Growth Kinetics of Ni3Ga7 in Ni/Ga System During Interfacial Reaction Diffusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
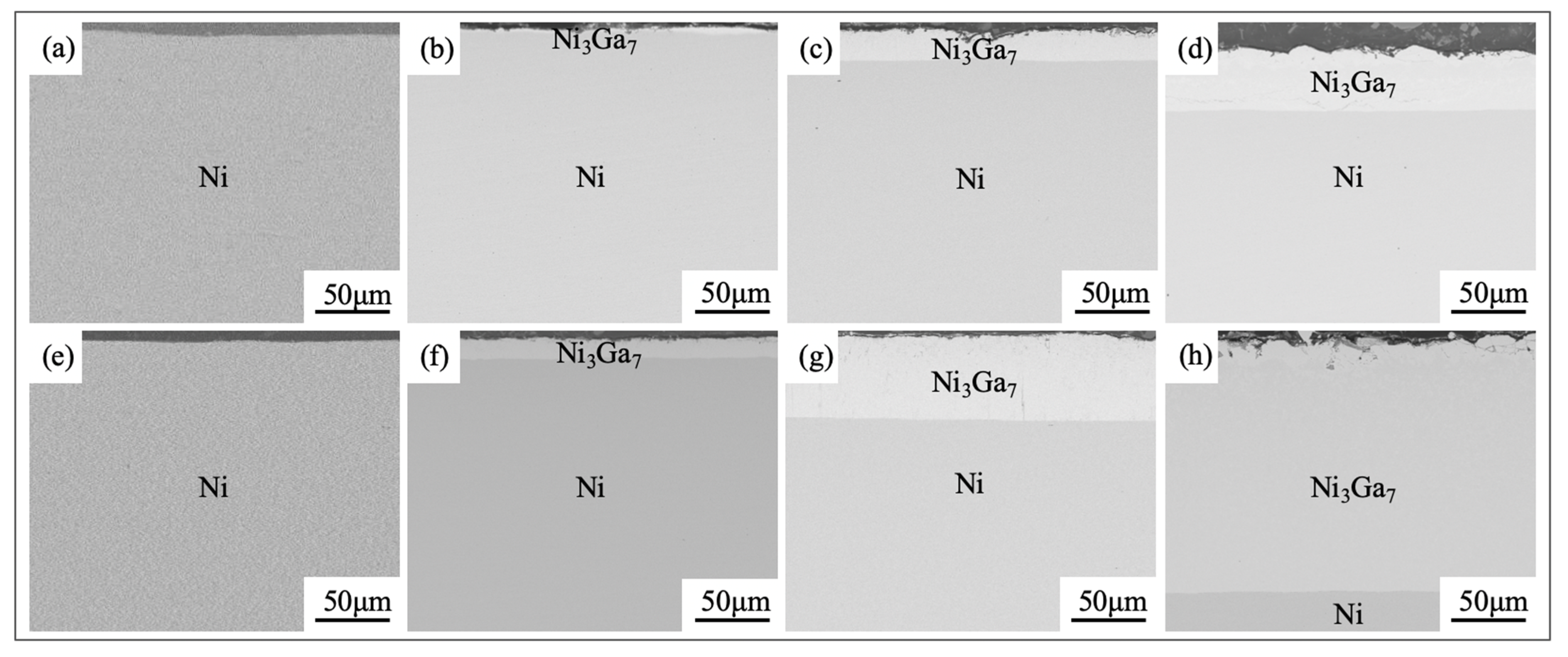
3.1. Microstructures and Phase Compositions
3.2. Diffusion Kinetics and Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wang, H.Z.; Zhao, R.Q.; Rao, W.; Liu, J. Liquid Metal Composites. Matter 2020, 2, 1446–1480. [Google Scholar] [CrossRef]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic Gallium-Indium (EGaIn): A Liquid Metal Alloy for the Formation of Stable Structures in Microchannels at Room Temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.J.; Cho, C.; Ko, S.H. Liquid Metal based Stretchable Room Temperature Soldering Sticker Patch for Stretchable Electronics Integration. Adv. Funct. Mater. 2023, 33, 2003286. [Google Scholar] [CrossRef]
- Chen, S.W.; Lin, J.M.; Yang, T.C.; Du, Y.H. Interfacial Reactions in the Cu/Ga/Co and Cu/Ga/Ni Samples. J. Electron. Mater. 2019, 48, 3643–3653. [Google Scholar] [CrossRef]
- Daalkhaijav, U.; Yirmibesoglu, O.D.; Walker, S.; Menguc, Y. Rheological Modification of Liquid Metal for Additive Manufacturing of Stretchable Electronics. Adv. Mater. Technol. 2018, 3, 1700351. [Google Scholar] [CrossRef]
- Ozutemiz, K.B.; Wissman, J.; Ozdoganlar, O.B.; Majidi, C. EGaIn−Metal Interfacing for Liquid Metal Circuitry and Microelectronics Integration. Adv. Mater. Interfaces 2018, 5, 1701596. [Google Scholar] [CrossRef]
- Jiang, M.Z.; Chen, S.; Zhang, P.; Sun, Y.W.; Ye, J.; Deng, Y.Q.; Li, L.; Liu, J. Liquid metal enabled plant injectable electronics. Mater. Today 2023, 66, 50–61. [Google Scholar] [CrossRef]
- Yuan, R.Z.; Cao, Y.J.; Zhu, X.Y.; Shan, X.H.; Wang, B.; Wang, H.Z.; Chen, S.; Liu, J. Liquid Metal Memory. Adv. Mate. 2024, 36, 2309182. [Google Scholar] [CrossRef]
- Ma, X.H.; Wu, X.T.; Cao, S.T.; Zhao, Y.F.; Lin, Y.; Xu, Y.R.; Ning, X.H.; Kong, D.S. Stretchable and Skin-Attachable Electronic Device for Remotely Controlled Wearable Cancer Therapy. Adv. Sci. 2023, 10, 2205343. [Google Scholar] [CrossRef]
- Deng, Y.G.; Liu, J. Corrosion development between liquid gallium and four typical metal substrates used in chip cooling device. Appl. Phys. A 2009, 95, 907–915. [Google Scholar] [CrossRef]
- Ralphs, M.; Kong, W.; Wang, R.Y.; Rykaczewski, K. Thermal Conductivity Enhancement of Soft Polymer Composites through Magnetically Induced Percolation and Particle–Particle Contact Engineering. Adv. Mater. Interfaces 2019, 6, 1801857. [Google Scholar] [CrossRef]
- Lin, S.K.; Wang, M.J.; Yeh, C.Y.; Chang, H.M.; Liu, Y.C. High-strength and thermal stable Cu-to-Cu joint fabricated with transient molten Ga and Ni under-bump-metallurgy. J. Alloy. Compd. 2017, 702, 561–567. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mat. Sci. Eng. R. 2005, 49, 1–60. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Paulasto-Krockel, M. Impurity and alloying effects on interfacial reaction layers in Pb-free soldering. Mat. Sci. Eng. R. 2010, 68, 1–38. [Google Scholar] [CrossRef]
- Lin, S.K.; Chang, H.M.; Cho, C.L.; Liu, Y.C.; Kuo, Y.K. Formation of solid-solution Cu-to-Cu joints using Ga solder and Pt under bump metallurgy for three-dimensional integrated circuits. Electron. Mater. Lett. 2015, 11, 687–694. [Google Scholar] [CrossRef]
- Mikheev, A.A.; Temnykh, V.I.; Kazakov, V.S.; Temnykh, E.V.; Mityaev, A.E.; Zeer, G.M.; Abkaryan, A.K. Kinetics and Products of Interaction of Zinc-Containing Gallium Pastes-Solders. Welding Int. 2012, 36, 314–315. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, B.H.; Chen, H.C.; Wang, C.M.; Wu, A.T. Interfacial Reactions of Low-Melting Sn-Bi-Ga Solder Alloy on Cu Substrate. J. Electron. Mater. 2016, 45, 197–202. [Google Scholar] [CrossRef]
- Froemel, J.; Baum, M.; Wiemer, M.; Gessner, T. Low-Temperature Wafer Bonding Using Solid-Liquid Inter-Diffusion Mechanism. J. Microelectromech. S. 2015, 24, 1973–1980. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tan, X.F.; McDonald, S.D.; Gu, Q.F.; Matsumura, S.; Nogita, K. Interfacial reactions between Ga and Cu-xNi (x = 0, 2, 6, 10, 14) substrates and the strength of Cu-xNi/Ga/Cu-xNi joints. Intermetallics 2021, 133, 107168. [Google Scholar] [CrossRef]
- Schaefer, M.; Laub, W.; Sabee, J.M.; Fournelle, R.A. A Numerical Method for Predicting Intermetallic Layer Thickness Developed During the Formation of Solder Joints. J. Electron. Mater. 1996, 25, 992–1003. [Google Scholar] [CrossRef]
- Schaefer, M.; Fournelle, R.A.; Liang, J. Theory for Intermetallic Phase Growth Between Cu and Liquid Sn-Pb Solder Based on Grain Boundary Diffusion Control. J. Electron. Mater. 1998, 27, 1167–1176. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zeng, G.; Yang, W.H.; McDonald, S.; Gu, Q.F.; Matsumura, S.; Nogita, K. Interfacial Reactions between Ga and Cu-10Ni Substrate at Low Temperature. ACS Appl. Mater. Interfaces 2020, 12, 21045–21056. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.M. Interfacial reaction in Cu/Sn/Cu system during the transient liquid phase soldering process. Acta Mater. 2011, 59, 1198–1221. [Google Scholar] [CrossRef]
- Lin, S.K.; Cho, C.L.; Chang, H.M. Interfacial reactions in Cu/Ga and Cu/Ga/Cu couples. J. Electron. Mater. 2014, 43, 204–211. [Google Scholar] [CrossRef]

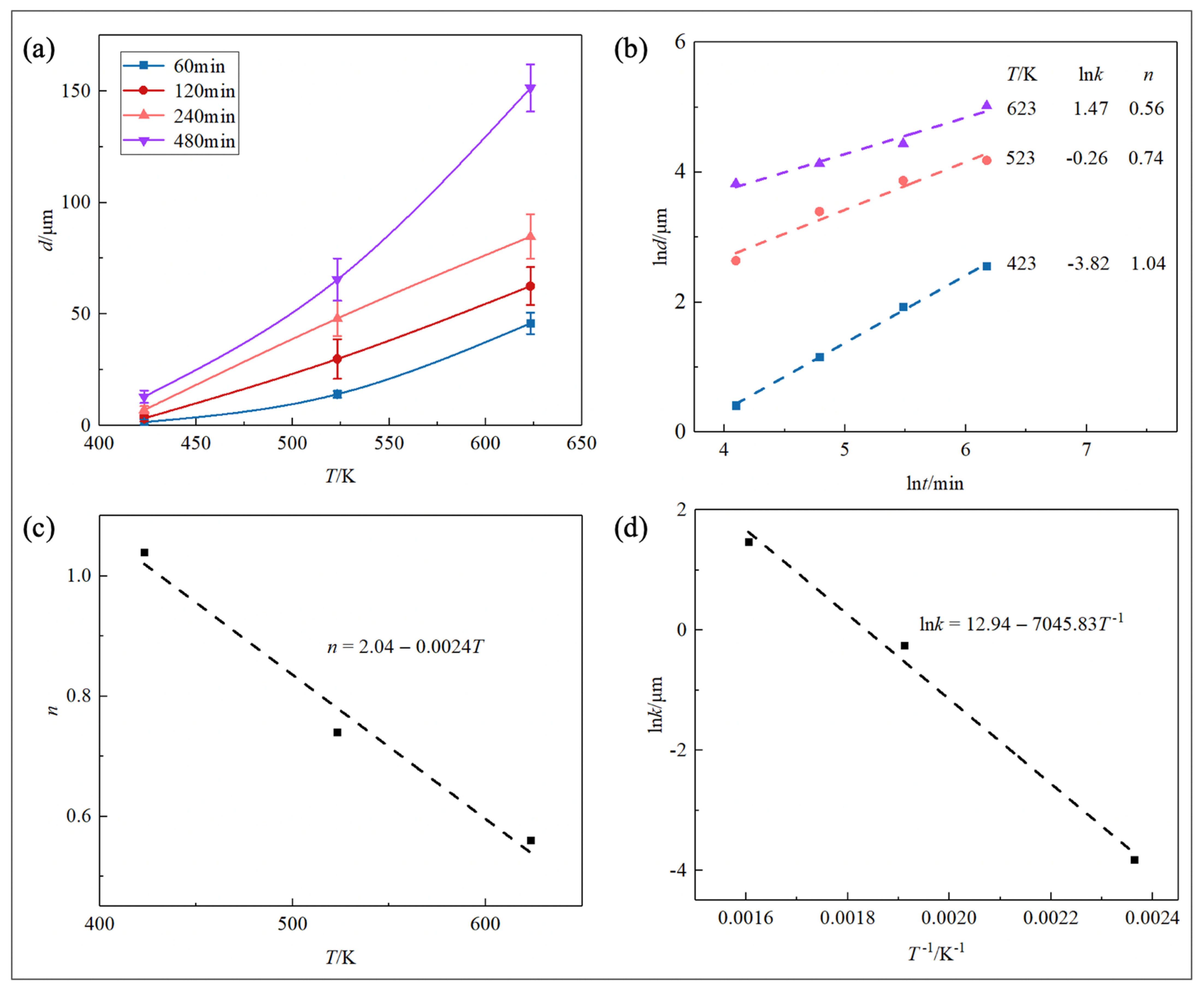
| Temperature (K) | Time (min) | Reaction Phase | kinetic Exponent (n) |
|---|---|---|---|
| 423 | 60 | Ni3Ga7 | 1.04 |
| 120 | Ni3Ga7 | 1.04 | |
| 240 | Ni3Ga7 | 1.04 | |
| 480 | Ni3Ga7 | 1.04 | |
| 523 | 60 | Ni3Ga7 | 0.74 |
| 120 | Ni3Ga7 | 0.74 | |
| 240 | Ni3Ga7 | 0.74 | |
| 480 | Ni3Ga7 | 0.74 | |
| 623 | 60 | Ni3Ga7 | 0.56 |
| 120 | Ni3Ga7 | 0.56 | |
| 240 | Ni3Ga7 | 0.56 | |
| 480 | Ni3Ga7 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Wang, T.; Zhang, S. Growth Kinetics of Ni3Ga7 in Ni/Ga System During Interfacial Reaction Diffusion. Crystals 2025, 15, 520. https://doi.org/10.3390/cryst15060520
Peng J, Wang T, Zhang S. Growth Kinetics of Ni3Ga7 in Ni/Ga System During Interfacial Reaction Diffusion. Crystals. 2025; 15(6):520. https://doi.org/10.3390/cryst15060520
Chicago/Turabian StylePeng, Jun, Tao Wang, and Shuai Zhang. 2025. "Growth Kinetics of Ni3Ga7 in Ni/Ga System During Interfacial Reaction Diffusion" Crystals 15, no. 6: 520. https://doi.org/10.3390/cryst15060520
APA StylePeng, J., Wang, T., & Zhang, S. (2025). Growth Kinetics of Ni3Ga7 in Ni/Ga System During Interfacial Reaction Diffusion. Crystals, 15(6), 520. https://doi.org/10.3390/cryst15060520





