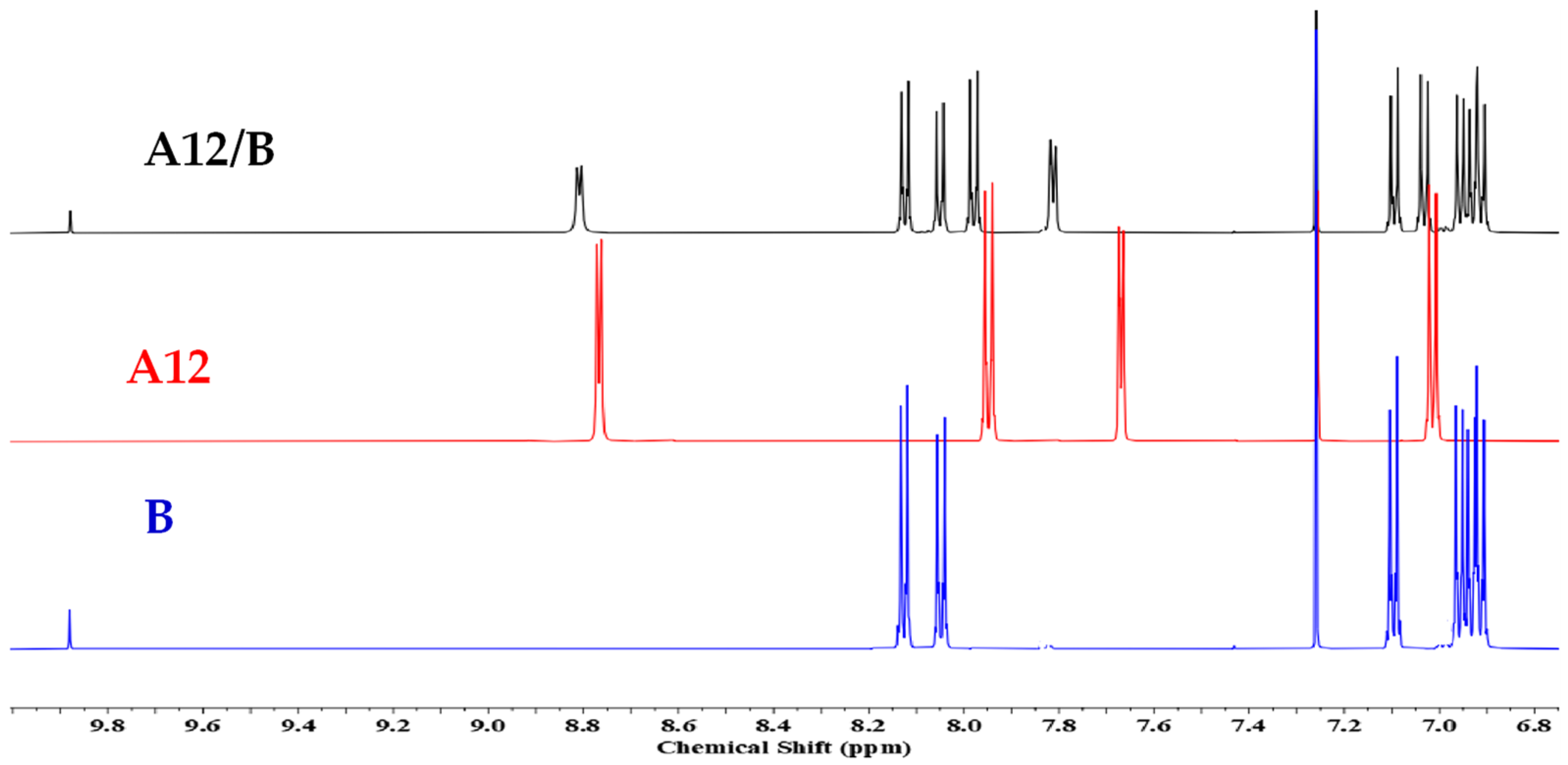
3.1. Hydrogen Bond Formation
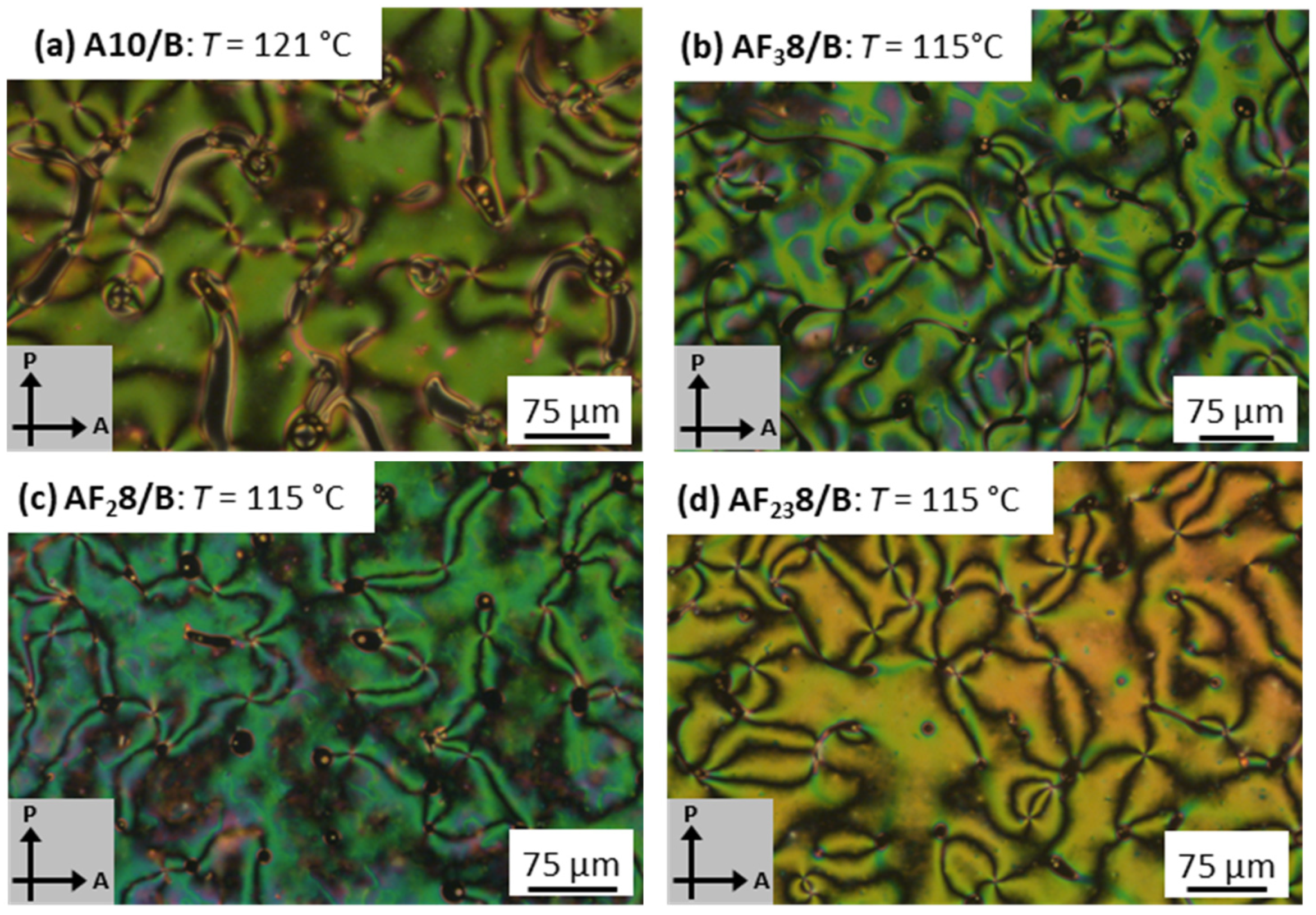
3.2. Mesomorphic Investigations
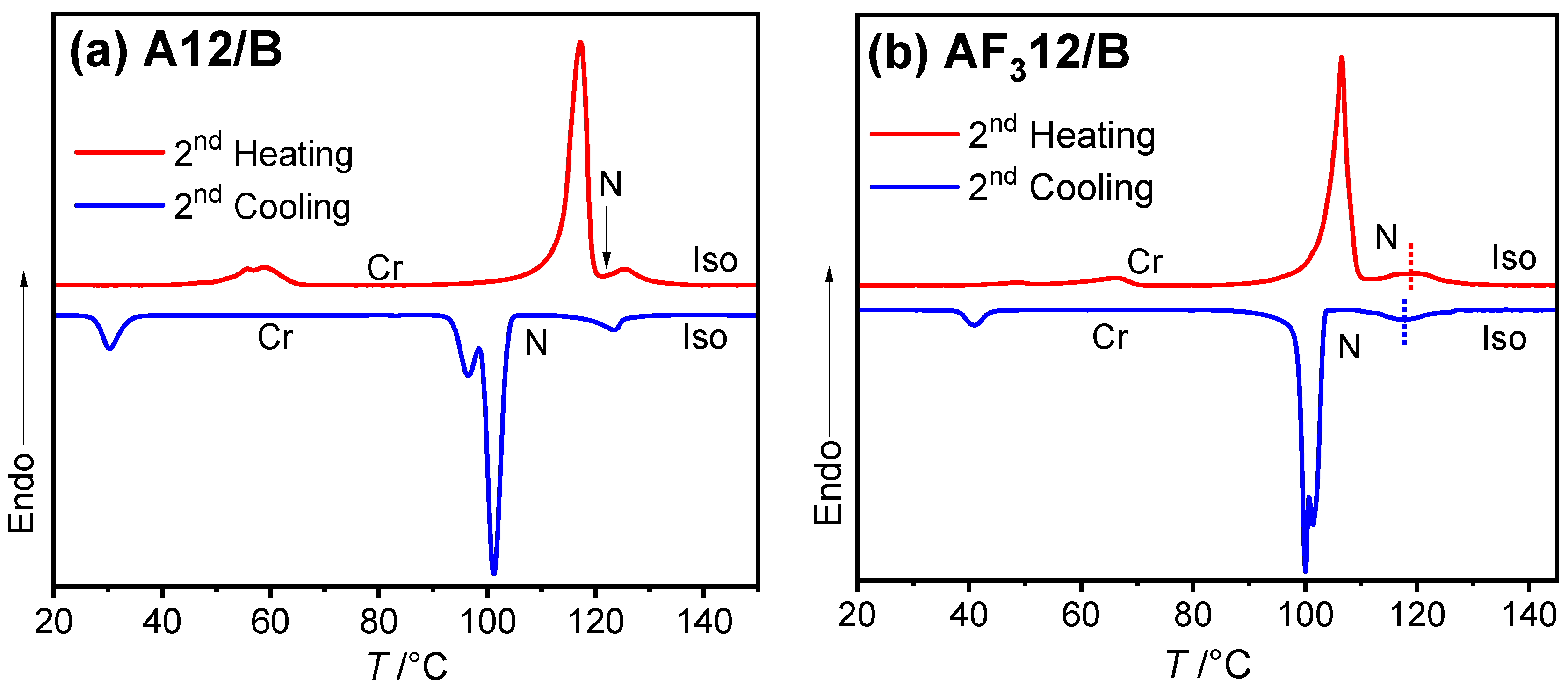
3.2.1. Nonfluorinated HBLCs (An/B)
3.2.2. Fluorinated HBLCs (AF3n/B, AF28/B, AF238/B)
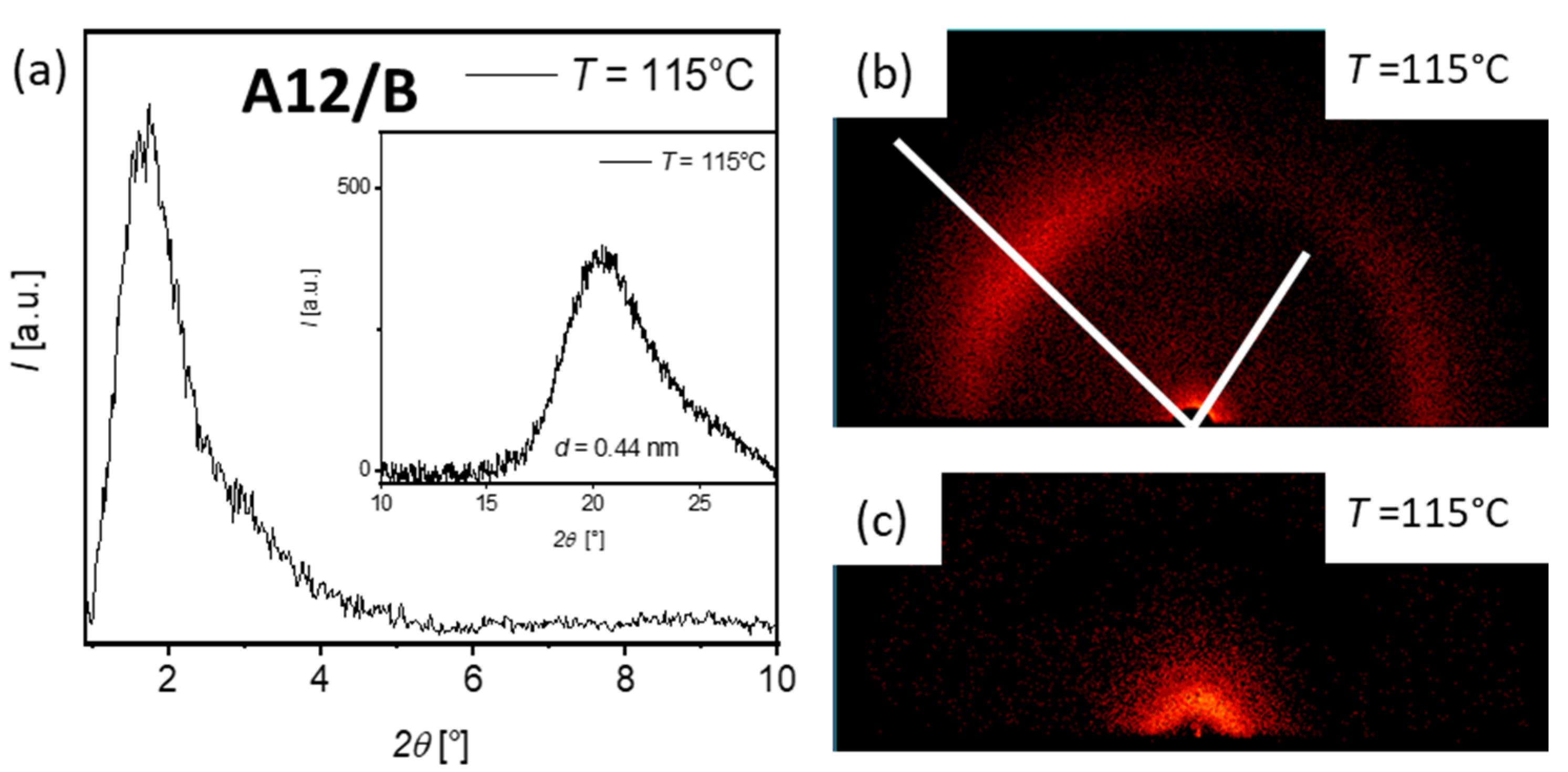
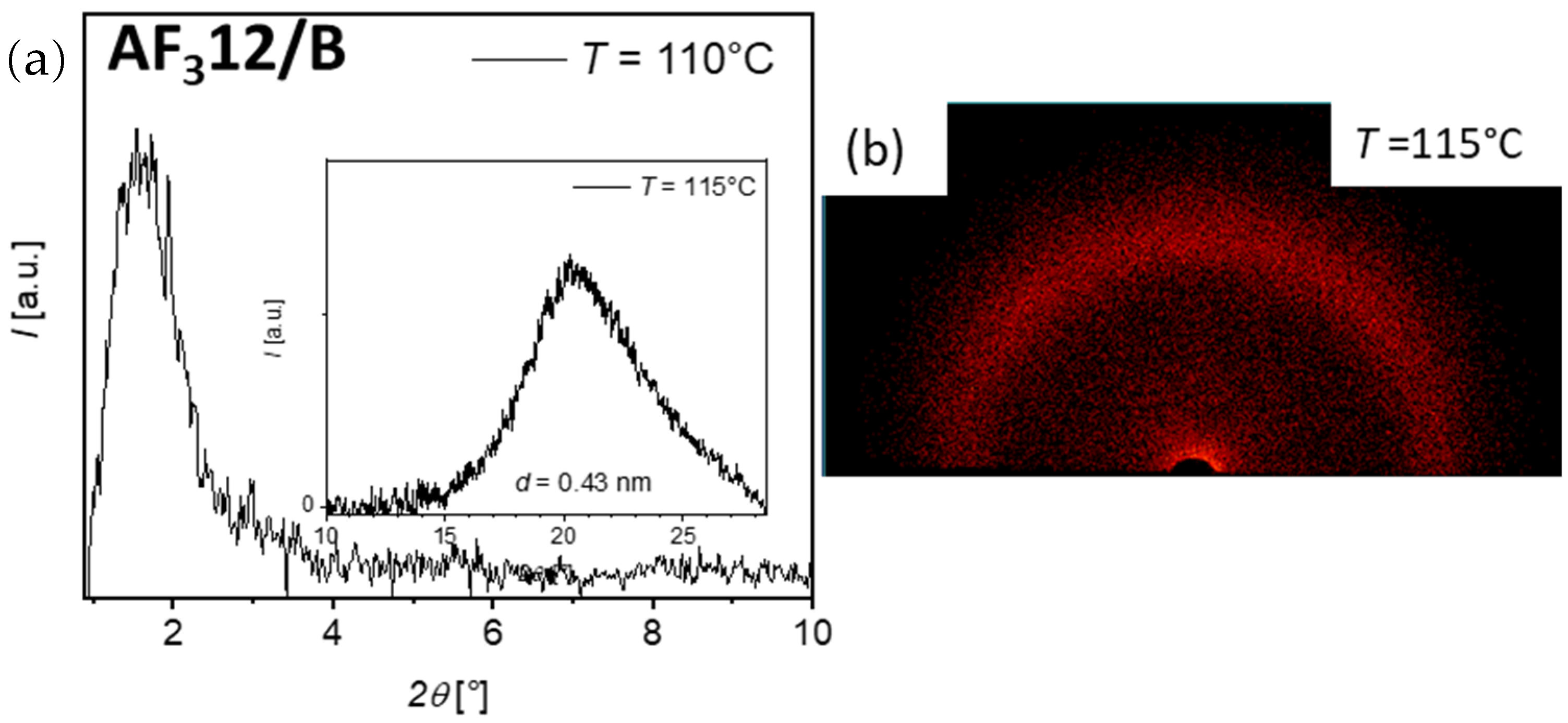
3.3. XRD Investigations
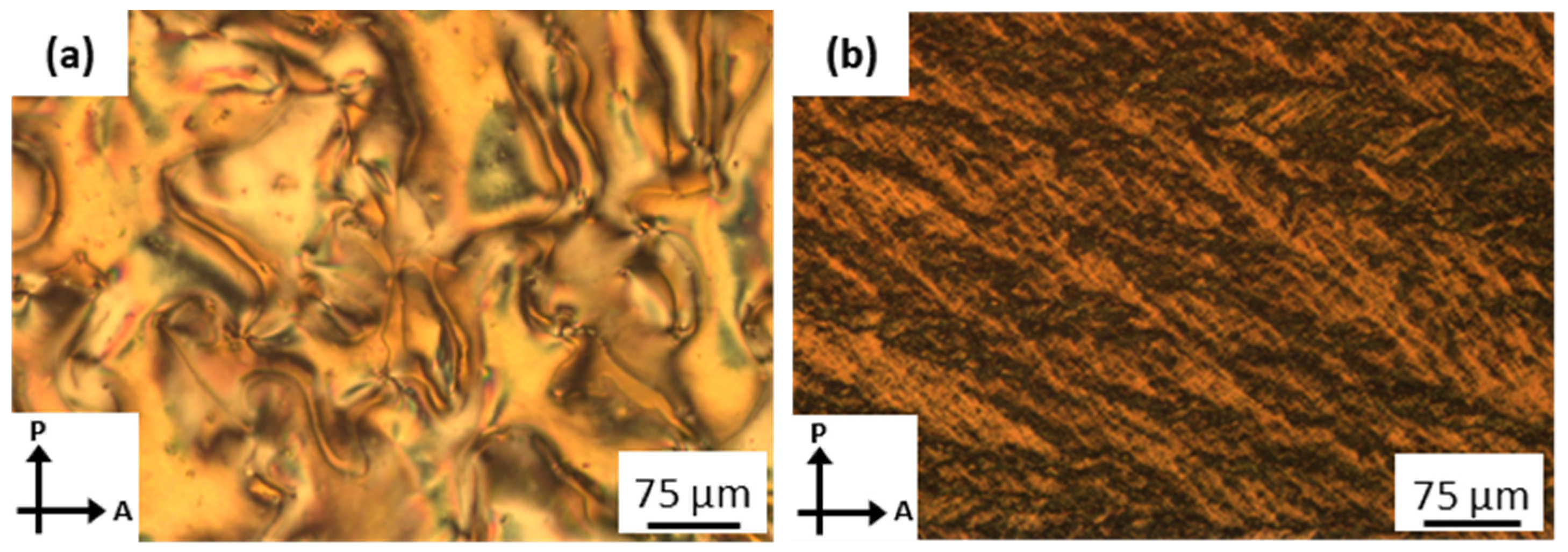
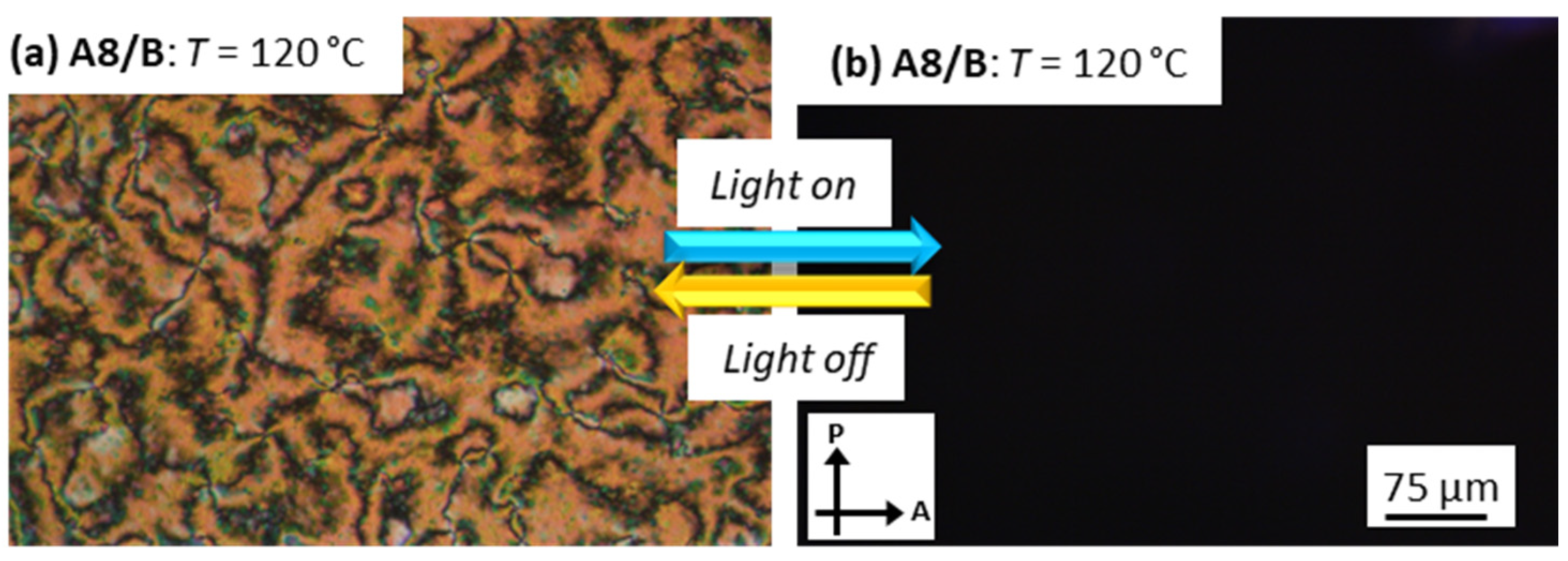
3.4. Photoisomerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887. [Google Scholar] [CrossRef]
- Buchs, J.; Vogel, L.; Janietz, D.; Prehm, M.; Tschierske, C. Chirality Synchronization of Hydrogen-Bonded Complexes of Achiral N-Heterocycles. Angew. Chem. 2017, 129, 286–290. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M.J. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Wallage, M.J.; Imrie, C.T. Supramolecular dimeric liquid crystals. The liquid crystalline behaviour of mixtures of alpha-(4-pyridyloxy)-omega-4-(4-butylphenylazo)phenoxy alkanes and 4-octyloxybenzoic acid. J. Mater. Chem. 1997, 7, 1163–1167. [Google Scholar] [CrossRef]
- Friot, B.; Boyd, D.; Willis, K.; Donnio, B.; Ungar, G.; Bruce, D.W. Hydrogen-bonded polycatenar mesogens. Liq. Cryst. 2000, 27, 605–611. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 5, pp. 513–540. [Google Scholar]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based Derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- de Oliveira, W.A.; Alaasar, M.; Cao, Y.; Westphal, E. 1,4-Bis(Acylhydrazone)-Based Polycatenar Liquid Crystals: Self-Assembly, Molecular Switching, and Gelation Properties. ACS Omega 2025, 10, 21637–21647. [Google Scholar] [CrossRef]
- Jansze, S.M.; Martínez-Felipe, A.; Storey, J.; Marcelis, A.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. 2015, 54, 643–646. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.P.; Martinez-Felipe, A.; Paterson, D.A.; Forsyth, E.; Lawrence, G.B.; Henderson, P.A.; Storey, J.M.D.; Gorecka, E.; et al. Hydrogen bonding and the design of twist-bend nematogens. J. Mol. Liq. 2020, 303, 112630. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Martinez-Felipe, A.; Storey, J.M.D.; Gorecka, E.; Imrie, C.T. Twist-Bend Nematogenic Supramolecular Dimers and Trimers Formed by Hydrogen Bonding. Crystals 2020, 10, 175. [Google Scholar] [CrossRef]
- Mandle, R. Supramolecular ferroelectric nematic materials. Liq. Cryst. 2022, 49, 2019–2026. [Google Scholar] [CrossRef]
- Alaasar, M.; Poppe, S.; Dong, Q.; Liu, F.; Tschierske, C. Mirror symmetry breaking in cubic phases and isotropic liquids driven by hydrogen bonding. Chem. Comm. 2016, 52, 13869–13872. [Google Scholar] [CrossRef]
- Alaasar, M.; Schmidt, J.-C.; Cai, X.; Liu, F.; Tschierske, C. Controlling liquid and liquid crystalline network formation by core-fluorination of hydrogen bonded supramolecular polycatenars. J. Mol. Liq. 2021, 332, 115870. [Google Scholar] [CrossRef]
- Alaasar, M.; Cai, X.; Kraus, F.; Giese, M.; Liu, F.; Tschierske, C. Controlling ambidextrous mirror symmetry breaking in photosensitive supramolecular polycatenars by alkyl-chain engineering. J. Mol. Liq. 2022, 351, 118597. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A. Liquid crystal dimers and higher oligomers: Between monomers and polymers. Chem. Soc. Rev. 2007, 36, 2096–2124. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A.; Yeap, G.-Y. Liquid crystal oligomers: Going beyond dimers. Liq. Cryst. 2009, 36, 755–777. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Y.; Tan, T.; Liu, F.; Alaasar, M. Manipulation of Supramolecular Chirality in Bicontinuous Networks of Bent-Shaped Polycatenar Dimers. Chem. Eur. J. 2025, 31, e202403586. [Google Scholar] [CrossRef]
- Zep, K.; Sitkowska, K.; Pociecha, D.; Górecka, E. Photoresponsive helical nanofilaments of B4 phase. J. Mater. Chem. C 2014, 2, 2323–2327. [Google Scholar] [CrossRef]
- Bandarab, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Alaasar, M. Azobenzene-containing bent-core liquid crystals: An overview. Liq. Cryst. 2016, 43, 2208–2243. [Google Scholar] [CrossRef]
- Alaasar, M.; Prehm, M.; Nagaraj, M.; Vij, J.K.; Tschierske, C. A liquid crystalline phase with uniform tilt, local polar order and capability of symmetry breaking. Adv. Mater. 2013, 25, 2186–2191. [Google Scholar] [CrossRef]
- Alaasar, M.; Poppe, S.; Dong, Q.; Liu, F.; Tschierske, C. Isothermal chirality switching in liquid-crystalline azobenzene compounds with non-polarized light. Angew. Chem. 2017, 56, 10801–10805. [Google Scholar] [CrossRef]
- Paterson, D.A.; Xiang, J.; Singh, G.; Walker, R.; Agra-Kooijman, D.M.; Martinez-Felipe, A.; Gan, M.; Storey, J.M.D.; Kumar, S.; Lavrentovich, O.D.; et al. Reversible isothermal twist-bend nematic-nematic phase transition driven by the photoisomerization of an azobenzene-based nonsymmetric liquid crystal dimer. J. Am. Chem. Soc. 2016, 138, 5283–5289. [Google Scholar] [CrossRef]
- Alaasar, M.; Schmidt, J.; Darweesh, A.F.; Tschierske, C. Azobenzene-based supramolecular liquid crystals: The role of core fluorination. J. Mol. Liq. 2020, 310, 113252. [Google Scholar] [CrossRef]
- Blanke, M.; Balszuweit, J.; Saccone, M.; Wölper, C.; Jiménez, D.D.; Mezger, M.; Voskuhl, J.; Giese, M. Photo-switching and -cyclisation of hydrogen bonded liquid crystals based on resveratrol. Chem. Commun. 2020, 56, 1105–1108. [Google Scholar] [CrossRef]
- Ren, H.; Yang, P.; Yu, H. Recent Progress in Azopyridine-Containing Supramolecular Assembly: From Photoresponsive Liquid Crystals to Light-Driven Devices. Molecules 2022, 27, 3977. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, M.; Siksnys, V. Molecular scissors under light control. Proc. Natl. Acad. Sci. USA 2010, 107, 1259–1260. [Google Scholar] [CrossRef]
- Lee, K.M.; White, T.J. Photomechanical Response of Composite Structures Built from Azobenzene Liquid Crystal Polymer Networks. Polymers 2011, 3, 1447–1457. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Castro, M.C.R.; Cuadrado, A.; Raposo, M.M.M.; Velasco, D. Molecular photo-oscillators based on highly accelerated heterocyclic azo dyes in nematic liquid crystals. Chem. Comm. 2014, 50, 6704–6706. [Google Scholar] [CrossRef] [PubMed]
- Fehrentz, T.; Schönberger, M.; Trauner, D. Optochemical Genetics. Angew. Chem. 2011, 50, 12156–12182. [Google Scholar] [CrossRef] [PubMed]
- Hird, M.; Goodby, J.W.; Lewis, R.A.; Toyne, K.J. The fascinating influence of fluoro substituents on the synthesis and properties of liquid crystals. Mol. Cryst. Liq. Cryst. 2003, 401, 1–18. [Google Scholar] [CrossRef]
- Kirsch, P.; Bremer, M. Understanding Fluorine Effects in Liquid Crystals. ChemPhysChem 2010, 11, 357–360. [Google Scholar] [CrossRef]
- Darweesh, A.F.; Anders, C.; Ranjitha, B.S.; Shanker, G.; Alaasar, M. On the impact of aromatic core fluorination in hydrogen-bonded liquid crystals. RSC Adv. 2025, 15, 6803–6816. [Google Scholar] [CrossRef] [PubMed]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Chem. Discotic liquid crystals: A new generation of organic semiconductors. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef]
- Baghla, A.; Sahai, M.; Yadav, N.; Gupta, S.P.; Punjani, V.; Manjuladevi, V.; Vij, J.K.; Pal, S.K. Unusual polar ordering and room-temperature blue phase stabilization in tetrafluorinated bent-shaped mesogens. Chem. Sci. 2025, 16, 8002–8013. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Z.; Pan, Q.; Liu, S.; Zhao, J. Effect of Fluorinated Substituents on Solubility and Dielectric Properties of the Liquid Crystalline Poly(ester imides). ACS Appl. Polym. Mater. 2022, 5, 141–151. [Google Scholar] [CrossRef]
- Gibb, C.J.; Hobbs, J.; Nikolova, D.I.; Raistrick, T.; Berrow, S.R.; Mertelj, A.; Osterman, N.; Sebastián, N.; Gleeson, H.F.; Mandle, R.J. Spontaneous symmetry breaking in polar fluids. Nat. Comm. 2024, 15, 5845. [Google Scholar] [CrossRef]
- Karcz, J.; Herman, J.; Rychłowicz, N.; Kula, P.; Gorecka, E.; Szydlowska, J.; Majewski, P.W.; Pociecha, D. Spontaneous chiral symmetry breaking in polar fluid–heliconical ferroelectric nematic phase. Science 2024, 384, 1096. [Google Scholar] [CrossRef]
- Shivakumar, K.I.; Pociecha, D.; Szczytko, J.; Kapuściński, S.; Monobe, H.; Kaszyński, P. Photoconductive bent-core liquid crystalline radicals with a paramagnetic polar switchable phase. J. Mater. Chem. C 2020, 8, 1083–1088. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C. Nematic phases driven by hydrogen-bonding in liquid crystalline nonsymmetric dimers. Liq. Cryst. 2019, 46, 124–130. [Google Scholar] [CrossRef]
- Alaasar, M.; Nirgude, T.; Anders, C. The influence of bromine substitution and linking groups on the phase behaviour of light-responsive rod-like molecules. J. Mol. Liq. 2024, 414, 126174. [Google Scholar] [CrossRef]

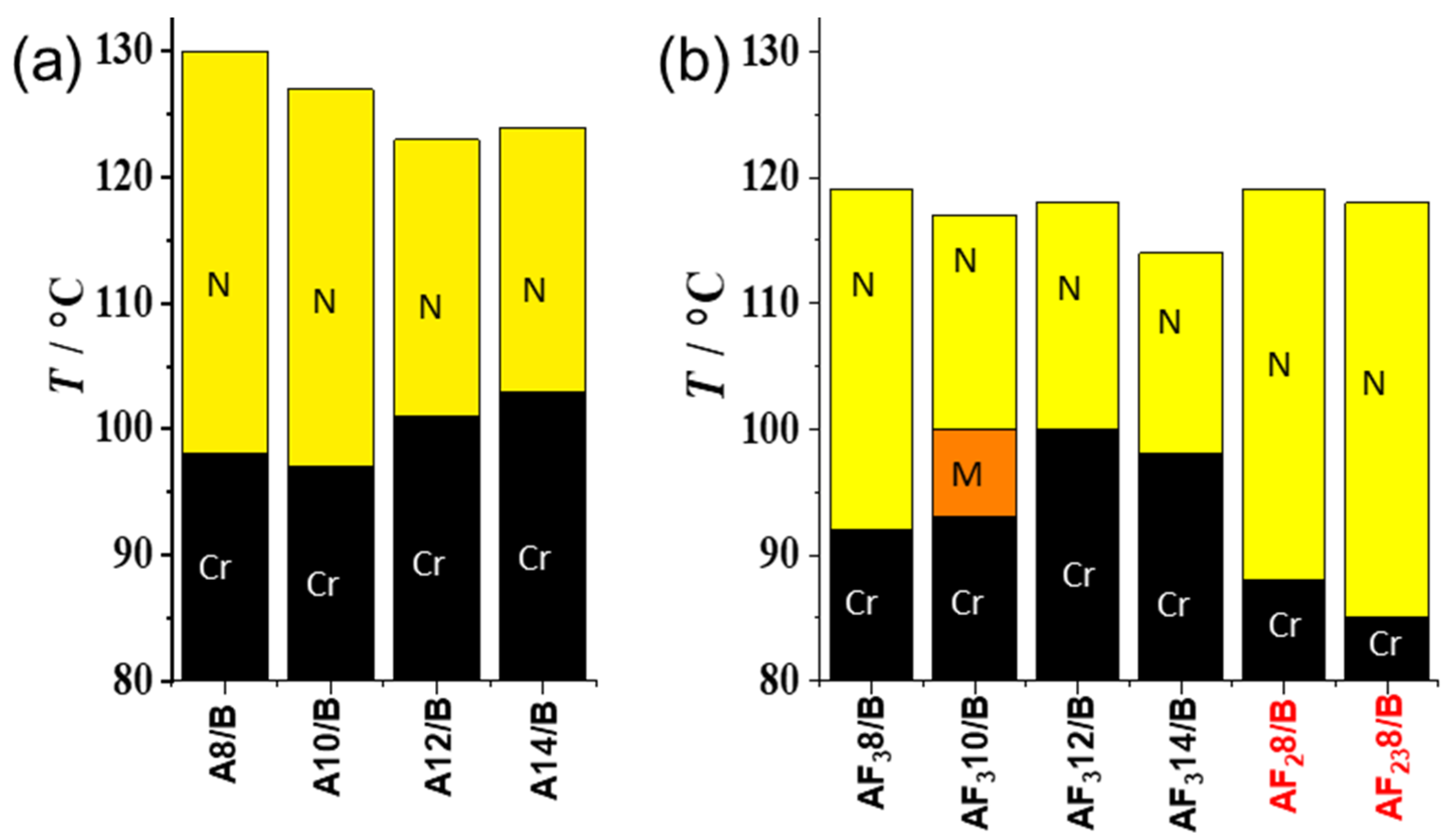
| Complex | n | Phase Sequence (T [°C]/∆H [kJ/mol]) |
|---|---|---|
| A8/B | 8 | H: Cr 115 [60.8] N 132 [5.1] Iso C: Iso 130 [−5.0] N 98 [−60.4] Cr |
| A10/B | 10 | H: Cr 111 [41.1] N 128 [3.6] Iso C: Iso 127 [−3.0] N 97 [−38.0] Cr |
| A12/B | 12 | H: Cr 117 [67.5] N 125 [3.0] Iso C: Iso 123 [−4.0] N 101 [−66.4] Cr |
| A14/B | 14 | H: Cr 118 [89.5] N 126 [6.5] Iso C: Iso 124 [−6.1] N 103 [−86.5] Cr |
| AF38/B | 8 | H: Cr1 104 [49.4] Cr2 110 [15.1] N 120 [3.3] Iso C: Iso 119 [−2.4] N 92 [−55.6] Cr |
| AF310/B | 10 | H: Cr 105 [58.7] N 117 [5.5] Iso C: Iso 117 [−2.6] N 100 [−0.5] M 93 [−58.2] Cr |
| AF312/B | 12 | H: Cr 107 [57.1] N 120 [3.2] Iso C: Iso 118 [−3.0] N 100 [−61.8] Cr |
| AF314/B | 14 | H: Cr 107 [67.5] N 115 [2.2] Iso C: Iso 114 [−2.4] N 98 [−67.2] Cr |
| AF28/B | 8 | H: Cr 96 [19.1] N 119 [1.2] Iso C: Iso 119 [−1.2] N 88 [−20.8] Cr |
| AF238/B | 8 | H: Cr 94 [48.4] N 120 [4.2] Iso C: Iso 118 [−3.5] N 85 [−46.0] Cr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anders, C.; Abu Bakar, M.; Nirgude, T.; Alaasar, M. Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers. Crystals 2025, 15, 576. https://doi.org/10.3390/cryst15060576
Anders C, Abu Bakar M, Nirgude T, Alaasar M. Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers. Crystals. 2025; 15(6):576. https://doi.org/10.3390/cryst15060576
Chicago/Turabian StyleAnders, Christian, Muhammad Abu Bakar, Tejal Nirgude, and Mohamed Alaasar. 2025. "Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers" Crystals 15, no. 6: 576. https://doi.org/10.3390/cryst15060576
APA StyleAnders, C., Abu Bakar, M., Nirgude, T., & Alaasar, M. (2025). Nematic Phases in Photo-Responsive Hydrogen-Bonded Liquid Crystalline Dimers. Crystals, 15(6), 576. https://doi.org/10.3390/cryst15060576







