Synergistic Effects of Microbial-Induced Carbonate Precipitation and Modified Biochar on the Engineering Properties of Loess
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Bacteria and Growth Conditions
2.2. Modified Biochar
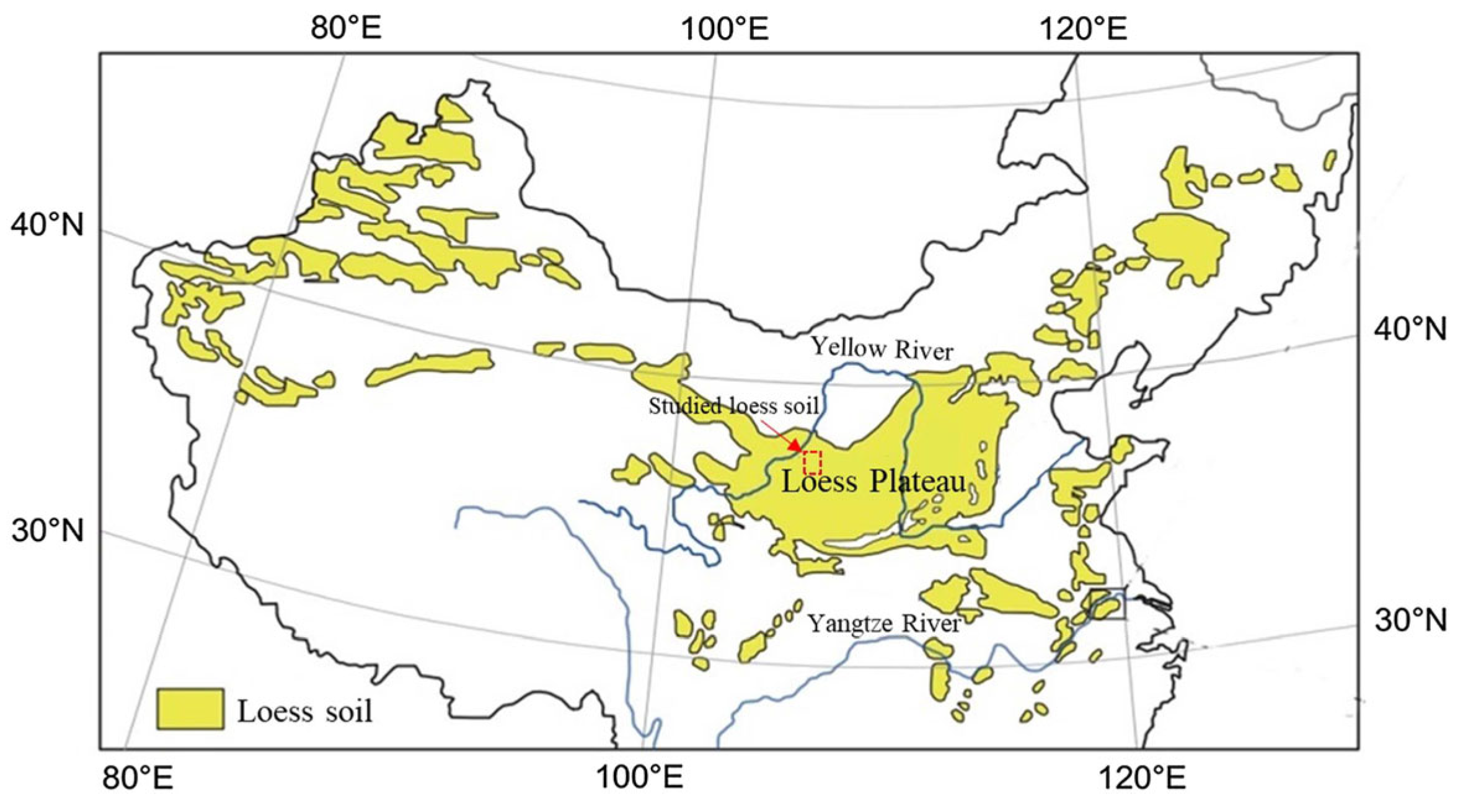
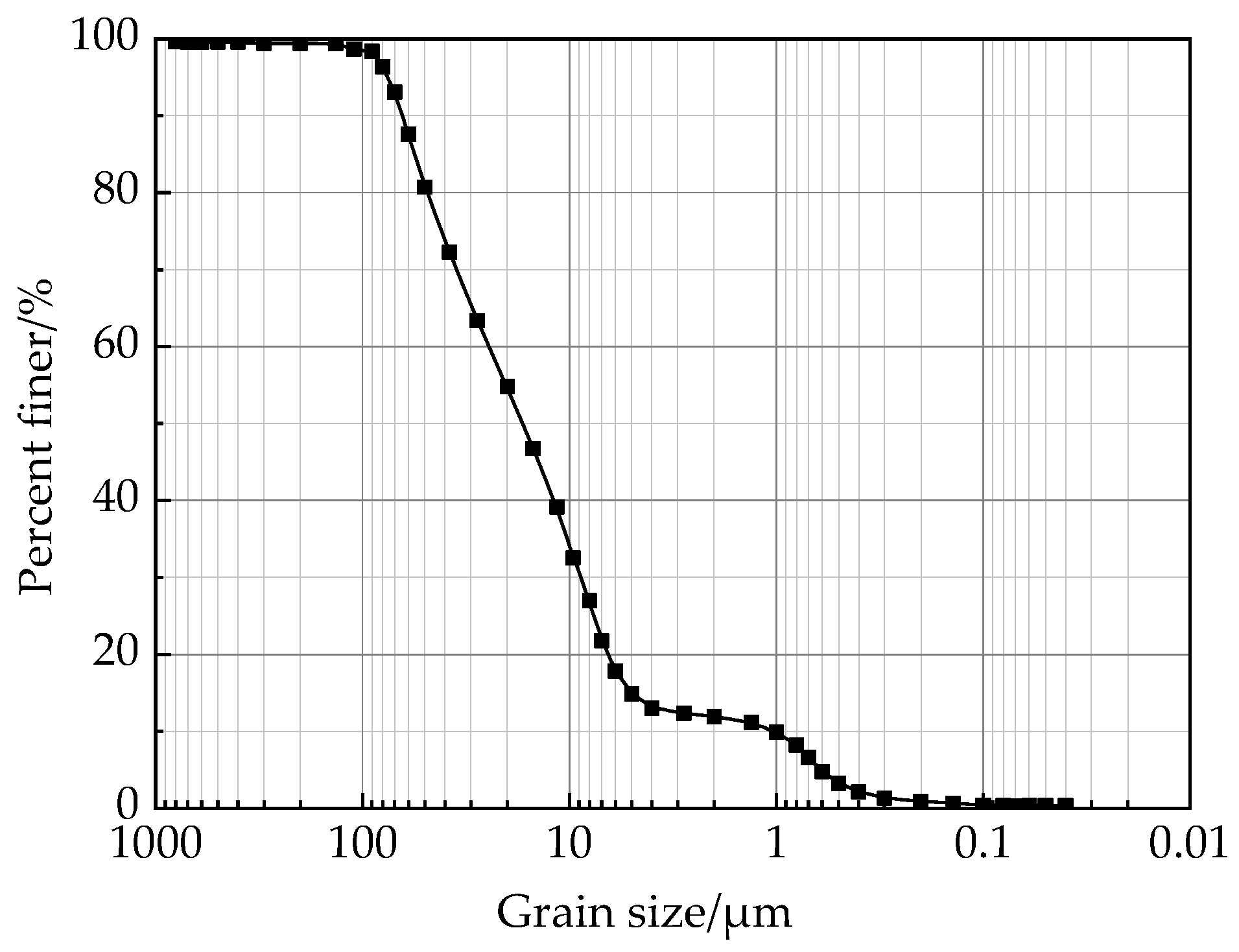
2.3. Loess Specimen and Experimental Programme
3. Soil Engineering Properties
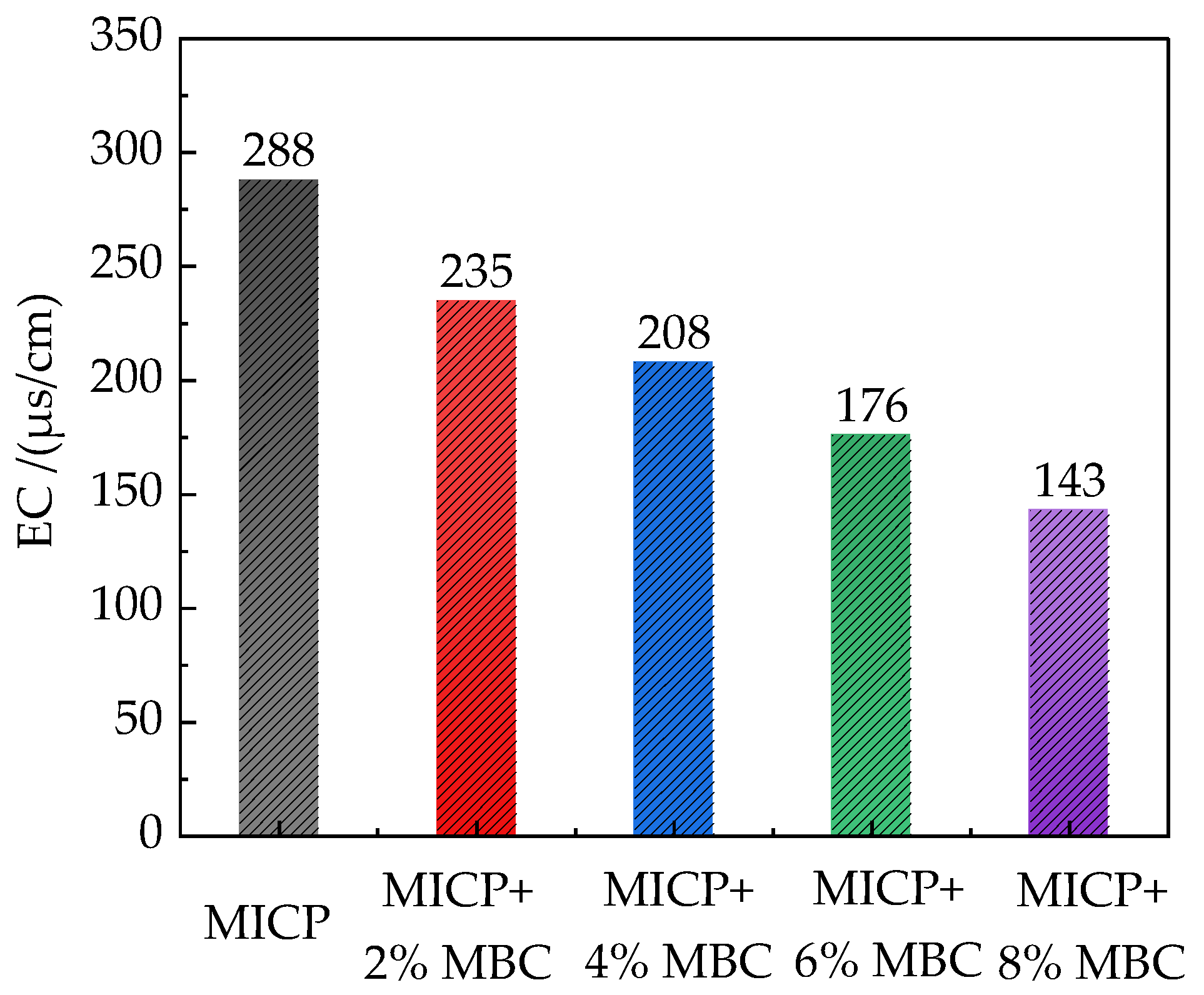
3.1. Soil Electrical Conductivity (EC) Assessment
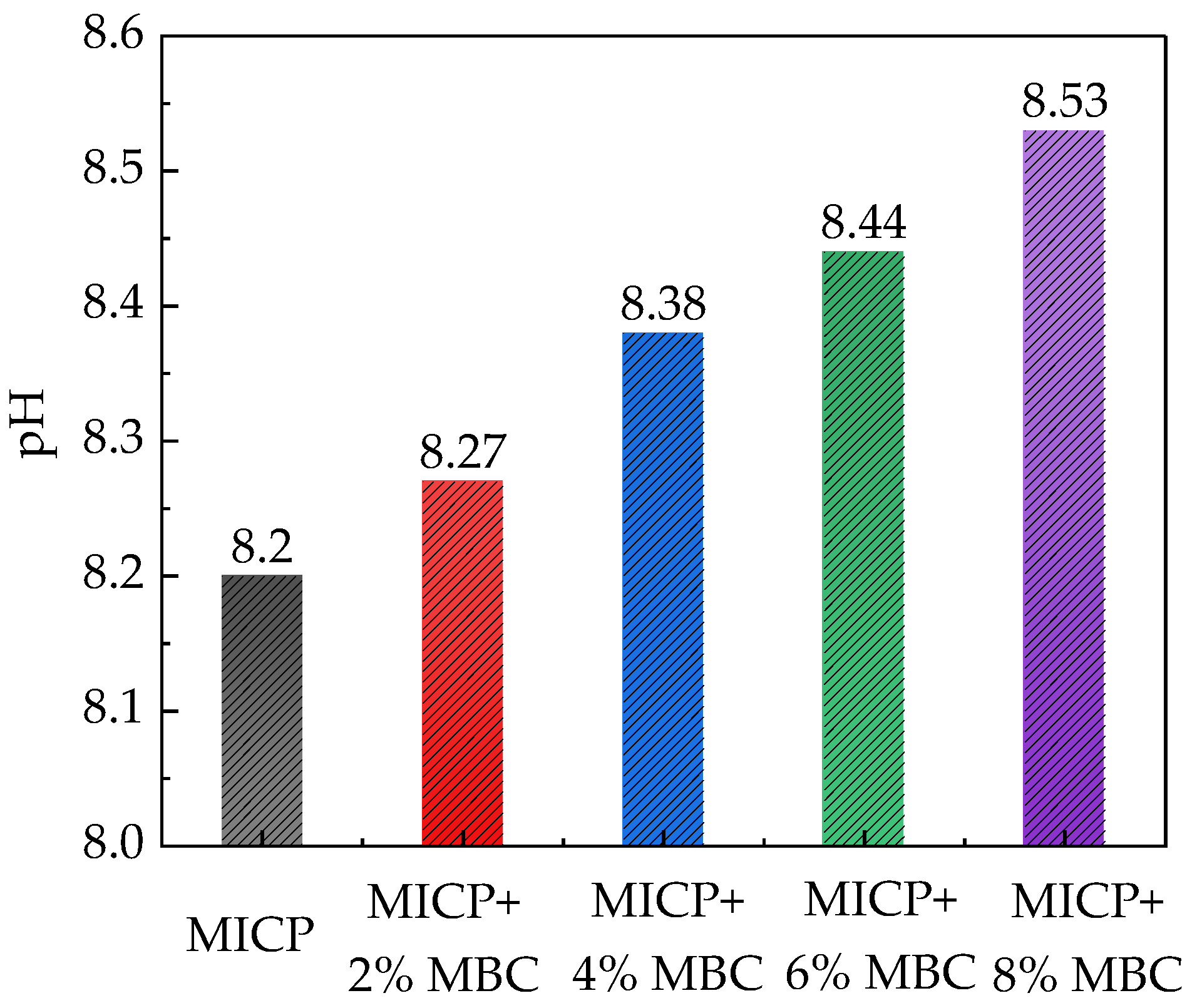
3.2. Soil pH Quantification Protocol
3.3. Permeability Characteristics of Treated Loess
3.4. Consolidated Undrained Shear Strength Evaluation
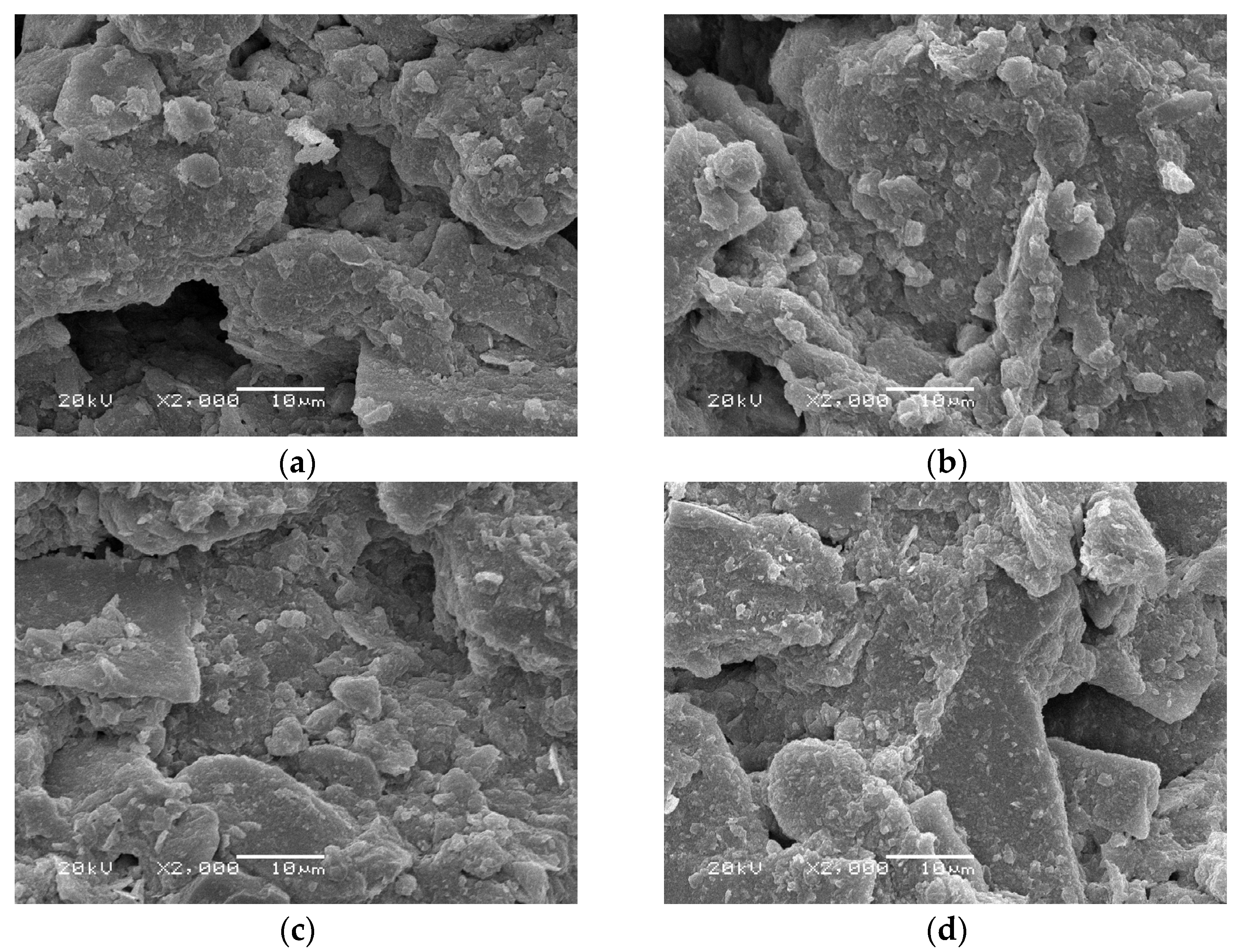
3.5. SEM Tests
4. Results and Discussions
4.1. Soil pH
4.2. Soil EC
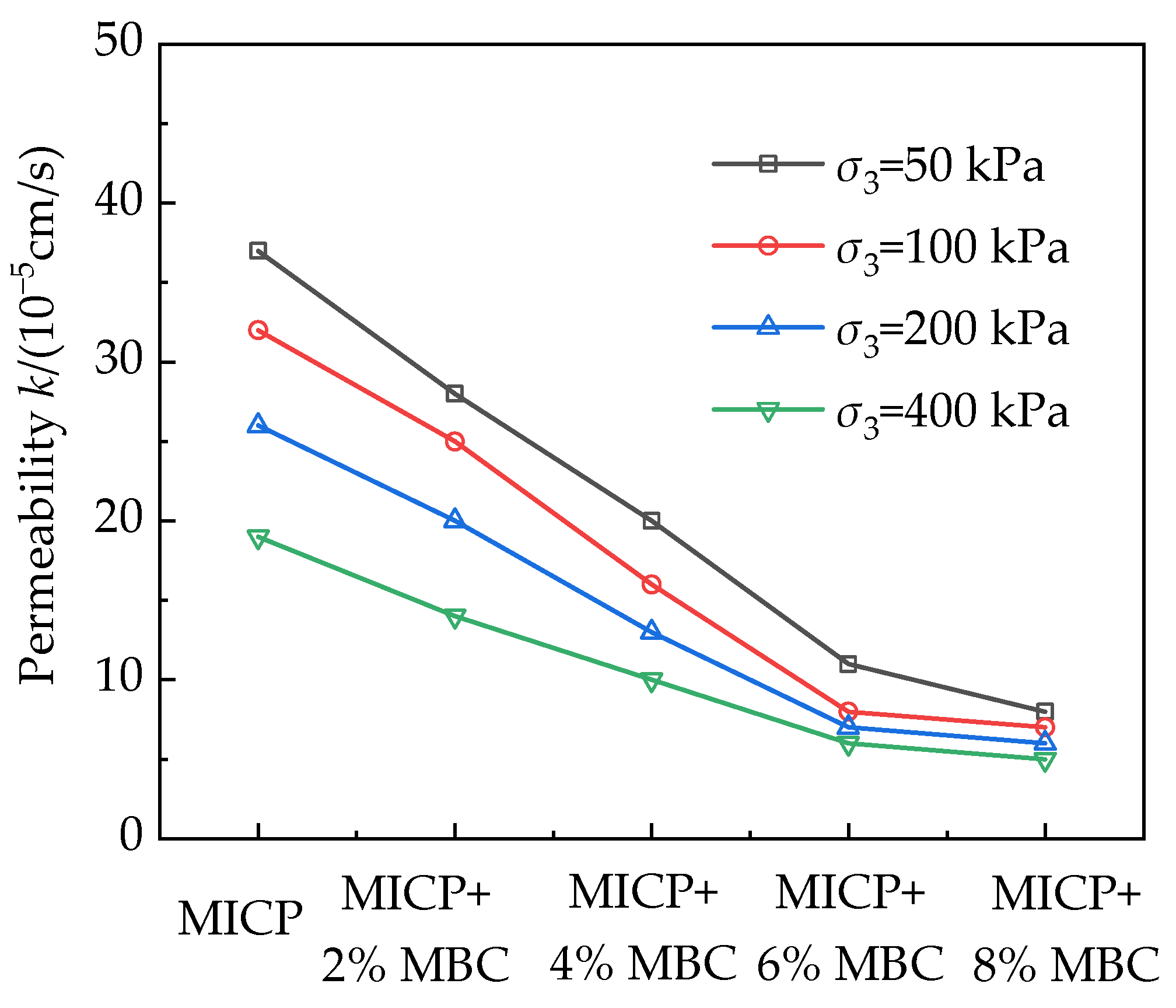
4.3. Permeability
4.3.1. Influence of Cementation Reagent Concentration on Permeability
4.3.2. Influence of MBC Content on Permeability
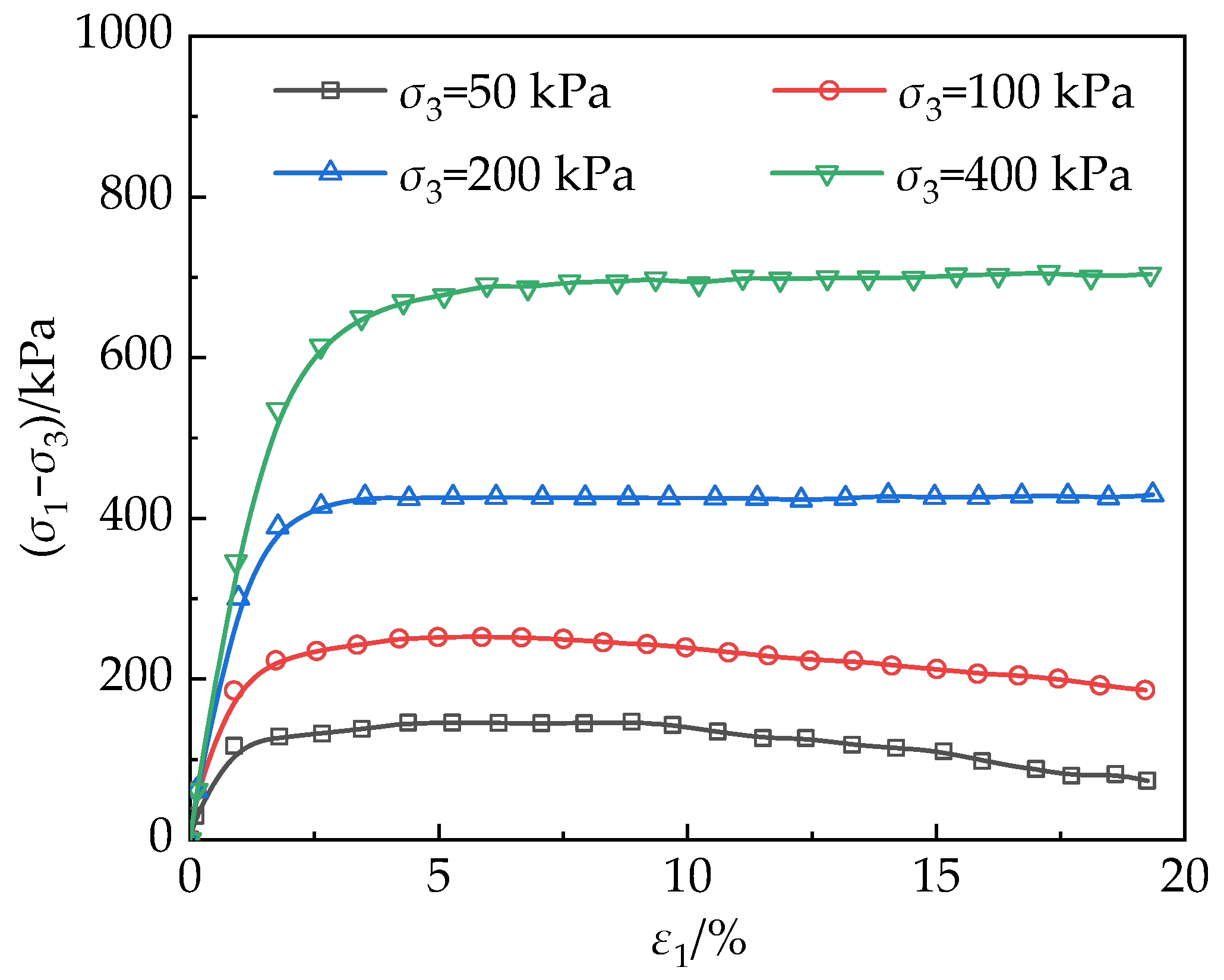
4.4. Consolidated Undrained Triaxial Shear Testing
4.4.1. Stress–Strain Behavior of Untreated Loess
4.4.2. Stress–Strain Behavior of MICP–MBC Modified Loess
4.5. Scanning Electron Microscope Microstructural Analysis
5. Conclusions
- (1)
- Combining MBC (4–6% w/w) with MICP (1.0 mol/L cementation reagent) increases shear strength by 52% and reduces hydraulic conductivity by 72% compared with untreated loess. The biochar’s nucleation sites optimize calcium carbonate distribution, while its porous structure balances pore occlusion and microbial activity.
- (2)
- Elevated confining pressures (200–400 kPa) transform loess from a brittle (strain-softening) to a ductile (strain-hardening) behavior by enhancing particle interlocking and suppressing dilatancy. Peak strength quadruples under 400 kPa confinement, emphasizing stress-dependent design for deep foundations.
- (3)
- SEM analysis revealed that MBC stabilizes dual-scale pore networks, in which macropores sustain microbial colonization, while mesopores are occluded by CaCO3-MBC composites. Residual salts are sequestered within biochar pores, mitigating efflorescence risks.
- (4)
- Optimal treatment parameters (6% MBC, 1.0 mol/L reagent, 200 kPa confinement) achieve 85% of maximum strength gain at 50% lower reagent cost, offering a scalable solution for eco-friendly infrastructure in loess regions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.H.; Miao, L.C.; Chen, R.F.; Wang, H.X.; Wu, L.Y.; Xia, J.X. Liquefaction resistance of biocemented loess soil. J. Geotech. Geoenviron. Eng. 2021, 147, 04021117. [Google Scholar] [CrossRef]
- Muñoz-Castelblanco, J.A.; Pereira, J.M.; Delage, P.; Cui, Y.J. The water retention properties of a natural unsaturated loess from northern France. Geotechnique 2012, 62, 95–106. [Google Scholar] [CrossRef]
- Wen, B.P.; Yan, Y.J. Influence of structure on shear characteristics of the unsaturated loess in Lanzhou, China. Eng. Geol. 2014, 168, 46–58. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Ma, F.; Wang, M.; Bai, X.; Zheng, Y.; Yin, H.; Zhang, G. Collapsibility, composition, and microstructure of loess in China. Can. Geotech. J. 2015, 53, 673–686. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, J.; Xiao, T.; Zhang, D. Effect of sisal fibers on the disintegration characteristics of sisal fiber-amended loess. Constr. Build. Mater. 2024, 453, 139032. [Google Scholar] [CrossRef]
- Cui, M.; Lai, H.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil improvement. Acta Geotech. 2020, 16, 481–489. [Google Scholar] [CrossRef]
- Lian, Q.; Yao, L.; Ahmad, Z.U.; Konggidinata, M.I.; Zappi, M.E.; Gang, D.D. Modeling mass transfer for adsorptive removal of Pb(II) onto phosphate modified ordered mesoporous carbon (OMC). J. Contam. Hydrol. 2020, 228, 103562. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Salifu, E.; MacLachlan, E.; Iyer, K.R.; Knapp, C.W.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef]
- Jiang, N.J.; Liu, R.; Du, Y.J.; Bi, Y.Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Unconfined compressive strength of MICP and EICP treated sands subjected to cycles of wetting-drying, freezing-thawing and elevated temperature: Experimental and EPR modelling. J. Rock Mech. Geotech. Eng. 2023, 15, 1226–1247. [Google Scholar] [CrossRef]
- Venuleo, S.; Laloui, L.; Terzis, D.; Hueckel, T.; Hassan, M. Effect of microbially induced calcite precipitation on soil thermal conductivity. Géotech. Lett. 2016, 6, 39–44. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Ji, G.S.; Huan, C.C.; Zeng, Y.; Lyu, Q.Y.; Du, Y.L.; Liu, Y.; Xu, L.S.; He, Y.; Tian, X.P.; Yan, Z.Y. Microbiologically induced calcite precipitation (MICP) in situ remediated heavy metal contamination in sludge nutrient soil. J. Hazard. Mater. 2024, 473, 134600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Song, H.W.; Mishra, S.; Zhang, W.; Zhang, Y.L.; Zhang, Q.R.; Yu, Z.G. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- Li, C.; Tian, L.; Dong, C.H.; Zhang, Y.F.; Wang, Y.X. Experimental study on zinc-lead composite contaminated soil solidified/stabilized by MICP technology combined with porous silicon adsorption materials. Rock Soil Mech. 2022, 43, 307–316. [Google Scholar]
- Wang, S.; Fang, L.Y.; Dapaah, M.F.; Niu, Q.J.; Cheng, L. Bio-remediation of heavy metal-contaminated soil by microbial-induced carbonate precipitation (MICP)—A critical review. Sustainability 2023, 15, 7622. [Google Scholar] [CrossRef]
- Duan, Y.T.; Xu, X.; Niu, L.; Wang, Z.; Huang, X.G.; Zheng, C.L. Study on the mechanism and stability of microbially induced struvite precipitation (MISP) for enhanced immobilization of uranium. J. Clean. Prod. 2024, 449, 141536. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2018, 14, 627–638. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Chen, R. Effects of different clay’s percentages on improvement of sand-clay mixtures with microbially induced calcite precipitation. Geomicrobiol. J. 2019, 36, 810–818. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuwano, R. Undrained cyclic triaxial testing on sand with non-plastic fines content cemented with microbially induced CaCO3. Soils Found. 2016, 56, 485–495. [Google Scholar] [CrossRef]
- Liu, X.J.; Fan, J.Y.; Yu, J.; Gao, X. Solidification of loess using microbial induced carbonate precipitation. J. Mt. Sci. 2021, 18, 265–274. [Google Scholar] [CrossRef]
- Tang, C.S.; Yin, L.; Jiang, N.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, J.; Tong, H.; Yuan, J.; Lei, D.; Li, Y. Effects of activated carbon on liquefaction resistance of calcareous sand treated with microbially induced calcium carbonate precipitation. Soil Dyn. Earthq. Eng. 2022, 161, 107419. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation, Japan. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, C.; Wang, C.Y.; Gao, Y. Improving the erosion resistance performance of pisha sandstone weathered soil using micp technology. Crystals 2021, 11, 1112. [Google Scholar] [CrossRef]
- Liu, X.J.; Pan, C.F.; Yu, J.; Fan, J.Y. Study on micro-characteristics of microbe-induced calcium carbonate solidified loess. Crystals 2021, 11, 1492. [Google Scholar] [CrossRef]
- He, P.; Guo, J.; Zhang, S. Investigating the potential of microbially induced carbonate precipitation combined with modified biochar for remediation of lead-contaminated loess. Sustainability 2024, 16, 7550. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Ministry of Water Resources of the People’s Republic of China, Standard for Geotechnical Test Methods. China Planning Press: Beijing, China, 2019.
- Liu, C.; Shi, B.; Zhou, J.; Tang, C. Quantification and characterization of microporosity by image processing, geometric measurement and statistical methods: Application on SEM images of clay materials. Appl. Clay Sci. 2011, 54, 97–106. [Google Scholar] [CrossRef]
- Klikova, K.; Holecek, P.; Nezerka, V.; Prosek, Z.; Konakova, D.; Demnerova, K.; Stiborova, H. Application of Sporosarcina pasteurii for the biomineralization of calcite in the treatment of waste concrete fines. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef]


| Tryptone g/L | NaCl g/L | Soytone g/L | Agar g/L | Urea g/L |
|---|---|---|---|---|
| 15 | 5 | 5 | 20 | 20 |
| Category | Incorporation Ratio (%) | Ratio of MBC (%) | Treatment Time (d) |
|---|---|---|---|
| MICP | 3 | / | 28 |
| MICP + 2%MBC | 3 | 2 | 28 |
| MICP + 4%MBC | 3 | 4 | 28 |
| MICP + 6%MBC | 3 | 6 | 28 |
| MICP + 8%MBC | 3 | 8 | 28 |
| Dry Density g/cm3 | Porosity % | Liquid Limit % | Plastic Limit % | Plastic Index % |
|---|---|---|---|---|
| 1.51 | 45.58 | 36.5 | 19 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Kong, L.; Fang, S. Synergistic Effects of Microbial-Induced Carbonate Precipitation and Modified Biochar on the Engineering Properties of Loess. Crystals 2025, 15, 504. https://doi.org/10.3390/cryst15060504
Yan Q, Kong L, Fang S. Synergistic Effects of Microbial-Induced Carbonate Precipitation and Modified Biochar on the Engineering Properties of Loess. Crystals. 2025; 15(6):504. https://doi.org/10.3390/cryst15060504
Chicago/Turabian StyleYan, Qibo, Lingwei Kong, and Shiyue Fang. 2025. "Synergistic Effects of Microbial-Induced Carbonate Precipitation and Modified Biochar on the Engineering Properties of Loess" Crystals 15, no. 6: 504. https://doi.org/10.3390/cryst15060504
APA StyleYan, Q., Kong, L., & Fang, S. (2025). Synergistic Effects of Microbial-Induced Carbonate Precipitation and Modified Biochar on the Engineering Properties of Loess. Crystals, 15(6), 504. https://doi.org/10.3390/cryst15060504




