Abstract
Green coating research and development has taken a new turn in recent years because of the combination of nanomaterials and anticorrosive and antifouling coatings. Because of its distinct physicochemical characteristics, graphene, a novel two-dimensional material, exhibits significant promise in anticorrosive and antifouling coatings. The fundamental characteristics of graphene are presented in this paper along with an overview of its uses in anticorrosive films, anticorrosive coatings, and antifouling coatings. The mechanism underlying graphene anticorrosive and antifouling coatings is also presented, along with the difficulties associated with them and their potential future development. It seeks to serve as a resource for the study and use of anticorrosion and antifouling coatings based on graphene.
1. Introduction
Anticorrosion and antifouling coatings are becoming more and more common in various types of infrastructure, particularly in marine, chemical, construction, and other industries, as industrialization continues to pick up speed. The performance of conventional anticorrosion and antifouling coatings is frequently limited by the material itself, and it is challenging to satisfy the ever-tougher criteria of use, even though they can somewhat withstand biological adhesion and environmental corrosion. The environment and human health will also be harmed by the formaldehyde, toluene diisocyanate (TDI), and volatile organic compounds (VOCs) found in conventional anticorrosion coatings. Most antifouling coatings can inhibit or kill marine organisms attached to the hull surface by adding heavy metals like copper, tin, and mercury to the hull surface, according to pertinent studies [1,2]. This can seriously harm the marine ecological environment and harm human health. Consequently, the creation of novel high-performance coatings has emerged as a focus of research.
In recent years, integrating nanomaterials and antifouling coatings has opened up a new path for researching environmental protection coatings [3,4,5,6,7]. As a new type of two-dimensional nanomaterial, graphene has a stable chemical structure, large specific surface area, good electrical conductivity, and extremely low permeability [8,9,10,11], showing great application potential in the field of coatings. For example, the high specific surface area and excellent dispersion of graphene enable it to effectively enhance the densification of the coating and reduce the permeability of water and oxygen, thus improving the corrosion resistance of the coating. In terms of antifouling, the excellent antibacterial properties of graphene also provide a new idea for the development of antifouling coatings.
Although the application of graphene in anticorrosion and antifouling coatings has made some progress, there are still some technical challenges, such as the dispersion of graphene and the compatibility with the base material, which need to be solved [12]. Therefore, this paper aims to review the progress of graphene in anticorrosive and antifouling coatings and discuss its application status, challenges, and future development direction to provide a reference for related research.
2. The Basic Properties of Graphene
Graphene, essentially a graphite sheet, is a new carbon material with a single-layer, two-dimensional honeycomb crystal structure formed by the close packing of carbon atoms and is the basic unit for the construction of carbon materials such as zero-dimensional fullerenes, one-dimensional carbon nanotubes, and two-dimensional graphite [13,14]. The carbon atoms in graphene are arranged by sp2 hybridization to form a hexagonal honeycomb two-dimensional structure. Among them, the carbon–carbon bond length is about 0.142 nm, and the single-layer graphene exhibits an atomic thickness of 0.335 nm, which is the finest material found in the field of nanomaterials so far [15]. As a two-dimensional crystal with a single atomic layer, carbon atoms in graphene are bonded to adjacent carbon atoms by sp2 hybridization to form regular hexagons, as shown in Figure 1.
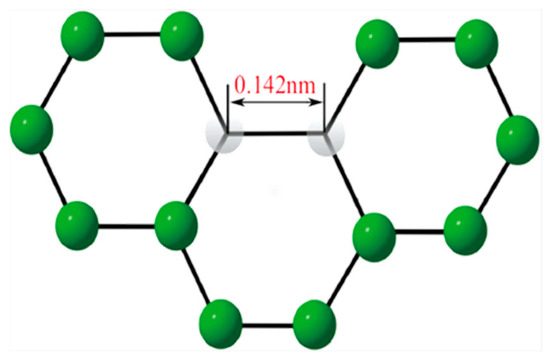
Figure 1.
The cellular crystal structure of carbon atoms in graphene [16].
Graphene has a very high specific surface area [17], which can form a dense barrier in the paint, effectively blocking corrosive substances such as oxygen, water, and salt ions, and improving the corrosion resistance of the coating. As one of the strongest materials known, graphene has a strength of up to 130 GPa [18], which significantly improves the wear and durability of coatings. In addition, the chemical properties of graphene are very stable, do not easily react with other substances, and can maintain the best performance in a variety of complex environments, such as moisture and salt spray, which is crucial to improving the chemical resistance of coatings. On the other hand, the extremely high surface energy and good hydrophobic effect of graphene make it difficult for microorganisms to grow or adhere to its surface, showing an excellent antifouling effect. Some studies have found that graphene has an inhibitory effect on some bacteria and microorganisms [19], making it a potential application in the field of antifouling coatings.
In terms of REDOX properties, the unique electronic structure of graphene endows it with excellent electrochemical activity. Due to it being a zero-bandgap semimetallic material [20], the delocalized large π bond system formed by its sp2 hybridization (non-intermolecular π-π stacking interaction) has excellent electron transport capacity [21], and the electron mobility can reach 2.5 × 105 cm2 V−1 s−1 at room temperature. This efficient electron migration characteristic can uniformly disperse the local microcurrent in the coating, thereby inhibiting the formation of electrochemical corrosion micro-cells on the surface of the metal substrate [22]. Studies have shown that the high electrical conductivity of graphene can also promote the rapid formation of a passive film on the metal surface, further isolating the corrosive medium from direct contact with the metal [23]. In addition, the active sites at the edge of graphene (such as defects, dangling bonds, etc.) can generate oxygen-containing functional groups such as hydroxyl groups (-OH), epoxy groups (-O-), and carboxyl groups (-COOH) under controllable oxidation conditions, providing a chemical basis for functional modification. Taking the preparation of graphene oxide (GO) by the Hummers method as an example, although the polar groups enriched on the base surface and edges destroy the π-conjugated structure, resulting in a decrease in conductivity, the abundant oxygen-containing groups significantly enhance the interfacial bonding force with the resin matrix. Although reduced graphene (rGO) removes most of the oxygen-containing functional groups through chemical or thermal reduction, restoring a highly conjugated sp2 carbon network, the polar groups remaining at its edges and defects (such as carbonyl C=O) can still serve as catalytic sites for REDOX reactions, promoting electron transfer at the coating–metal interface. It is worth noting that by regulating the REDOX degree of graphene (such as controlling the residual proportion of oxygen-containing functional groups during the reduction process), its hydrophilicity and hydrophobicity, electrical conductivity, and the density of active sites can be precisely adjusted. GO—with a high degree of oxidation—is more easily dispersed in polar resins, enhancing the adhesion of the coating, while rGO—with a low degree of oxidation—retains higher electrical conductivity, strengthening the inhibition of corrosion micro-cells. This adjustable REDOX property provides a key idea for designing anticorrosion coatings that have both a high interfacial bonding force and electrochemical protection ability.
Although graphene has shown excellent performance in the field of anticorrosion and antifouling coatings, there are still some challenges. Due to van der Waals forces, graphene sheets easily agglomerate and precipitate, which affects their dispersion in composite materials and ultimately compromises the stability of the paint [24]. In addition, graphene’s high stability and strong hydrophobicity limit its dispersion efficiency and degree of functionalization. To solve these problems, researchers pretreated graphene before coating preparation and prepared graphene composites using a variety of modification techniques [25,26,27,28,29,30]. Graphene-modified nanoparticles and resin-loaded graphene composites can be obtained through modification, which can effectively overcome the defects of graphene coatings and significantly improve the dispersion and functionalization level of graphene.
3. The Application of Graphene in Anticorrosive Coatings
Anticorrosive coatings are mainly used to protect marine engineering structures, such as ships, docks, offshore bridges, offshore drilling platforms, and other steel facilities, to prevent or reduce the corrosion of metals and other materials in the marine environment, and to protect the service life of engineering structures. There are two main ways to apply graphene in the field of anticorrosion. One is to directly deposit single- or multi-layer graphene films on the surface of metal substrates as barrier coatings. The second is to add graphene and its derivatives to the organic polymer matrix as functional fillers and to distribute them in the organic coating through dispersion to form graphene/polymer composite anticorrosive coatings.
3.1. Graphene Anticorrosive Film
When a continuous graphene film is used to protect the metal, the graphene film can be regarded as a molecular diffusion barrier, forming an effective isolation layer between the metal surface and the corrosive medium, reducing the corrosion rate of the metal. At the same time, graphene is cross-arranged in the coating to form a dense grid-like structure, forming a complex hydrophobic network, which can effectively inhibit the diffusion path of corrosive media and prevent the penetration of corrosive media. At present, the main methods for preparing graphene films on metal surfaces include chemical vapor deposition (CVD), mechanical transfer, and electrophoretic deposition.
3.1.1. Chemical Vapor Deposition (CVD) Method
The graphene film prepared by the CVD method has good quality and few defects, and can directly grow a continuous, large area of graphene film on the surface of Cu, Ni, and other metals [31,32], showing great application potential in the field of anticorrosion film.
The Cu-based CVD method was invented by Chen et al. [33]. It is currently the most common method for the large-area preparation of graphene films. In the 2011 study [34], the team successfully prepared graphene films on the surface of Cu and Cu/Ni alloys using the CVD method for the first time, effectively preventing the erosion of Cu and Cu/Ni alloy substrates by air oxidation. In this experiment, the researchers heated a graphene-coated metal foil with an uncoated metal foil in 200 °C air for four hours. The results showed that the composition of the metal foil coated with the graphene film remained stable, confirming the excellent performance of the graphene film in protecting the metal from oxidation (Figure 2). This study marks the first application of graphene films prepared by the CVD method in the field of metal corrosion protection.
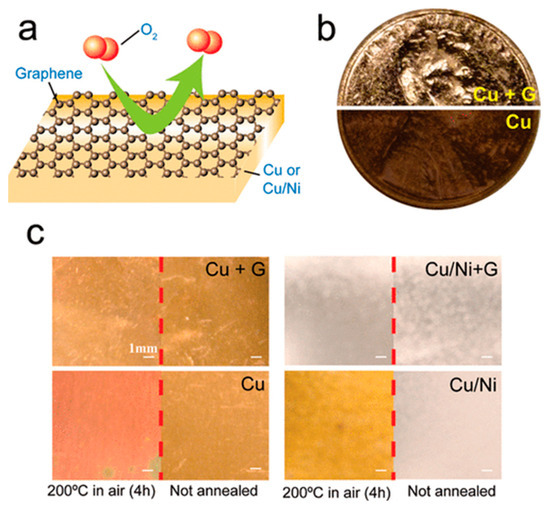
Figure 2.
Anticorrosive properties of graphene films [34]. (a) Illustrations describing graphene sheets as chemically inert diffusion barriers. (b) Coins coated with graphene (top) and uncoated with graphene (bottom) after H2O2 treatment (30%, 2 min). (c) Photographs of Cu and Cu/Ni foils without graphene coating taken before and after annealing in air (200 °C, 4 h).
The CVD method can be carried out directly on the metal surface, and the prepared graphene film is not only of high purity but also the preparation process is simple, allowing the direct growth of graphene on different substrates for the corrosion protection of metals. Raman and his team [35] used the CVD method and compared the properties of coated and uncoated Cu samples by electrochemical testing. The results showed that the electrochemical degradation resistance of Cu was increased by 1.5 orders of magnitude, and the anodic dissolution of Cu was effectively inhibited, which provided good protection for the substrate. Prasai et al. [36] used electrochemical testing methods to explore the effect of graphene films on Cu and Ni corrosion inhibition. The results show that the corrosion rate of Cu in oxygenated Na2SO4 solution is reduced by 7 times by the graphene film grown by the CVD method, and the corrosion rate of Ni is reduced by 20 times by the multi-layer graphene film grown on Ni. Pu et al. [37] grew graphene on SUS304 stainless steel and Ni/SUS304 double-layer structure by the CVD method and studied the effect of graphene coating on improving the corrosion resistance of stainless steel. In the brine polarization experiment of 3.5 wt%, the corrosion current density of the uncoated sample was 5 times higher than that of the graphene-coated SUS304 stainless steel. In addition, the Ni layer as a buffer layer can effectively reduce the diffusion of carbon atoms to the stainless steel matrix, catalyze the formation of graphene, and help the graphene to completely cover the stainless steel surface, thus providing better corrosion resistance.
3.1.2. Mechanical Transfer Method
For metal substrates that lack catalytic activity, such as Au and Ag, they cannot effectively promote the decomposition of carbon source gas and the growth of graphene, so graphene cannot be prepared directly by the CVD method [38,39].
To solve this problem, the researchers used mechanical transfer technology to transfer graphene films prepared on Cu, Ni, and other metal surfaces to the target metal. Zhao et al. [40] prepared a single-layer graphene film on copper foil by the CVD method, and successfully transferred the film to the surface of the Ag film with the help of polymethyl methacrylate (PMMA)-assisted mechanical transfer technology. Tafel’s polarization analysis showed that the corrosion rate of the graphene-coated Ag film was reduced by 66.3 times, effectively inhibiting the reaction of the anode and cathode. Figure 3 visually shows that the graphene protective layer significantly enhances the corrosion resistance of Ag films to gases and liquids. Zheng et al. [41] proposed to prepare a hydrophobic graphene coating on an Al alloy substrate. The method is a combination of biomimetic microstructure treatment and the CVD method to prepare and transfer graphene films, which significantly improves the hydrophobicity and anticorrosion properties of Al alloy.
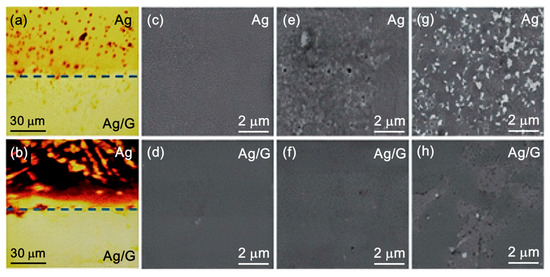
Figure 3.
Optical images of a Ag film half-coated with graphene [40]. (a) After heat treatment in air at 150 °C for 5 h; (b) after dipping in 34.5% H2O2 aqueous solution for 2 min—SEM images of the bare Ag film (Ag) and the Ag film with a graphene coating (Ag/G); (c,d) pristine film; (e,f) after heat treatment in air at 150 °C for 5 h; (g,h) after dipping in 34.5% H2O2 aqueous solution for 2 min.
3.1.3. Electrophoretic Deposition
Compared to the CVD process for graphene preparation, electrophoretic deposition has shown a wide range of applicability to both metallic and non-metallic substrates.
The technique uses the direct current electric field effect to induce graphene to form a deposited film on the metal surface. Mallick et al. [42] successfully prepared graphene anticorrosion coating on Nitinol substrate by cathodic electrophoretic deposition technology and thoroughly studied the corrosion resistance of the coating after deposition and annealing treatment. The experimental results showed that the corrosion potential of deposited and annealed samples increased by 135 mV and 249 mV, respectively, compared with the uncoated Nitinol substrate, which confirmed that the corrosion resistance of graphene films to the Nitinol substrate was significantly improved. Hares et al. deposited graphene oxide films on the surface of copper tubes by electrophoretic deposition technology to enhance their corrosion resistance [43]. At 20 V voltage and 60 s deposition time, 0.5 mg/mL GO concentration can form a uniform and continuous graphene film on the surface of the copper tube. The corrosion current density of GO-treated copper pipes in 3.5 wt% NaCl corrosion solution is significantly reduced, and their corrosion resistance is twice that of untreated copper pipes. Overall, electrophoretic deposition is more universal and cost-effective than CVD for substrates, but there are still challenges in achieving large-scale, large-area, high-quality graphene preparation.
3.1.4. Defects and Improvement of Graphene Films
In theory, the perfect graphene film can completely block the corrosive medium (Figure 4), but the existing graphene film preparation technology cannot avoid the production of defects. In addition, researchers have found that defective graphene films can even exacerbate metal corrosion [44,45,46,47,48]. When the graphene film on the metal surface is defective, the graphene at the defect forms a corrosion micro battery with the exposed metal, and the metal acts as an anode to promote the electrochemical reaction, thereby accelerating the corrosion rate of the metal (Figure 5).
Figure 4.
The barrier effect of graphene films on corrosive media [44].

Figure 5.
The defects in the graphene film promote corrosion of the metal [44].
To solve the problem of corrosion acceleration faced by the application, the researchers have improved it and achieved remarkable results. Hsieh et al. [49] found that the nanoscale structural defects in graphene prepared by the CVD method were the key factors leading to its inadequate protection performance. By adjusting the particle size generated by atomic layer deposition, selective passivation of defects is achieved, which can effectively inhibit the penetration of liquids through these structural defects and thus improve the corrosion resistance of graphene films. On the other hand, Anisur et al. [50] pointed out that during the preparation of graphene films by CVD, the hydrogen environment and cooling rate have significant effects on the defect density and barrier properties of the coatings. Slow cooling conditions will inhibit the formation of graphene films, while fast cooling conditions are conducive to the growth of graphene. Electrochemical tests further showed that under the condition of rapid cooling without hydrogen flow, the formation of film wrinkles could be reduced. Even after soaking in 0.1 mol/L NaCl solution for 1008 h, the sample could still maintain excellent corrosion resistance, significantly improving the durability of the coating.
In summary, graphene anticorrosive film has shown significant application value in the field of metal protection, mainly as an efficient protective barrier of the metal substrate, by effectively slowing the corrosion rate of metal and blocking the penetration path of corrosive media, showing an extraordinary anticorrosion effect. Graphene anticorrosive films not only significantly enhance the corrosion resistance of metals, but also effectively hinder the diffusion of corrosive media by their excellent hydrophobic properties and dense grid structure, providing an innovative solution for the long-term anticorrosion of metal structures such as marine engineering facilities.
3.2. Graphene Composite Anticorrosion Coatings
Compared with graphene anticorrosive film, graphene organic composite anticorrosive coating can not only take into account the excellent permeability resistance, chemical stability, and mechanical properties of graphene but also accounts for the strong adhesion and film formation of organic resin to metal matrix, which has a good synergistic effect. Polymers commonly used in anticorrosive coatings include epoxy resin [51,52,53,54,55], zinc-rich coatings [56,57,58], polyaniline [59,60], polyurethane [61,62,63,64], acrylic resin [65,66,67], polypyrrole [68,69], etc. The main preparation methods include in situ polymerization, solution blending, and melt blending.
3.2.1. Graphene/Epoxy Anticorrosion Coatings
As a high-performance anticorrosive coating, epoxy resin anticorrosive coating is widely used in the field of industrial anticorrosion because of its ability to effectively block water and corrosive media, and resist acid, base, and specific solvent erosion. However, these coatings have some limitations in heat resistance, wear resistance, and impact resistance, which may lead to a decline in anticorrosion properties. By adding graphene-based materials to epoxy resin coatings, the mechanical strength and thermal stability of the coatings can be significantly enhanced, and the corrosion resistance can be effectively improved.
There are van der Waals forces and π-π stacking effects inside the graphene material, and when individual graphene oxide is added to the epoxy resin, they often clump together, failing to achieve the desired anticorrosion effect. To solve this problem, the researchers modified the surface of graphene oxide using organic compounds to improve its dispersion in epoxy resins. Ramezanzadeh et al. [27] functionalized GO using p-phenylenediamine (PPDA) to enhance the interaction between GO sheets and epoxy resin. Functional graphene oxide (FGO) was dispersed in the epoxy coating by the wet transfer method to prepare FGO/EP composites. The experiment showed that the introduction of amino functional groups improved the compatibility and dispersion of GO in the epoxy resin matrix, thus enhancing the thermal stability and mechanical properties of the coating, and effectively slowing down the penetration of corrosive electrolyte into the steel substrate. This study provides a scientific basis for the development of new, high-performance anticorrosive coating materials and has important significance for improving the durability and reliability of coatings. Ding et al. [70] synthesized a novel hydroxyl epoxyphosphate monomer (PGHEP), which achieved efficient dispersion of graphene in waterborne epoxy resin through π-π interaction. This system breaks through the damage to the conjugated structure of graphene caused by traditional covalent modification. The obtained PGHEP-G/epoxy composite coating shows excellent anticorrosion performance in the 0.5 wt% PGHEP-G coating. Its minimum frequency impedance modulus reaches 8.25 × 105 Ω·cm2, which is increased by more than 10 times compared with the pure epoxy coating. The corrosion products decreased significantly after 360 h of the salt spray test. The phosphate groups of PGHEP can form a passivation film on the metal surface. Combined with the physical barrier effect of graphene, it effectively inhibits the penetration of corrosive media. In terms of composite modification, Yang et al. [71] functionalized graphene (PTCA-G) with 3,4,9, 10-perylene tetraformic acid (PTCA), achieving the synergistic regulation of π-π interaction and hydrophobic effects. Fourier Transform Infrared spectroscopy (FT-IR) confirmed that PTCA molecules were adsorbed on the surface of graphene in a vertical configuration, forming a dense hydrophobic layer. SEM/TEM showed that PTCA-G had a layered structure and was uniformly dispersed (Figure 6). When the volume ratio of PTCA to graphene is 10:4, the impedance value of the epoxy coating containing PTCA-G reaches 6.776 × 104 Ω·cm2, which is nearly 19 times higher than that of the pure epoxy coating. This performance improvement can be attributed to the excellent dispersion and barrier effect of PTCA-G, providing a new strategy for the design of high-performance anticorrosion coatings. The modified system of benzyl glycidyl ether (BGE) proposed by Duan has initiated a new modification idea of “dual effects with one dose” [72]. BGE molecules not only act as steric hindrance agents to prevent graphene agglomeration, but also can form chemical cross-linking with the resin matrix through epoxy groups. This system not only achieves the perfect dispersion of graphene but also introduces a unique “internal anti-plasticization” effect, significantly enhancing the performance of the composite materials and providing a simple and efficient approach for the preparation of high-performance graphene/epoxy nanocomposites.
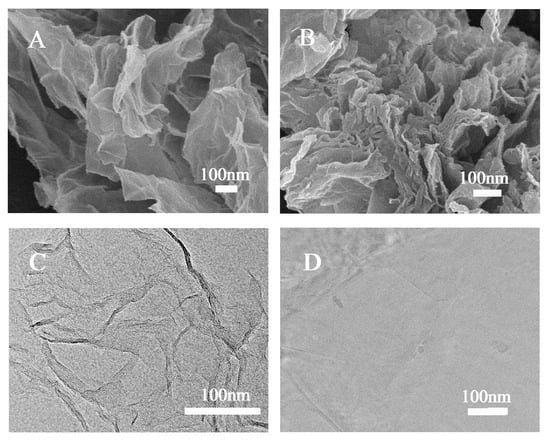
Figure 6.
SEM (A) and TEM (C) images of graphene and SEM (B) and TEM (D) images of PTCA-G composites [71].
Wang et al. [51] fabricated graphene/epoxy resin (OG/EP) composite coatings with highly oriented structures by spin coating. These coatings demonstrated outstanding anticorrosion performance in harsh corrosive environments. EIS tests showed that after being immersed in 3.5 wt% NaCl solution for 60 days, the |Z|0.01Hz values of OG/EP coatings containing 0.5 wt% and 1 wt% graphene significantly increased: the OG-0.5/EP coating rose from the initial 7.26 × 1010 Ω·cm2 to 1.23 × 1011 Ω·cm2, while the OG-1/EP coating increased from 1.73 × 1011 Ω·cm2 to 1.81 × 1011 Ω·cm2, both significantly outperforming the randomly arranged graphene/epoxy resin (RG/EP) coatings and pure epoxy resin (Spin-EP, Spray-EP) coatings. Experimental analysis revealed that the key mechanism for this performance enhancement lies in the unique horizontal orientation structure of graphene nanosheets in the OG/EP coatings (Figure 7). This structure fundamentally inhibits the formation of conductive networks by preventing direct contact between graphene sheets and the interface connection between graphene and the metal substrate. At the same time, this oriented arrangement maximizes the physical barrier effect of graphene against corrosive media, effectively restricting electron transfer and suppressing the anodic oxidation of the steel substrate and the cathodic oxygen reduction reaction. Ma et al. [52] prepared graphene oxide by closed oxidation method and dispersed the aqueous solution of graphene oxide in epoxy resin by wet transfer technology to prepare GO/EP anticorrosion coatings. The experimental data showed that after soaking in a 3.5% NaCl solution for 20 days, the surface of the GO/EP coating was only slightly roughened. The addition of GO not only improves the hydrophobicity and stability of the epoxy resin coating but also reduces the oxygen and water permeability of the GO/EP coating by 51.2% and 65.5%, respectively, showing excellent anticorrosion properties. To further exploit the anticorrosive potential of graphene in coatings, Zhao et al. designed a biomimetic graphene–epoxy resin (B-G-EP) coating with a pearl layer structure [53]. Through flow-induced orientation technology, the graphene and epoxy resin chains are carefully assembled into a layered structure to ensure that the graphene is effectively coated by the epoxy resin. Electrochemical impedance spectroscopy analysis shows that the low-frequency modulus of the bionic composite coating is 25 times higher than that of the pure epoxy resin coating, and can still maintain a high impedance value after long-term immersion, and the corrosion resistance is significantly better than that of the pure epoxy resin coating and the randomly distributed graphene–epoxy coating.
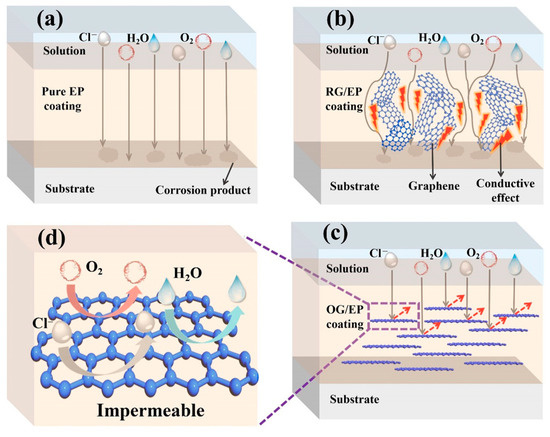
Figure 7.
Anticorrosion mechanisms of different coatings: (a) pure EP coating; (b) RG/EP coating; (c) OG/EP coating; (d) magnified view of OG/EP coating [51].
Water-based epoxy (WEP) coatings meet the requirements of environmental friendliness and corrosion resistance. However, because water-based epoxy coatings contain different dispersants and hydrophilic functional groups, there may be holes and defects in the film formation process, resulting in poorer long-term corrosion resistance than organic epoxy coatings. To solve this problem, Wang et al. [54] tried to add non-toxic nano-fillers to enhance the barrier effect and anticorrosion properties of the epoxy coating. Dopamine-modified mesoporous titanium dioxide (meso-TiO2) particles were introduced to enhance their compatibility with a water-based epoxy matrix. Electrochemical impedance spectroscopy (EIS) and salt spray test results showed that the coatings containing 1.0 wt% dopamine–mesoporous titanium dioxide showed the best corrosion resistance. On the other hand, He et al. [55] proposed to optimize the corrosion resistance of water-based epoxy/graphene coatings by combining non-covalent and covalent bonds, wherein graphene modified by non-covalent bonds can exhibit excellent dispersion properties in water and resin substrates.
3.2.2. Graphene/Zinc-Rich Anticorrosion Coatings
Zinc-rich coating is a kind of coating with excellent anticorrosion properties. The core of its anticorrosion mechanism is mainly the sacrificial anode effect of zinc powder, which provides effective cathodic protection for metal substrates such as steel. Therefore, the mass content of zinc powder in zinc-rich coatings is usually higher, up to more than 80% [73]. High zinc powder content will increase the porosity of the coating, resulting in a decrease in adhesion between the coating and the substrate, thus reducing the corrosion resistance of the coating.
Adding graphene not only improves the anticorrosion effect of zinc-rich epoxy coatings, but also a trace amount of graphene can significantly reduce the amount of zinc powder used, effectively reduce dust pollution in the construction process, and meet the market demand for the development of lightweight coatings. Jia et al. [57] prepared a graphene-modified, zinc-rich epoxy primer and studied its salt spray resistance. The results show that the graphene-modified, zinc-rich epoxy coating can significantly reduce the amount of zinc powder and improve the utilization rate of zinc powder. Under the condition that the amount of graphene is 0.75% and the amount of zinc powder is 35%, after a 3000 h salt spray test, the corrosion expansion width of the scratch is less than 1.5 mm, showing the best salt spray resistance. Graphene is easy to agglomerate in zinc-rich anticorrosive coatings, so solving the dispersion problem of graphene becomes the key to improving the anticorrosive properties of coatings. To solve this problem, Yang et al. [59] improved the dispersion of graphene in zinc-rich epoxy coatings by modifying ionic liquids. The 1-(2-aminoethyl) -3-methylimidazole bromide ionic liquid was modified with γ-Glycidyl Ether Oxypropyl Trimethoxysilane (KH560), and the modified ionic liquid was grafted on the surface of zinc powder. The effective dispersion of graphene in zinc-rich primer was realized through the adsorption of the ionic liquid. The results show that the corrosion resistance of 40% zinc-content graphene anticorrosive coating prepared by the dispersion method is equivalent to that of 80% zinc content, and its acid resistance and alkali resistance are better than that of 80% zinc-rich primer. The effect of graphene dispersion on the anticorrosion property of coatings was verified. The effective dispersion of graphene in zinc-rich primer was realized by the adsorption of ionic liquid.
3.2.3. Graphene/Polyaniline Anticorrosion Coatings
Polyaniline (PANI), as an electrically active polymer material [74], can effectively inhibit metal corrosion through an electrochemical reaction. However, PANI is less soluble and dispersible in coatings, and additional steps may be required to improve its processability and adhesion during synthesis and application. To further improve the anticorrosion properties and mechanical strength of PANI, composites can be formed with other materials, such as clay or graphene.
Chang et al. [59] combined 4-amino-benzoyl groups with graphene by an acylation reaction, and then combined functional graphene with aniline monomer by a polymerization reaction to prepare a new anticorrosion coating based on polyaniline/graphene composite material. Electrochemical tests show that the coated steel has higher corrosion potential and lower corrosion current density. Compared with clay, uniformly dispersed graphene has a higher aspect ratio, enhances the barrier properties to water and oxygen, and shows better corrosion resistance than pure polyaniline and polyaniline/clay composites. Kim et al. [60] developed a graphene/polyaniline (G/Pn) composite coating prepared by solution treatment (Figure 8) for metal corrosion protection and monitoring. G/Pn dispersion solution with high dispersion stability was prepared by ultrasonic-assisted, polyaniline-stripping graphene in organic solvent and coated on the copper metal surface. In 1 mol/L sulfuric acid solution and 3.5 wt% NaCl solution, the material showed 46.6% and 68.4% anticorrosion efficiency, respectively. G/Pn coating not only acts as an efficient physical and chemical barrier to effectively isolate corrosive substances from copper surfaces, but also further enhances the electrical conductivity of polyaniline and strengthens its corrosion mechanism through its electrical conductivity. In addition, the G/Pn coating also gives the copper surface hydrophobicity, further enhancing its protection in the water environment. These properties not only make G/Pn coatings an effective solution in the field of metal corrosion protection but also promise the development of smart protective layers that can monitor metal corrosion status in real time, providing a new direction for the development of smart materials.
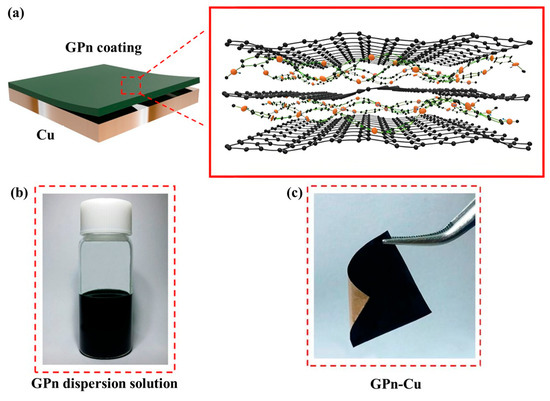
Figure 8.
Schematic illustrations of the alternating layered structure of GPn (a), and photos of the colloidal stability of the GPn dispersion (b) and GPn-Cu (c) [60].
3.2.4. Graphene/Polyurethane Anticorrosion Coatings
Polyurethane (PU) is a kind of polymer material with good mechanical properties, excellent wear resistance, impact resistance, and toughness. Combining graphene nanosheets with polyurethane can significantly enhance the overall performance of the coating.
Li et al. [61] prepared functionalized graphene oxide (TGO) by microwave reduction and titanate functionalization, and mixed it with waterborne polyurethane to prepare TGO/PU composite coating. It was found that the PU composite coating containing 0.4 wt% self-aligned TGO still maintained good corrosion resistance after soaking in 3.5 wt% NaCl solution for 96 h, and no corrosion occurred under the coating. In contrast, coatings with 0.2 wt% unaligned TGO exhibited lower corrosion resistance. By adding self-aligned TGO to the PU matrix, the anticorrosive properties of coatings can be significantly improved, which provides an effective method for developing high-performance anticorrosive coatings. Ramezanzadeh et al. [62] prepared functionalized polyisocyanate-graphene oxide/polyurethane (PI-Go/PU) composite coatings by covalently grafting polyisocyanate (PI) resin onto the surface of graphene oxide nanosheets to improve the barrier and anticorrosion properties of polyurethane coatings. EIS results showed that the addition of 0.1 wt% Pi-Go nanosheets significantly improved the anticorrosion performance of PU coating, and the composite coating showed excellent anticorrosion performance after soaking in 3.5 wt% NaCl solution, without any corrosion under the coating, providing a new material choice for the development of new polyurethane anticorrosion coatings. On the other hand, to improve the corrosion resistance and mechanical properties of PU coating, Haghdadeh et al. [63] used 3-glycidyl ether oxy propyl trimethoxysilane to functionalize GO and introduced it into the PU matrix. The stability and dispersion of the modified nanosheets in the polyurethane matrix were improved, and the interface bonding strength between the polyurethane coating and the functional graphene oxide (FGO) nanosheets was significantly enhanced. The addition of FGO significantly improves the mechanical properties of PU coating, such as tensile stress and loss factor, and significantly improves the anticorrosion properties of PU coating.
In addition, Li et al. [64] proposed an innovative method to significantly improve the corrosion resistance of waterborne polyurethane (WPU) coatings by incorporating graphene and its derivatives. The effects of graphene, graphene oxide, reduced graphene oxide, and functional graphene (FG) as additives on the anticorrosion properties of WPU coatings were studied. Figure 9 shows SEM images of the fracture surfaces of each coating. It can be seen that the fracture surface of pure polyurethane coating is smooth and flat, while the fracture surface of polyurethane composite coating with GO, RGO, and FG added becomes rough due to the distribution of the graphene layer. Compared with the composite coating with FG, the fracture surface of the composite coating with GO and RGO is smoother, indicating that the polyurethane matrix with GO and RGO has better uniformity and compatibility. The results of electrochemical impedance spectroscopy further confirmed that the addition of graphene and its derivatives can effectively enhance the corrosion resistance of WPU coating. Especially when the addition of RGO is 0.2 wt%, the coating shows the best anticorrosion performance.
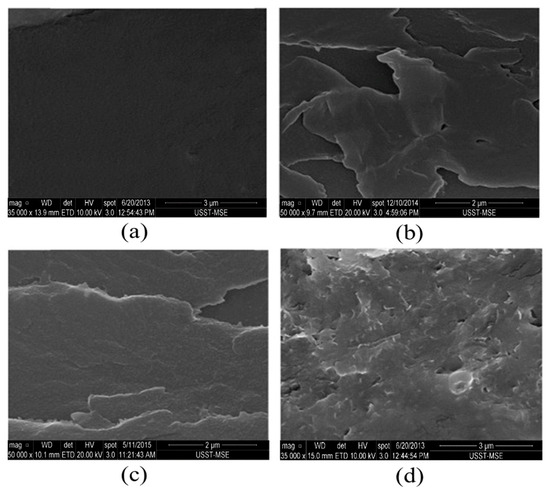
Figure 9.
SEM images of the manufacturing surface of pure PU- (a) GO- (b), RGO- (c), and FG- (d) reinforced PU composite coatings [64].
3.2.5. Graphene/Acrylic Anticorrosion Coatings
Water-based acrylic acid, using water as a solvent, effectively reduces the emission of volatile organic compounds and shows good adhesion on a variety of substrate surfaces. The introduction of graphene into the water-based acrylic coating system can significantly enhance its water resistance, adsorption capacity, and acid and alkali resistance.
Zhang et al. [65] studied the effect of amino-functionalized graphene oxide synthesized by the Hoffman rearrangement reaction on the properties of polyacrylate emulsions and films. The research shows that the introduction of amino-functionalized graphene oxide greatly improves the mechanical properties and thermal stability of the composite materials, and provides a new idea for the application of graphene in polymer nanocomposites. Huai et al. [66] uses graphene-modified, water-based acrylic resin to produce coatings. The results show that the addition of graphene significantly enhances the chemical solvent resistance, thermal stability, and functional group stability of the acrylic resin coating. At the same time, the wear resistance and hardness of the coating are increased by more than 1.5 times. The total volatile organic compounds and formaldehyde emission of the cured coating are far lower than the national limit standards, showing environmentally friendly performance. Li et al. [67] provided a new preparation method for polyacrylic acid emulsion, which improved the dispersion, compatibility, and corrosion resistance of polyacrylic acid/graphene composite coating by introducing micron-scale sulfonated graphene oxide (SRGO). Graphene oxide was prepared by an improved Hummer method, and then SRGO was obtained by reduction and sulfonation. The test results indicate that adding just 0.2% of a low dose of SRGO can significantly enhance the anticorrosive properties of polyacrylic acid/graphene composite coatings and foster the use of micron-scale graphene in industrial polyacrylic acid-based anticorrosive coatings.
The introduction of graphene has had a significant positive impact on the application of water-based acrylic anticorrosion coatings, significantly improving the overall performance of coatings by enhancing their water resistance, anticorrosion properties, dispersion, and compatibility, as well as improving their mechanical properties and thermal stability. In addition, the presence of graphene also helps to reduce the amount of volatile organic compounds and formaldehyde released during the curing process of the coating, showing environmentally friendly properties. These improvements not only improve the durability and protection of coatings but also promote the use of graphene in industrial polyacrylate-based anticorrosion coatings, bringing new opportunities for innovation and sustainable development to the coatings industry.
3.2.6. Graphene/Polypyrrole Anticorrosion Coatings
Polypyrrole (PPy) is a conductive polymer with a strong tolerance to various chemical media such as acids, bases, and salts [75]. The introduction of graphene can give the polypyrrole anticorrosion coating other excellent properties, improve the dispersion of the coating, make the polypyrrole anticorrosion coating in a variety of chemical media more stable, and improve the overall performance.
Qiu et al. [68] prepared a polypyrrole intercalated graphene composite coating based on π-π bond interaction between polypyrrole and graphene. In this study, polypyrrole-graphene (PPy-G) composite material was mixed with epoxy resin and coated on the surface of Q235 steel to promote the formation of Fe2O3 and Fe3O4 protective passivation films on the surface of carbon steel. Electrochemical tests show that compared with pure epoxy coating, PPy-G composite exhibits better anticorrosion properties, especially when the amount of graphene is 0.5 wt%, the impedance modulus reaches the highest, and the corrosion current density decreases to the lowest. Polypyrrole can polymerize on the surface of graphene and between layers, resulting in a disorderly arrangement and increased dispersion of graphene sheets. This polymerization effect not only fills the pore defects of the anticorrosion material but also improves the overall density of the material. Zhang et al. [69] synthesized the graphene/polypyrrole composite using the improved in situ polymerization method and prepared a new type of graphene/polypyrrole water-based anticorrosive coating (rGO/PPy/EP) with this as the filler and combined with water-based epoxy resin as the film-forming substrate. EP, rGO/EP, and rGO/PPy/EP were, respectively, coated on the surface of Q235 carbon steel and soaked in 3.5 wt% NaCl solution for 20 days to observe the changes in the surface corrosion morphology, as shown in Figure 10. The test shows that the addition of rGO/PPy EP coating significantly improves the corrosion resistance of the working electrode, and the coating surface becomes rougher, which further confirms the excellent corrosion resistance and stability of the composite coating. The coating not only has good dispersibility and dense structure, but also the protection effect of graphene/polypyrrole water-based anticorrosive coating with a mass fraction of 1% on bare steel reaching 91.02%, showing an excellent anticorrosive effect.
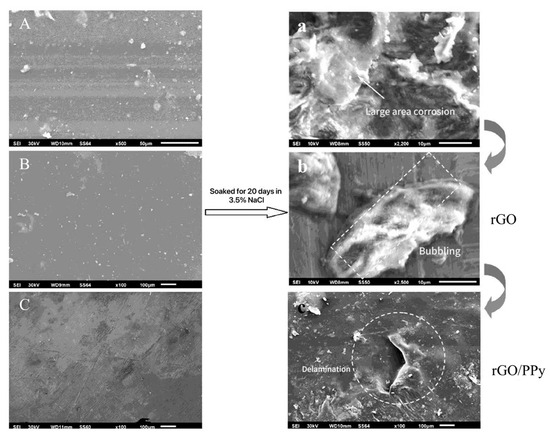
Figure 10.
Surface SEM images of EP, rGO/EP, and rGO/PPy/EP in 3.5 wt% NaCl solution after 20 days: (A,a) EP coating before and after corrosion; (B,b) R GO/EP coating before and after corrosion; (C,c) R GO/PPy/EP coating before and after corrosion [69].
The development of graphene anticorrosive coatings has been restricted by their easy aggregation in coatings, uneven dispersion, and insufficient adhesion with coatings. Graphene is treated by surface modification so that graphene can be more evenly dispersed in the coating, enhance the interaction between graphene and resin, and effectively prevent the penetration of corrosive media, thus improving the corrosion resistance of the coating.
4. The Application of Graphene in Antifouling Coatings
Antifouling coating is a special kind of marine coating. Its main purpose is to prevent marine organisms from attaching and fouling marine structures and keep the ship bottom or marine engineering smooth and clean. The influence of ship fouling on navigation resistance and speed is very significant. Studies have shown that when the ship has serious fouling problems [76,77], the navigation resistance of the ship will increase by 80% and the navigation speed will decrease by 11%; even if slight fouling is caused by diatom adhesion, the navigation resistance will increase by 11~20%. Therefore, it is extremely important to take effective measures to prevent marine biological fouling. The development of organotin self-polishing antifouling coatings (TBT-SPC) in the 1960s and 1970s brought new technologies to the field of marine antifouling. However, subsequent studies have found that organotin compounds cause great harm to the ecological environment, and the International Maritime Organization has banned the use of organotin self-polishing antifouling coatings since 2008 [78]. Since then, the research and development of low-toxicity and non-toxic antifouling coatings has become a research hotspot.
In recent years, the integration of various nanomaterials and antifouling technologies has opened up a new path for environmentally friendly antifouling coatings [79,80,81,82,83,84,85]. Through the introduction of nanomaterials, antifouling coatings with low toxicity and low surface energy can be produced to reduce the adhesion of marine organisms on the structural surface, showing excellent anti-pollution performance. In addition, the addition of nanomaterials can also enhance the antibacterial ability of the coating, and further promote the improvement of its anti-pollution efficiency. Graphene, as a two-dimensional nanomaterial, has great potential in the field of antifouling coatings due to its unique physical and chemical properties. Parra et al. [79] explored an abiotic killing strategy based on nanotechnology. Specifically, it reduces bacterial adhesion by changing the properties of the material’s surface, rather than by killing bacteria. Compared with traditional antifouling technologies using biocides, the effect of graphene coatings is limited to the surface of the coating and has less impact on other organisms in the surrounding water, providing a scientific basis for the development of new, environmentally friendly antifouling materials.
4.1. Tin-Free Self-Polishing Antifouling Coatings
Tin-free self-polishing antifouling coatings are simple in construction, have excellent anticorrosion effects, and are mainly used on the surface of ships and marine facilities to prevent the attachment and growth of marine organisms such as algae, barnacles, and mollusks. Under the action of hydrodynamic force, this coating can gradually release antifouling agents on the surface of the coating and achieve self-polishing during use, maintaining the smoothness of the coating and effectively prevent the attachment of marine organisms.
Compared with traditional tin-coated antifouling coatings, tin-free self-polishing antifouling coatings usually use copper compounds (such as Cu2O) as the main antifouling agent. To achieve an antifouling effect similar to that of organotin self-polishing antifouling coatings, the content of copper compounds is usually high, which has a certain impact on the environment [86]. The amount of Cu2O can be reduced by adding nanoparticles to antifouling coatings, and marine antifouling coatings with low copper content can be prepared by using the synergistic action of nanoparticles and original antifouling agents. Gu et al. [83] introduced Cu2O nanoparticles into the interlayer of GO nanosheets and synthesized rGO@Cu2O nanocomposites in situ by the liquid-phase method and applied them to acrylic resin to prepare a new type of marine self-polishing antifouling coating. This coating demonstrates excellent hydrophobicity, adhesion, and outstanding antifouling performance. Antifouling tests have shown that in the marine environment, uncoated panels are extensively covered by marine organisms within 90 days, and the Cu2O-coated surface also shows biological adhesion after 110 days. In contrast, rGO@Cu2O showed excellent stain resistance with almost no biofouling during the 365-day test period. This excellent antifouling performance is mainly attributed to the biological toxicity of Cu+ in the rGO@Cu2O coating and the unique mesoporous structure of rGO. On the one hand, Cu+ is ultimately oxidized to Cu2+ in seawater, which can effectively inactivate the key enzymes of marine organisms and cause the flocculation of biological cell proteins, thereby inhibiting biological attachment. On the other hand, the unique layered nanostructure and extremely large specific surface area of rGO not only enable Cu2O nanoparticles to be uniformly dispersed in the coating, but also effectively inhibit the rapid release of Cu2O into seawater, significantly extending the antifouling period. This composite coating has shown great application potential in the field of marine antifouling, providing important theoretical basis and practical guidance for the development of new environmentally friendly and efficient marine antifouling coatings.
To investigate whether nanomaterials can enhance the antifouling effect, Liu et al. [85] used an ultrasound–mechanical blending method to add nano-zinc oxide and graphene oxide to the tin-free self-polishing antifouling coating and systematically investigated the effects of nanomaterials on the surface properties of the coating, the antifouling performance of the solid sea, the adhesion force, and the release rate of copper ions. All samples showed varying degrees of biological fouling over time, but the samples with graphene oxide nanoparticles added showed better fouling resistance (Figure 11). The results show that the antifouling performance of tin-free self-polishing antifouling coatings can be improved by adding only a small amount of nanomaterials without sacrificing physical and chemical properties, such as surface properties and bond strength. This finding provides important theoretical support and guidance for the modification of tin-free self-polishing antifouling coatings by nanomaterials and the development of low-copper antifouling coatings. Li et al. [87] studied the preparation of acrylic-modified graphene oxide composites and their synergistic mechanisms in terms of corrosion and pollution resistance. The composite material exhibits hydrolytic properties in slightly alkaline solution, self-polishing antifouling properties in natural seawater, and good corrosion resistance and mechanical strength. This research provides a new type of marine ship protective coating, which can have both anticorrosion and antifouling functions, which is of great significance for improving ship performance and reducing maintenance costs.

Figure 11.
Images of tested panels coated with antifouling coatings with different types of nanoparticles [85].
4.2. Low-Surface-Energy Antifouling Coating
Surface energy is one of the important indices that affect the antifouling performance of hulls. Low-surface-energy antifouling coatings have very low surface energy, and it is difficult for fouling organisms to attach to the surface of the material. Even after attachment, they easily fall off under the action of water flow or other external forces, which significantly reduces the corrosion and loss of fouling organisms [86]. Compared with traditional antifouling coatings, low-surface-energy antifouling coatings do not contain toxic substances, but reduce the surface energy of the coating to inhibit the attachment and growth of marine organisms, and weaken their adhesion, thus greatly improving the antifouling efficiency. Researchers are constantly developing and researching new low-surface-energy antifouling coatings to improve their performance and application effect, and low-surface-energy antifouling coatings are gradually becoming the future of development in the field of marine antifouling technology.
Jin et al. [88] prepared a graphene silane composite film with low surface energy and adjustable elastic modulus by the blending method, and tested the adhesion of bacteria in static and flowing states. Under flow conditions, silane membranes containing 0.36 wt% graphene can effectively reduce the attachment of fouling organisms (Figure 12), confirming the antifouling effectiveness of structural surfaces in dynamic environments. However, in the static state of water flow, the composite film did not show obvious antifouling properties. To obtain a static antifouling effect and overcome the defects of low-surface-energy antifouling coating, Soleimani et al. [89] used Avicennia marina and AgNO3 to reduce graphene oxide and prepared silver-supported redox-graphene for the first time (GOH@Ag). The antifouling coatings of GOH@Ag, bare GO, and multi-walled carbon nanotubes (MWCNTs) were added to the polydimethylsiloxane (PDMS) coating at the weight percentages of 0.01%, 0.1%, and 0.5%, respectively, and their surface properties were characterized. The results show that the PrG coating containing 0.5 wt% GOH@Ag exhibits the lowest pseudo barnacle adhesion strength (0.16 MPa) and the lowest surface energy, which can effectively promote the removal of attached fouling organisms from the structural surface. In addition, with its large surface area, the GOH nanosheets can attract bacteria to their surface, killing some of the fouling organisms by releasing Ag+, thus achieving a static antifouling effect.
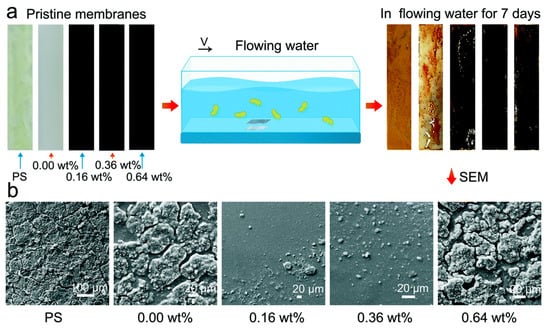
Figure 12.
(a) A comparison of the specimens before and after the bacterial attachment test in flowing water; (b) SEM measurement of the specimens after the bacterial attachment test under hydrodynamic conditions [88].
The introduction of graphene provides the possibility for the preparation of coatings with the synergistic effect of various antifouling technologies. With the unique two-dimensional structure and physical barrier properties of graphene, a dense physical barrier can be formed in the coating, effectively blocking the contact between marine organisms and the coating surface. Because of its extremely high specific surface area and excellent electrical conductivity, graphene can be used as an effective negative carrier for other antifouling agents, and enhance the overall antifouling performance of the coating by fine-regulating the release rate of antifouling agents. In addition, the addition of graphene can also improve the surface energy and hydrophobic properties of the coating, further reducing the adhesion ability of marine organisms, and achieving long-term antifouling.
5. Discussions
Although graphene has shown great potential in the field of anticorrosion and antifouling coatings, it still faces some technical and practical challenges. At present, the application of graphene in the field of corrosion protection is in its infancy, and the preparation of defect-free graphene anticorrosive films and the avoidance of local current corrosion when the coating is damaged are important challenges in the application of graphene in the field of anticorrosive protection. Although the dispersion of graphene in coatings can be improved through a variety of modification techniques, methods to achieve a uniform distribution of graphene without sacrificing other properties of coatings still need further research. The dispersion of graphene and the compatibility with the base material are still the key factors restricting its application. Secondly, the preparation cost of graphene is relatively high, and finding a way to reduce the cost during large-scale production to achieve commercial applications is an urgent problem to be solved.
On the other hand, the environmental impact and safety assessment of graphene is also an important aspect. Despite the excellent physicochemical properties of graphene, its potential effects on the environment and human health still need to be studied in depth to ensure its safety in practical applications. In terms of antifouling coatings, the high chemical stability and hydrophobicity of graphene help to improve antifouling performance, but it may also lead to poor bonding with film-forming substances, affecting the overall performance of the coating. Therefore, optimizing the synergistic effect of graphene and other antifouling agents to improve the comprehensive performance of coatings is an important direction of future research.
6. Conclusions
In this paper, the application progress of graphene in anticorrosion coatings and antifouling coatings was reviewed, and its modification in anticorrosion films and organic antifouling coatings and its influence on antifouling performance were analyzed. With its excellent physical and chemical properties, graphene has shown significant advantages in improving the corrosion resistance and fouling resistance of coatings. However, the application of graphene in coatings still faces many challenges, such as preparation cost, dispersion, environmental impact, and so on. It can be seen that the future research of graphene in the field of anticorrosion and antifouling coatings will be a multi-faceted, interdisciplinary, comprehensive study aimed at solving the current technical challenges and achieving the wide application and industrial development of graphene in this field.
Future research should focus on how to reduce the production cost of graphene, improve its dispersibility and compatibility in coatings, and evaluate its environmental and safety impacts in depth. Researchers need to further optimize the composite and functional design of graphene-based materials to improve their adaptability and reliability in extreme environments, including the development of coatings with multiple functions, such as self-healing and self-cleaning, to improve coating performance. Given the short antifouling period of antifouling coatings used in marine ships, future research should focus on the development of new antifouling coatings to extend the antifouling period and reduce the damage to the anticorrosive coating when removing attached marine organisms. As a result, it is important to develop products with a longer antifouling cycle and that are compatible with anticorrosive coatings or antifouling coatings, to reduce maintenance costs, extend the life of the ship, protect the environment, and improve the operating efficiency. At the same time, with the increasingly strict environmental regulations, the research on graphene-modified anticorrosion and antifouling coatings will pay more attention to environmental protection performance, to reduce the harm caused to the environment in the process of graphene modification and achieve sustainable development.
Author Contributions
Q.Z. and X.L.: Writing—original draft. Q.Z.: Funding acquisition, supervision, and writing—review and editing. Y.J.: Writing—review and editing. F.X.: Supervision and writing—review and editing. W.W.: Investigation, supervision, and writing—review and editing. J.D.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Natural Science Foundation of Shandong Province under grant no. ZR2022QE131.
Data Availability Statement
Data are contained in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Schiff, K.; Diehl, D.; Valkirs, A. Copper emissions from antifouling paint on recreational vessels. Mar. Pollut. Bull. 2004, 48, 371–377. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, P.; Li, G.; Lu, D.D.; Sun, R.; Wong, C. Core–shell SiO2@RGO hybrids for epoxy composites with low percolation threshold and enhanced thermo-mechanical properties. J. Mater. Chem. A 2014, 2, 18246–18255. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; He, Y.; Liang, L.; Lv, L.; Ran, X.; Pan, Y.; Luo, Z. Preparation of graphene oxide modified by titanium dioxide to enhance the anti-corrosion performance of epoxy coatings. Surf. Coat. Technol. 2015, 276, 471–478. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Zhou, F.; Zhan, S.; Tian, Y. Antifouling improvement of graphene/TiO2 modified polyurethane coatings. J. Control. Release 2017, 259, e33. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Xia, Y.; Li, H.; He, Z. Two-dimensional hybrid materials: MoS2-RGO nanocomposites enhanced the barrier properties of epoxy coating. Appl. Surf. Sci. 2018, 444, 511–521. [Google Scholar] [CrossRef]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Graphene: Super Gas Barrier and Selectivity of Graphene Oxide-Polymer Multilayer Thin Films. Adv. Mater. 2013, 25, 493. [Google Scholar] [CrossRef]
- Compton, O.C.; Kim, S.; Pierre, C.; Torkelson, J.M.; Nguyen, S.B.T. Crumpled Graphene Nanosheets as Highly Effective Barrier Property Enhancers. Adv. Mater. 2010, 22, 4759–4763. [Google Scholar] [CrossRef]
- Unalan, I.U.; Wan, C.; Figiel, L.; Olsson, R.T.; Trabattoni, S.; Farris, S. Exceptional oxygen barrier performance of pullulan nanocomposites with ultra-low loading of graphene oxide. Nanotechnology 2015, 26, 275703. [Google Scholar] [CrossRef]
- Bhm, S. Graphene against corrosion. Nat. Nanotechnol. 2014, 9, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Chen, Z.G.; Li, M.D.; Yang, B.; Xin, M.L.; Li, S.P.; Yin, Z.J. Surface Functional Modification of Graphene and Graphene Oxide. Acta Chim. Sin. 2016, 74, 789–799. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Han, T.W.; He, P.F.; Luo, Y.; Zhang, X.Y. Research progress in the mechanical properties of graphene. Adv. Mech. 2011, 41, 279–293. [Google Scholar]
- Liu, Y.Q. Graphene: From Foundation to Application; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Heteroatom modified graphenes: Electronic and electrochemical applications. J. Mater. Chem. C 2014, 2, 6454–6461. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Szroeder, P.; Banaszak-Piechowska, A.; Sahalianov, I. Tailoring Electrocatalytic Properties of sp2-Bonded Carbon Nanoforms Through Doping. Molecules 2025, 30, 1265. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Cui, M.; Li, W.; Pu, J.; Xue, Q.; Wang, L. N-doping of graphene: Toward long-term corrosion protection of Cu. J. Mater. Chem. A 2018, 6, 24136–24148. [Google Scholar] [CrossRef]
- Lee, J.H.; Avsar, A.; Jung, J.; Tan, J.Y.; Watanabe, K.; Taniguchi, T.; Natarajan, S.; Eda, G.; Adam, S.; Neto, A.H.C.; et al. Van der Waals force: A dominant factor for reactivity of graphene. Nano Lett. 2015, 15, 319–325. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Liu, F.; Xu, L.; Zhang, Z.C.; Han, E.H.; Ke, W.; Arukalam, I.O. Superhydrophobicity, conductivity and anticorrosion of robust siloxane-acrylic coatings modified with graphene nanosheets. Prog. Org. Coat. 2019, 127, 239–251. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Liu, T.; Liu, Z.; Pu, J.; Liu, W.; Zhao, H.; Li, X.; Wang, L. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene. Carbon 2019, 142, 164–176. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Qing, F.; Shen, C.; Jia, R.; Zhan, L.; Li, X. Catalytic substrates for graphene growth. MRS Bull. 2017, 42, 819–824. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Colombo, L.; Ruoff, R.S. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 2009, 9, 4268–4272. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Raman, S.R.; Banerjee, C.P.; Lobo, E.D.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Show moreProtecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. Acs Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Pu, N.W.; Shi, G.N.; Liu, Y.M.; Sun, X.L.; Chang, J.K.; Sun, C.L.; Ger, M.D.; Chen, C.Y.; Wang, P.C.; Peng, Y.Y.; et al. Graphene grown on stainless steel as a high-performance and ecofriendly anti-corrosion coating for polymer electrolyte membrane fuel cell bipolar plates. J. Power Sources 2015, 282, 248–256. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, C.; Xu, X.; Deng, B.; Han, N.; Liu, M.; Hong, M.; Ning, Y.; Liu, K.; Zhao, J.; et al. Unique transformation from graphene to carbide on Re (0001) induced by strong carbon–metal interaction. J. Am. Chem. Soc. 2017, 139, 17574–17581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Sun, L.; Liu, Z.; Ding, F.; Liu, Z. Roles of Transition Metal Substrates in Graphene Chemical Vapor Deposition Growth. Acta Phys. Chim. Sin. 2022, 38, 26–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Y.; Hui, Y.Y.; Tang, L.; Jie, W.; Jiang, Y.; Xu, L.; Lau, S.P.; Chai, Y. Highly impermeable and transparent graphene as an ultra-thin protection barrier for Ag thin films. J. Mater. Chem. C 2013, 1, 4956–4961. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Bai, Y.; Zhang, J.; Han, Z.; Ren, L. Fabrication of biomimetic hydrophobic patterned graphene surface with ecofriendly anti-corrosion properties for Al alloy. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 64–71. [Google Scholar] [CrossRef]
- Mallick, M.; Arunachalam, N. Electrophoretic deposited graphene based functional coatings for biocompatibility improvement of Nitinol. Thin Solid Film. 2019, 692, 137616. [Google Scholar] [CrossRef]
- Hares, E.; El-Shazly, A.H.; El-Kady, M.F.; Hammad, A.S. Electrophoretic deposition of graphene oxide nanosheets on copper pipe for corrosion protection. Arab. J. Sci. Eng. 2019, 44, 5559–5569. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloys Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Z.; Shenoy, G.J.; Li, L.; Liu, H. Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano 2013, 7, 6939–6947. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. The structural stability of graphene anticorrosion coating materials is compromised at low potentials. Chem. A Eur. J. 2015, 21, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ji, Y.; Shi, Y.; Hui, F.; Duan, H.; Lanza, M. A review on the use of graphene as a protective coating against corrosion. Ann. J. Mater. Sci. Eng. 2014, 1, 16. [Google Scholar]
- Hsieh, Y.P.; Hofmann, M.; Chang, K.W.; Jhu, J.G.; Li, Y.Y.; Chen, K.Y.; Yang, C.C.; Chang, W.S.; Chen, L.C. Complete corrosion inhibition through graphene defect passivation. ACS Nano 2014, 8, 443–448. [Google Scholar] [CrossRef]
- Anisur, M.R.; Banerjee, P.C.; Easton, C.D.; Raman, R.K.S. Controlling hydrogen environment and cooling during CVD graphene growth on nickel for improved corrosion resistance. Carbon 2018, 127, 131–140. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, C.; Zhang, X.; Lin, D.; Xu, F.; Zhu, Y.; Wang, H.; Gong, J.; Wang, T. Highly orientated graphene/epoxy coating with exceptional anti-corrosion performance for harsh oxygen environments. Corros. Sci. 2020, 176, 109049. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.; Zhang, M.S.; Zhang, L.; Chen, Z. Preparation of graphene oxide modified epoxy resin coating and research on its anti-corrosive performance. Chem. Ind. Eng. Prog. 2021, 40, 4456. [Google Scholar]
- Zhao, H.; Ding, J.; Zhou, M.; Yu, H. Enhancing the anticorrosion performance of graphene–epoxy coatings by biomimetic interfacial designs. ACS Appl. Nano Mater. 2021, 4, 6557–6561. [Google Scholar] [CrossRef]
- Wang, N.; Diao, X.; Zhang, J.; Kang, P. Corrosion resistance of waterborne epoxy coatings by incorporation of dopamine treated mesoporous-TiO2 particles. Coatings 2018, 8, 209. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Xiao, G.; Zhong, F.; Wu, Y.; He, Z. Improved corrosion protection of waterborne epoxy/graphene coating by combining non-covalent and covalent bonds. React. Funct. Polym. 2019, 137, 104–115. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Jia, X.L.; Li, J.X.; Feng, H.L.; Liu, H.F. Preparation of Graphene Modified Epoxy Zinc-rich Primer and Research on Salt Spray Resistance. China Coat. 2021, 36, 31–35. [Google Scholar]
- Yang, L.H.; Liu, J.J.; Chen, Q.; Zhao, Y.J.; Guo, D.L.; Xiao, P. Effect of Zinc Powder Grafted Ionic Liquid on Anticorrosive Properties of Epoxy Coatings. Paint. Coat. Ind. 2022, 52, 37–43. [Google Scholar]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Kim, S.; Le, T.H.; Park, C.S.; Park, G.; Kim, K.H.; Kim, S.; Kwon, O.S.; Lim, G.T.; Yoon, H. A solution-processable, nanostructured, and conductive graphene/polyaniline hybrid coating for metal-corrosion protection and monitoring. Sci. Rep. 2017, 7, 15184. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Li, J.; Yang, J. Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J. Mater. Chem. A 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.H.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Haghdadeh, P.; Ghaffari, M.; Ramezanzadeh, B.; Bahlakeh, G.; Saeb, M.R. The role of functionalized graphene oxide on the mechanical and anti-corrosion properties of polyurethane coating. J. Taiwan Inst. Chem. Eng. 2018, 86, 199–212. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Gao, D.; Zhou, Y.; Li, C.; Zha, J.; Zhang, J. Preparation of amino-functionalized graphene oxide by Hoffman rearrangement and its performances on polyacrylate coating latex. Prog. Org. Coat. 2016, 94, 9–17. [Google Scholar] [CrossRef]
- Huai, K.K.; Gu, Q.Q.; Guo, H.Y.; Wang, N.N.; Wang, X. Preparation and Property of Water-Based Acrylic Resin Coating Modified with Graphene. Mater. Prot. 2017, 50, 40–44. [Google Scholar]
- Li, H.; Xia, Y.; Wu, S.; Zhang, D.; Oliver, S.; Li, X.; Chen, X.; Lei, L.; Shi, S. Micron-dimensional sulfonated graphene sheets co-stabilized emulsion polymerization to prepare acrylic latex used for reinforced anticorrosion coatings. Prog. Org. Coat. 2022, 165, 106762. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liu, C.; Wang, X. Preparation and anticorrosion performance of an innovative graphene/polypyrrole waterborne anticorrive coatings. Chem. Ind. Eng. Prog. 2017, 36, 4562–4568. [Google Scholar]
- Ding, J.; ur Rahman, O.; Peng, W.; Dou, H.; Yu, H. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings. Appl. Surf. Sci. 2018, 427, 981–991. [Google Scholar] [CrossRef]
- Yang, T.; Cui, Y.; Li, Z.; Zeng, H.; Luo, S.; Li, W. Enhancement of the corrosion resistance of epoxy coating by highly stable 3, 4, 9, 10-perylene tetracarboxylic acid functionalized graphene. J. Hazard. Mater. 2018, 357, 475–482. [Google Scholar] [CrossRef]
- Duan, W.; Chen, Y.; Ma, J.; Wang, W.; Cheng, J.; Zhang, J. High-performance graphene reinforced epoxy nanocomposites using benzyl glycidyl ether as a dispersant and surface modifier. Compos. Part B Eng. 2020, 189, 107878. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Gui, T.J.; Wang, X.; Zhao, X.D.; Liu, J.; Li, B.J.; Song, L.Y.; Li, W.H. Review of Theoretical and Applied Research of Graphene in Anti-corrosion Film and Organic Anti-corrosion Coatings. Acta Chim. Sin. 2019, 77, 1140–1155. [Google Scholar] [CrossRef]
- Yu, H.Z.; Chen, M.G.; Bei, C.X. Properties and applicative view of polyaniline. Polym. Mater. Sci. Eng. 2003, 19, 18–21+26. [Google Scholar]
- Zhang, M.M.; Liu, X.Y.; Qian, W. Research Development of Polypyrrole Electrode Materials in Supercapacitors. Mater. Rep. 2018, 32, 378–383. [Google Scholar]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, C.M.; Brennan, A.B. Bio-inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 211–229. [Google Scholar] [CrossRef]
- Evans, S.M.; Birchenough, A.C.; Brancato, M.S. The TBT ban: Out of the frying pan into the fire? Mar. Pollut. Bull. 2000, 40, 204–211. [Google Scholar] [CrossRef]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J. Nanobiotechnology 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Yee MS, L.; Khiew, P.S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.Y.; Leong, C.O. Green synthesis of graphene-silver nanocomposites and its application as a potent marine antifouling agent. Colloids Surf. B Biointerfaces 2016, 148, 392–401. [Google Scholar]
- Punitha, N.; Saravanan, P.; Mohan, R.; Ramesh, P.S. Antifouling activities of β-cyclodextrin stabilized peg based silver nanocomposites. Appl. Surf. Sci. 2017, 392, 126–134. [Google Scholar] [CrossRef]
- Knowles, B.R.; Wagner, P.; Maclaughlin, S.; Higgins, M.J.; Molino, P.J. Silica nanoparticles functionalized with zwitterionic sulfobetaine siloxane for application as a versatile antifouling coating system. ACS Appl. Mater. Interfaces 2017, 9, 18584–18594. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, L.; Huang, D.; Jiang, L.; Liu, L.; Li, F.; Pang, A.; Guo, X.; Tao, B. In situ synthesis of graphene@ cuprous oxide nanocomposite incorporated marine antifouling coating with elevated antifouling performance. Open J. Org. Polym. Mater. 2019, 9, 47–62. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Yang, J.; Zhou, S. Preparation and synergistic antifouling effect of self-renewable coatings containing quaternary ammonium-functionalized SiO2 nanoparticles. J. Colloid Interface Sci. 2020, 563, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, M.; Gadang, P.; Huang, Y.L.; Duan, J.Z.; Hou, B.R. Preparation and performance of nanomaterials modified self-polishing marine antifouling coatings. Water Resour. Hydropower Eng. 2021, 52, 1–10. [Google Scholar]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wang, F.; Liang, W.; Yang, H.; Wu, D. Fabrication of acrylic acid modified graphene oxide (AGO)/acrylate composites and their synergistic mechanisms of anticorrosion and antifouling properties. Prog. Org. Coat. 2022, 168, 106910. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, T.; Bing, W.; Dong, S.; Tian, L. Antifouling performance and mechanism of elastic graphene–silicone rubber composite membranes. J. Mater. Chem. B 2019, 7, 488–497. [Google Scholar] [CrossRef]
- Soleimani, S.; Jannesari, A.; Yousefzadi, M.; Ghaderi, A.; Shahaddi, A. Eco-friendly foul release coatings based on a novel reduced graphene oxide/Ag nanocomposite prepared by a green synthesis approach. Prog. Org. Coat. 2021, 151, 106107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).