Sodium Dithiocuprate(I) Dodecahydrate [Na3(H2O)12][CuS2], the First Crystal Structure of an Exclusively H-Bonded Dithiocuprate(I) Ion, and Its Formation in the Alkaline Sulfide Treatment of Copper Ore Concentrates
Abstract
1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Leaching Experiments
2.3. Deliberate Preparation of Crystals of Na3CuS2·12(H2O)
3. Results and Discussion
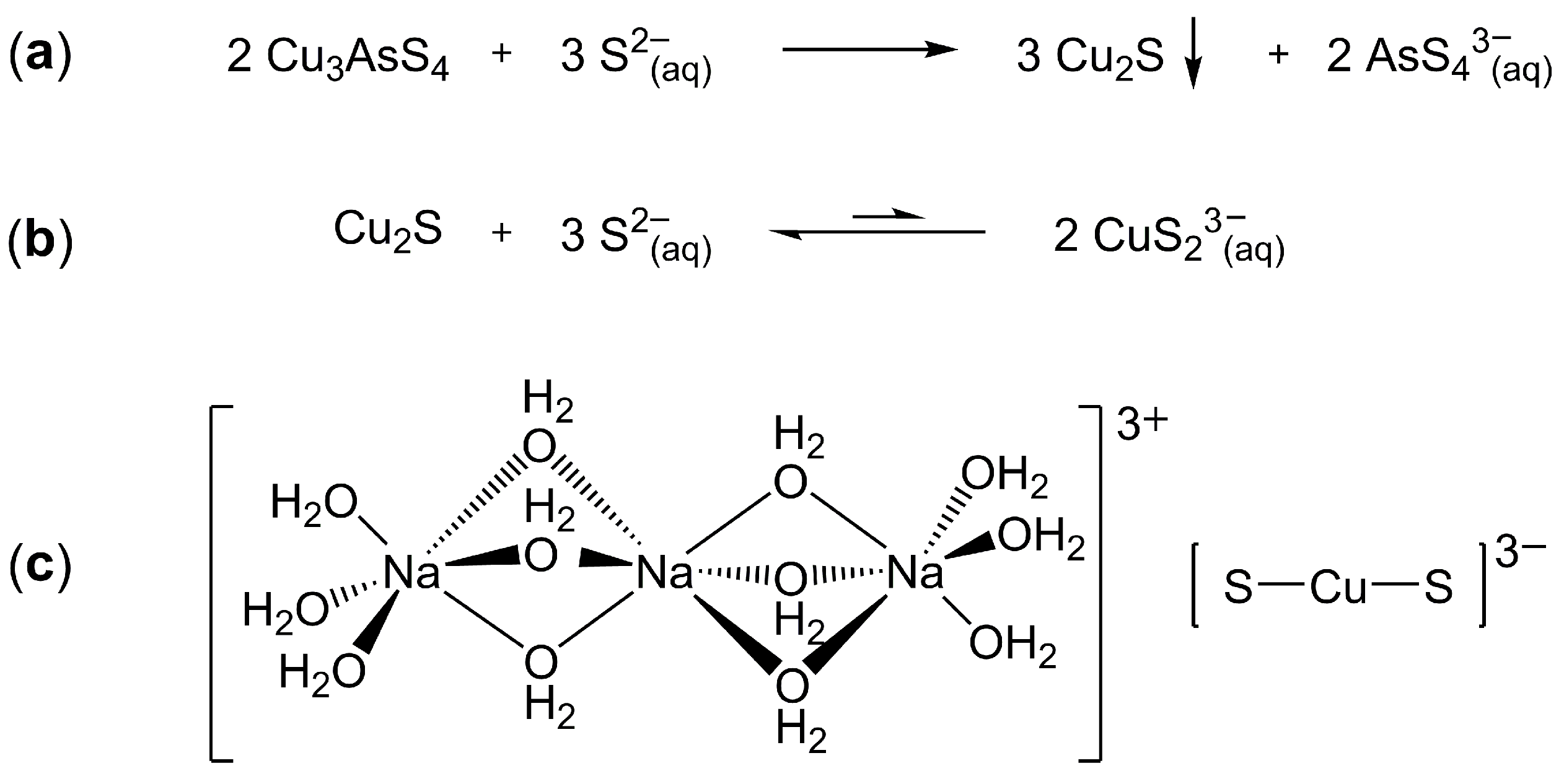
3.1. Preparation of Crystals of Na3CuS2·12(H2O)
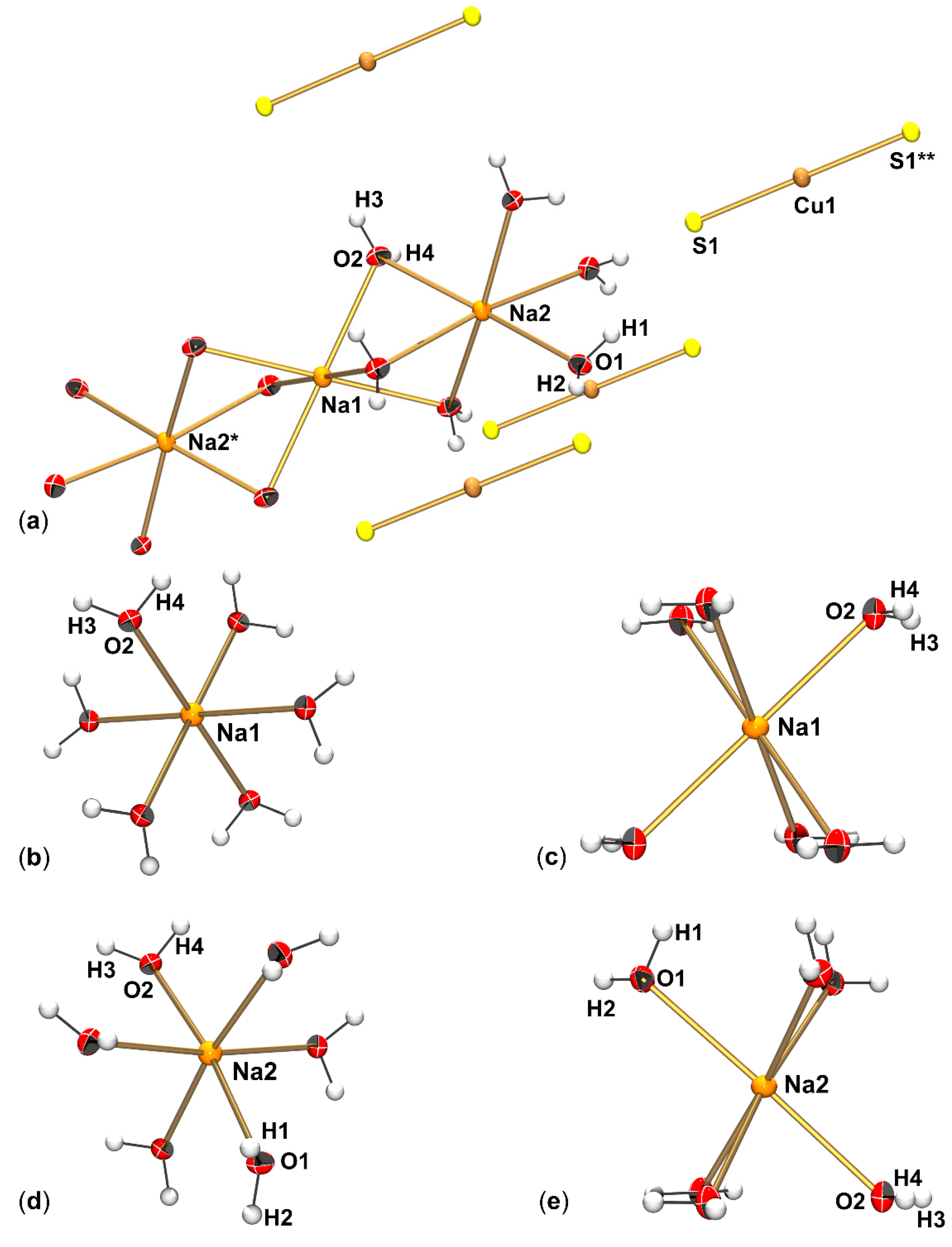
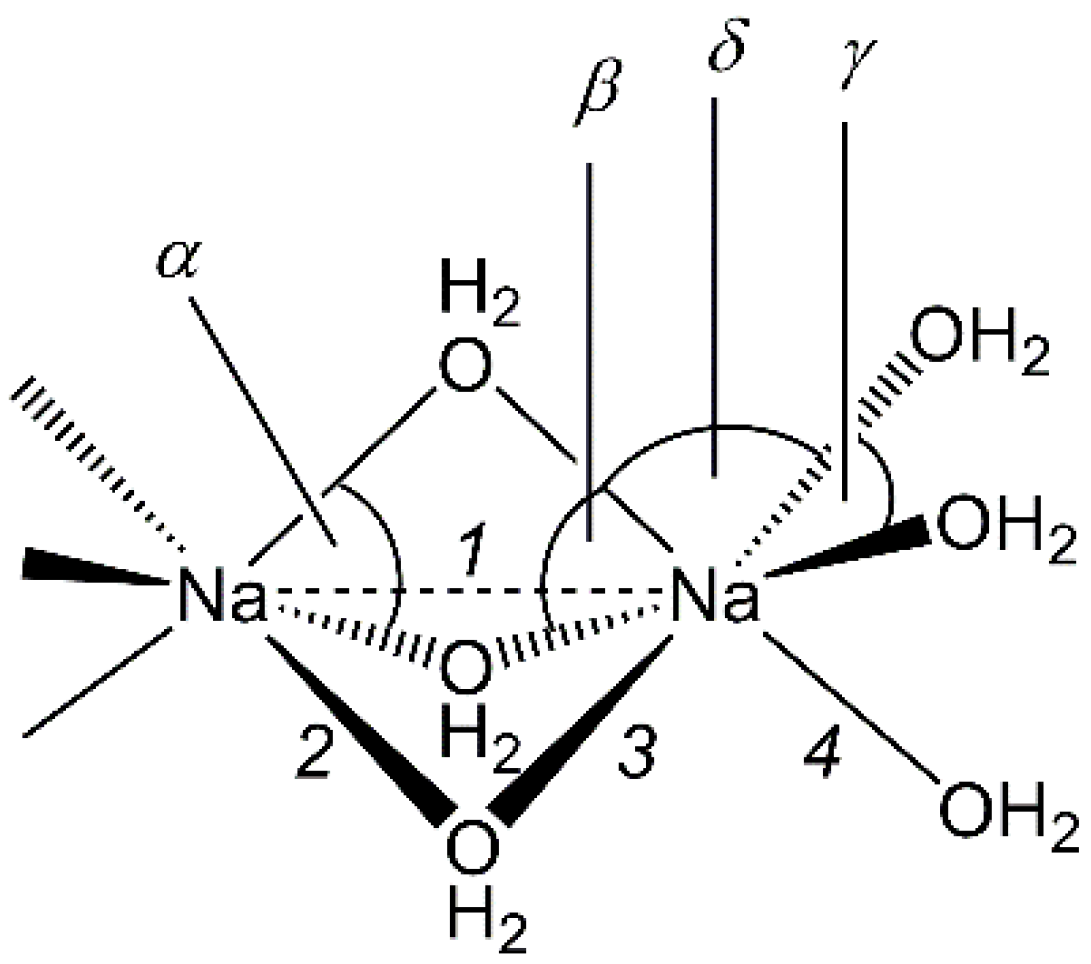
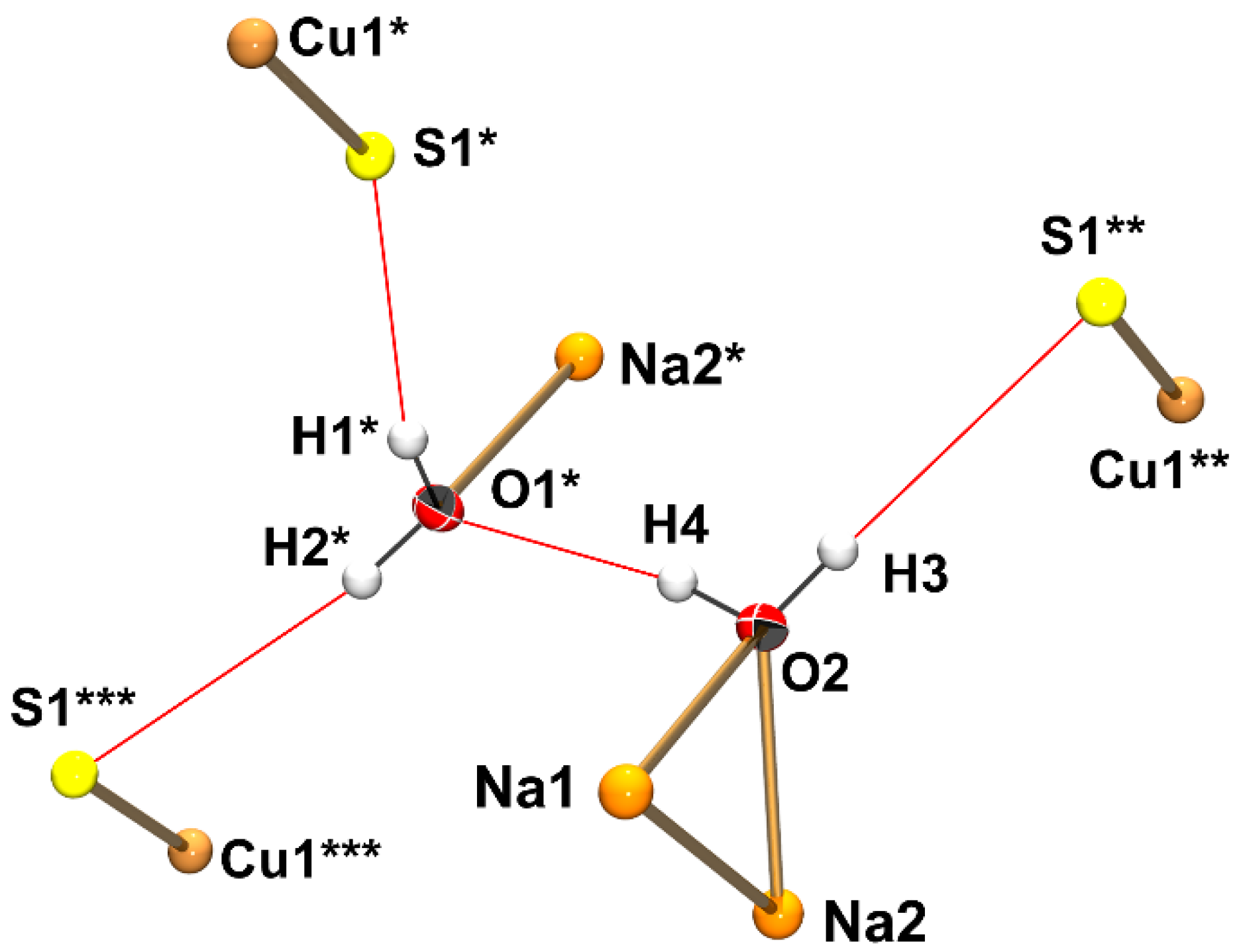
3.2. Crystallographic Analysis of Na3CuS2·12(H2O)
3.3. Raman Spectroscopic Analysis of Na3CuS2·12(H2O)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | 1 | 2 | 3 |
|---|---|---|---|
| Formula | Cu2H48Na6O24S4 1 | Cu2H48Na6O24S4 1 | Cu2H48Na6O24S4 1 |
| Mr | 825.64 | 825.64 | 825.64 |
| T (K) | 200(2) | 160(2) | 160(2) |
| Λ (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal size (mm3) | 0.15 × 0.10 × 0.05 | 0.42 × 0.38 × 0.10 | 0.35 × 0.30 × 0.08 |
| Crystal system | trigonal | trigonal | trigonal |
| Space group | c | c | c |
| a = b (Å) | 8.5067(5) | 8.4801(2) | 8.4804(2) |
| C (Å) | 38.618(4) | 38.6136(13) | 38.5146(11) |
| V (Å3) | 2420.2(4) | 2404.76(14) | 2398.77(13) |
| Z | 3 1 | 3 1 | 3 1 |
| ρcalc (g·cm−1) | 1.699 | 1.710 | 1.715 |
| μMoKα (mm−1) | 1.74 | 1.74 | 1.75 |
| F (000) | 1284 | 1284 | 1284 |
| θmax (°), Rint | 26.0, 0.0310 | 35.0, 0.0310 | 27.0, 0.0474 |
| Completeness | 99.8% | 100% | 100% |
| Reflns collected | 6016 | 11,355 | 12,829 |
| Reflns unique | 534 | 1189 | 590 |
| Reflns [I > 2σ(I)] | 391 | 970 | 495 |
| Restraints | 0 | 0 | 0 |
| Parameters | 46 2 | 46 2 | 45 2 |
| GoF | 1.021 | 1.074 | 1.100 |
| R1, wR2 [I > 2σ(I)] | 0.0188, 0.0377 | 0.0163, 0.0378 | 0.0287, 0.0907 |
| R1, wR2 (all data) | 0.0311, 0.0398 | 0.0243, 0.0398 | 0.0355, 0.0954 |
| Largest peak/hole (e·Å−3) | 0.25, −0.23 | 0.22, −0.43 | 0.90, −0.76 |
| T = 240 (2) K | T = 120 (2) K | ||
|---|---|---|---|
| a = b (Å) | 8.5046 (7) | 8.4672 (7) | |
| ∆a (Å) 1 | 0.0374 | ||
| ∆a % 1 | 0.44 | ||
| C (Å) | 38.665 (4) | 38.594 (4) | |
| ∆c (Å) 1 | 0.071 | ||
| ∆c % 1 | 0.18 | ||
| V (Å3) | 2421.9 (4) | 2396.2 (4) | |
| ∆V (Å3) 1 | 25.7 | ||
| ∆V % 1 | 1.01 |
References
- Sefarzedeh, M.; Moats, M.; Miller, J.D. Recent Trends in the Processing of Enargite Concentrates. Miner. Process. Extr. Metall. Rev. 2014, 35, 283–367. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghareman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2014, 174, 258–281. [Google Scholar] [CrossRef]
- Charitos, A.; Wrobel, M.; Runkel, M.; Hammerschmidt, J.; Demopoulos, G.P. Partial Roasting Combined with Scorodite Technology for Efficient Arsenic Removal and Stabilization from Cu-Concentrates. In Proceedings of the 63rd Conference of Metallurgists, Halifax, NS, Canada, 18 August 2024. [Google Scholar] [CrossRef]
- Brendler, E.; Meiner, K.; Wagler, J.; Thiere, A.; Charitos, A.; Stelter, M. 75As Nuclear Magnetic Resonance Spectroscopic Investigation of the Thioarsenate Speciation in Strongly Alkaline Sulfidic Leaching Solutions. Molecules 2024, 29, 2848. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.N.; Young, C.; Huang, H.; Hope, G. Spectroelectrochemistry of enargite III: Alkaline sulfide leaching. Min. Metall. Explor. 2015, 32, 14–21. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Anderson, C.; Dahlgren, E.; Huang, H.; Miranda, P.; Jeffrey, M.; Chandra, I. Fundamentals and Applications of Alkaline Sulfide Leaching and Recovery of Gold. In Proceedings of the 29th IPMI Annual Precious Metals Conference, Westmount, QC, Canada, 10 May 2014. [Google Scholar]
- Parada, F.; Jeffrey, M.I.; Asselin, E. Leaching kinetics of enargite in alkaline sodium sulphide solutions. Hydrometallurgy 2014, 146, 48–58. [Google Scholar] [CrossRef]
- Bedlivy, D.; Preisinger, A. Die Struktur von Na2S · 9H2O und Na2Se · 9H2O. Z. Kristallogr. 1965, 121, 114–130. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures, SHELXL-2019/3; University of Göttingen: Göttingen, Germany, 2019. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- POV-RAY (Version 3.7), Trademark of Persistence of Vision Raytracer Pty. Ltd., Williamstown, Victoria (Australia). Copyright Hallam Oaks Pty. Ltd., 1994–2004. Available online: http://www.povray.org/download/ (accessed on 28 June 2021).
- Labspec6 Spectroscopy Suite, Version 6.5.2.11; Horiba France SAS: Paris, France, 2020.
- Origin(Pro), Version 2019b; OriginLab Corporation: Northampton, MA, USA, 2019.
- Meiner, K.; Khulan, B.; Weigelt, A.; Thiere, A.; Vogt, D.; Stelter, M.; Kassahun, A.; Meima, J.; Charitos, A. Investigations on the selective arsenic reduction from copper concentrates by alkaline sulfide leaching and arsenic precipitation. In Proceedings of the International Copper 2022 Conference (as part of the 59th Conference of Metallurgists), Santiago de Chile, Chile, 14 November 2022; Hydrometallurgy Volume. p. 336. [Google Scholar]
- Knerr, S.; Brendler, E.; Gericke, R.; Kroke, E.; Wagler, J. Two Modifications of Nitrilotris(methylenephenylphosphinic) Acid: A Polymeric Network with Intermolecular (O=P–O–H)3 vs. Monomeric Molecules with Intramolecular (O=P–O–H)3 Hydrogen Bond Cyclotrimers. Crystals 2024, 14, 662. [Google Scholar] [CrossRef]
- Goldstein, B.M. Disorder in the structure of trisodium phosphorothioate dodecahydrate. Acta Crystallogr. Sect. B 1982, 38, 1116–1120. [Google Scholar] [CrossRef]
- Glaser, J. Crystal and Molecular Structure of Trisodiumhexachlorothallium(III) Dodekahydrate, Na3TlCl6.12H2O. Acta Chem. Scand. 1980, 34, 141–146. [Google Scholar] [CrossRef]
- Trots, D.M.; Senyshyn, A.; Mikhailova, D.A.; Vad, T.; Fuess, H. Phase transitions in jalpaite, Ag3CuS2. J. Phys. Condens. Matter 2008, 20, 455204. [Google Scholar] [CrossRef][Green Version]
- Kohner-Kerten, A.; Tshuva, E.Y. Preparation and X-ray characterization of two-coordinate Cu(I) complex of aliphatic thiolato ligand: Effect of steric bulk on coordination features. J. Organomet. Chem. 2008, 693, 2065–2068. [Google Scholar] [CrossRef]
- Guschlbauer, J.; Vollgraff, T.; Xie, X.; Weigend, F.; Sundermeyer, J. A Series of Homoleptic Linear Trimethylsilylchalcogenido Cuprates, Argentates and Aurates Cat[Me3SiE−M−ESiMe3] (M = Cu, Ag, Au; E = S, Se). Inorg. Chem. 2020, 59, 17565–17572. [Google Scholar] [CrossRef]
- Goreshnik, E.; Petrusenko, S. Cation Charge as a Tool to Change Dimensionality in Organic–Inorganic Hybrids Based on Copper Thiocyanate Templated by 1,4-Diazabicyclo[2.2.2]octane. Molecules 2023, 28, 3608. [Google Scholar] [CrossRef]
- Garner, C.D.; Nicholson, J.R.; Clegg, W. Preparation, Crystal Structure, and Spectroscopic Characterization of [NEt4]2[Cu(SPh)3]. Inorg. Chem. 1984, 23, 2148–2150. [Google Scholar] [CrossRef]
- Karanović, L.; Cvetković, L.; Poleti, D.; Balić-Žunić, T.; Makovicky, E. Crystal and absolute structure of enargite from Bor (Serbia). N. Jb. Miner. Mh. 2002, 6, 241–253. [Google Scholar] [CrossRef]
- Mereiter, K.; Preisinger, A.; Zellner, A.; Mikenda, W.; Steidl, H. Hydrogen bonds in Na2S·5H2O: X-ray diffraction and vibrational spectroscopic study. J. Chem. Soc. Dalton Trans. 1984, 13, 1275–1277. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond lengths in organic and metal-organic compounds revisited: X—H bond lengths from neutron diffraction data. Acta Crystallogr. Sect. B 2010, 66, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Brafman, O.; Mitra, S.S. Raman Effect in Wurtzite- and Zinc-Blende-Type ZnS Single Crystals. Phys. Rev. 1968, 171, 931–934. [Google Scholar] [CrossRef]
- Berkh, K.; Majzlan, J.; Meima, J.A.; Plášil, J.; Rammlmair, D. The effect of chemical variability and weathering on Raman spectra of enargite and fahlore. Eur. J. Mineral. 2023, 35, 737–754. [Google Scholar] [CrossRef]
- Angermaier, K.; Zeller, E.; Schmidbaur, H. Crystal structures of chloro(trimethylphosphine) gold(I), chloro(tri-ipropylphosphine)gold(I) and bis(trimethylphosphine) gold(I) chloride. J. Organomet. Chem. 1994, 472, 371–376. [Google Scholar] [CrossRef]
- Ainscough, E.W.; Bowmaker, G.A.; Brodie, A.M.; Freeman, G.H.; Hanna, J.V.; Jameson, G.B.; Otter, C.A. Structural and spectroscopic characterization of silver(I) tribenzylphosphane complexes including chloro and bromo derivatives with unusual stoichiometries and an iodo complex with a Ag13I13 cluster core. Polyhedron 2011, 30, 638–646. [Google Scholar] [CrossRef]
- Asplund, M.; Jagner, S.; Nilsson, M. Crystal Structures of Tetrabutylammonium Dichlorocuprate(I) and Tetrabutylammonium Dibromocuprate(I), [N(C4H9)4][CuCl2] and [N(C4H9)4][CuBr2]. Acta Chem. Scand. 1983, 37, 57–62. [Google Scholar] [CrossRef]
- Effenberger, H.; Pertlik, F. Crystal structure of NaCu5S3. Monshefte. Chem. 1985, 116, 921–926. [Google Scholar] [CrossRef]
- Savelsberg, G.; Schäfer, H. Zur Kenntnis von Na2Cu4S3 und KCu3Te2. Mat. Res. Bull. 1981, 16, 1291–1297. [Google Scholar] [CrossRef]
- Klepp, K.O.; Sing, M.; Boller, H. Preparation and crystal structure of Na4Cu2S3, a thiocuprate with discrete anions. J. Alloys Compds. 1992, 184, 265–273. [Google Scholar] [CrossRef]
- Zhou, X.; Kolluru, V.S.C.; Xu, W.; Wang, L.; Chang, T.; Chen, Y.-S.; Yu, L.; Wen, J.; Chan, M.K.Y.; Chung, D.Y.; et al. Private communication of the experimental crystal structure determination, 2022. (Private Communication to the Cambridge Structure Database, deposition number 2184426, ICSD 175836). [CrossRef]
- Hofmann, K.A.; Höchtlen, F. Krystallisirte Polysulfide von Schwermetallen. Ber. Dtsch. Chem. Ges. 1904, 37, 245–249. [Google Scholar] [CrossRef]
- Burschka, C. The Crystal Structure of NH4CuS4. Z. Natforsch. B 1980, 35, 1511–1514. [Google Scholar] [CrossRef]
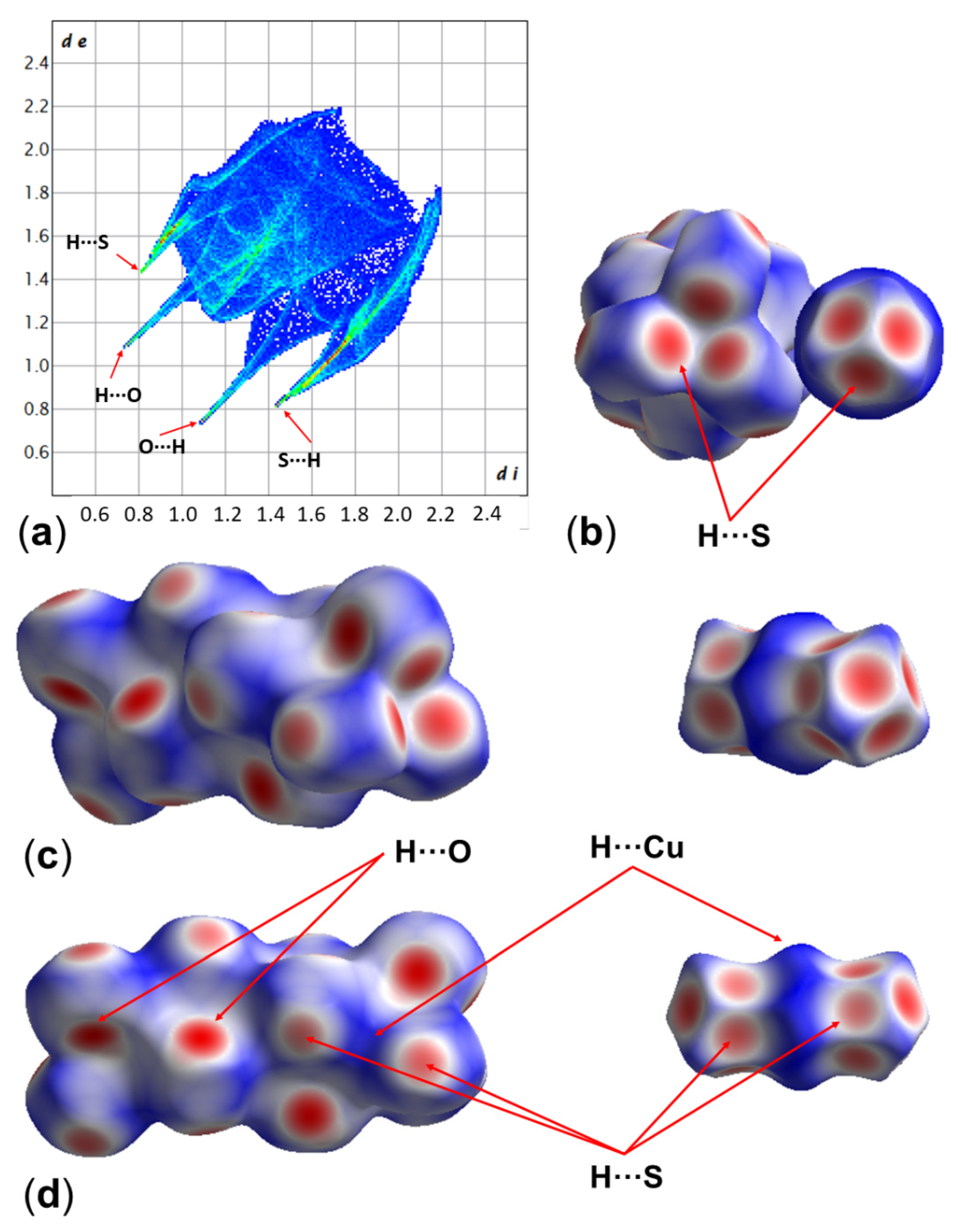

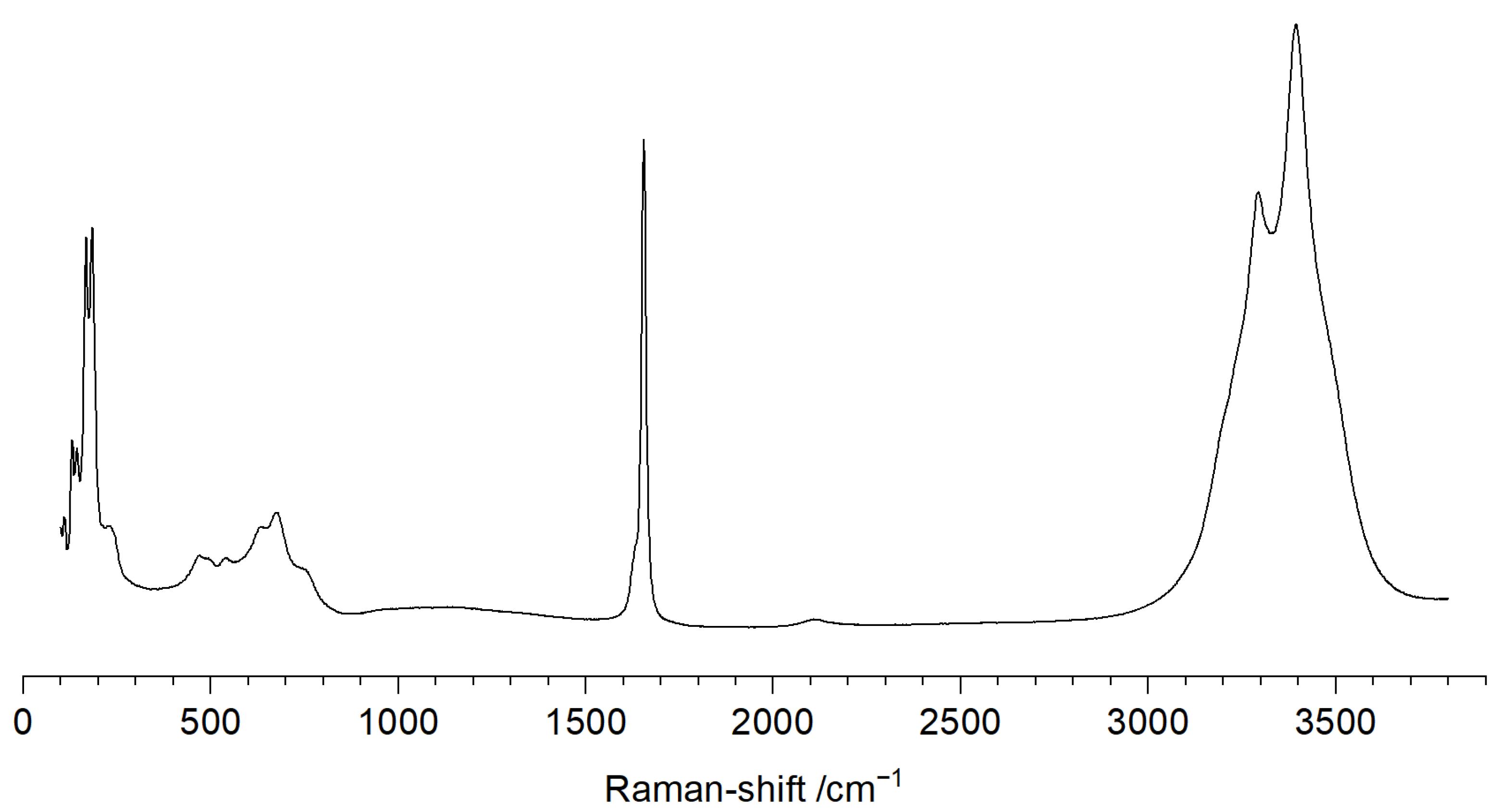
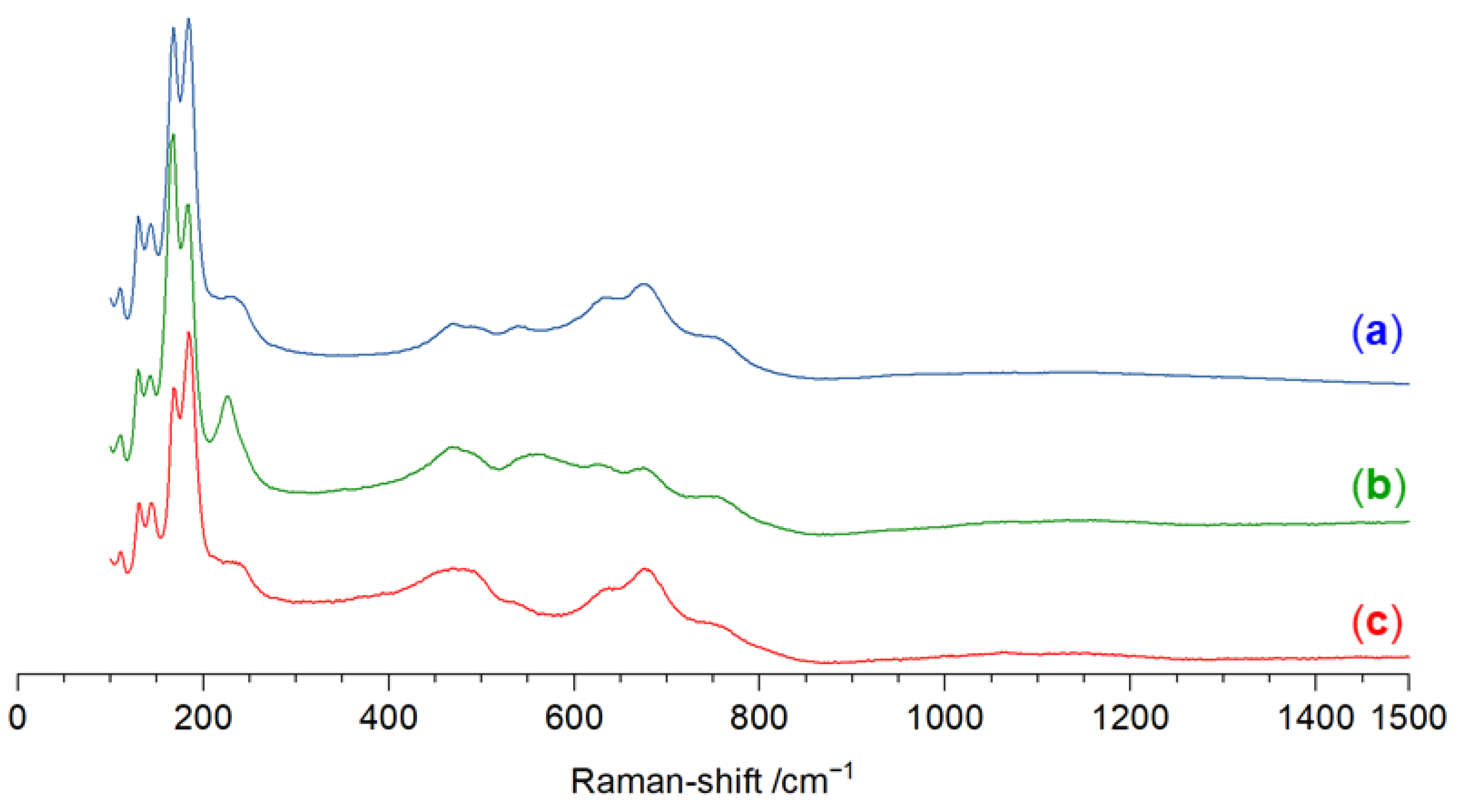
| Parameter | Value |
|---|---|
| [AsFeed-Conc.]/wt.-% | 4.0 |
| [CuFeed-Conc.]/wt.-% | 14.3 |
| [SFeed-Conc.]/wt.-% | 40.8 |
| Leaching yield of As (solid based)/% | 97 |
| Cu residual concentration of leaching residues/wt.-% | 16.7 |
| As residual concentration of leaching residues/wt.-% | 0.15 |
| Volumed feed/mL | 200 |
| Volume after leaching/mL | 186 |
| Mass of feed concentrate/g | 20.0 |
| Mass of leached concentrate/g | 11.9 |
| [AsICP-gel] in g/L | 3.52 |
| [CuICP-gel]/mg/L | 126.2 |
| Parameter | Na3CuS2·12(H2O) | Na3SPO3·12(H2O) | Na3TlCl6·12(H2O) |
|---|---|---|---|
| Crystal | |||
| Crystal system | trigonal | trigonal | trigonal |
| Space group | c | c | m |
| a [Å] | 8.4801(2) | 9.061(2) | 10.345(5) |
| c [Å] | 38.6136(13) | 34.34(2) | 18.007(5) |
| Atom distances | |||
| 1 | 3.28 | 3.18 | 3.24 |
| 2 | 2.40 | 2.40 | 2.41 |
| 3 | 2.40 | 2.38 | 2.44 |
| 4 | 2.41 | 2.38 | 2.40 |
| Angles | |||
| α | 78.3 | 80.4 | 80.7 |
| β | 78.6 | 81.1 | 79.7 |
| γ | 81.1 | 90.6 | 89.9 |
| δ | 96.1, 104.7 | 93.3, 94.7 | 86.1, 106.0 |
| ∆δ | 8.6 | 1.4 | 19.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagler, J.; Meiner, K.; Gattnar, F.; Thiere, A.; Stelter, M.; Charitos, A. Sodium Dithiocuprate(I) Dodecahydrate [Na3(H2O)12][CuS2], the First Crystal Structure of an Exclusively H-Bonded Dithiocuprate(I) Ion, and Its Formation in the Alkaline Sulfide Treatment of Copper Ore Concentrates. Crystals 2025, 15, 501. https://doi.org/10.3390/cryst15060501
Wagler J, Meiner K, Gattnar F, Thiere A, Stelter M, Charitos A. Sodium Dithiocuprate(I) Dodecahydrate [Na3(H2O)12][CuS2], the First Crystal Structure of an Exclusively H-Bonded Dithiocuprate(I) Ion, and Its Formation in the Alkaline Sulfide Treatment of Copper Ore Concentrates. Crystals. 2025; 15(6):501. https://doi.org/10.3390/cryst15060501
Chicago/Turabian StyleWagler, Jörg, Karsten Meiner, Florian Gattnar, Alexandra Thiere, Michael Stelter, and Alexandros Charitos. 2025. "Sodium Dithiocuprate(I) Dodecahydrate [Na3(H2O)12][CuS2], the First Crystal Structure of an Exclusively H-Bonded Dithiocuprate(I) Ion, and Its Formation in the Alkaline Sulfide Treatment of Copper Ore Concentrates" Crystals 15, no. 6: 501. https://doi.org/10.3390/cryst15060501
APA StyleWagler, J., Meiner, K., Gattnar, F., Thiere, A., Stelter, M., & Charitos, A. (2025). Sodium Dithiocuprate(I) Dodecahydrate [Na3(H2O)12][CuS2], the First Crystal Structure of an Exclusively H-Bonded Dithiocuprate(I) Ion, and Its Formation in the Alkaline Sulfide Treatment of Copper Ore Concentrates. Crystals, 15(6), 501. https://doi.org/10.3390/cryst15060501







