Large-Diameter Bulk Crystal Growth and Scintillation Characterization of Thallium-Based Ternary Halide Crystals for Detection and Imaging
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. 16 mm Diameter Crystal Growth
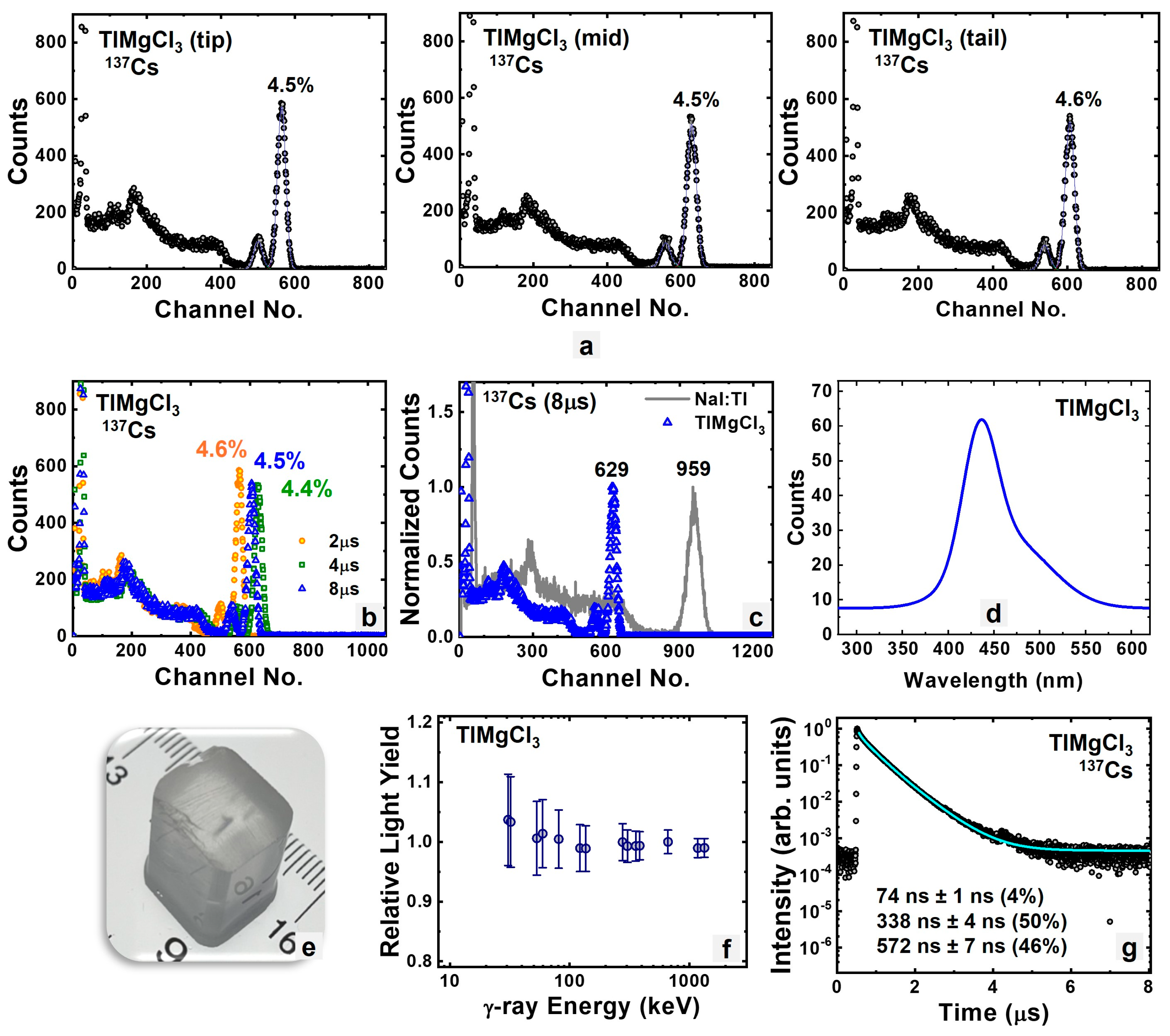
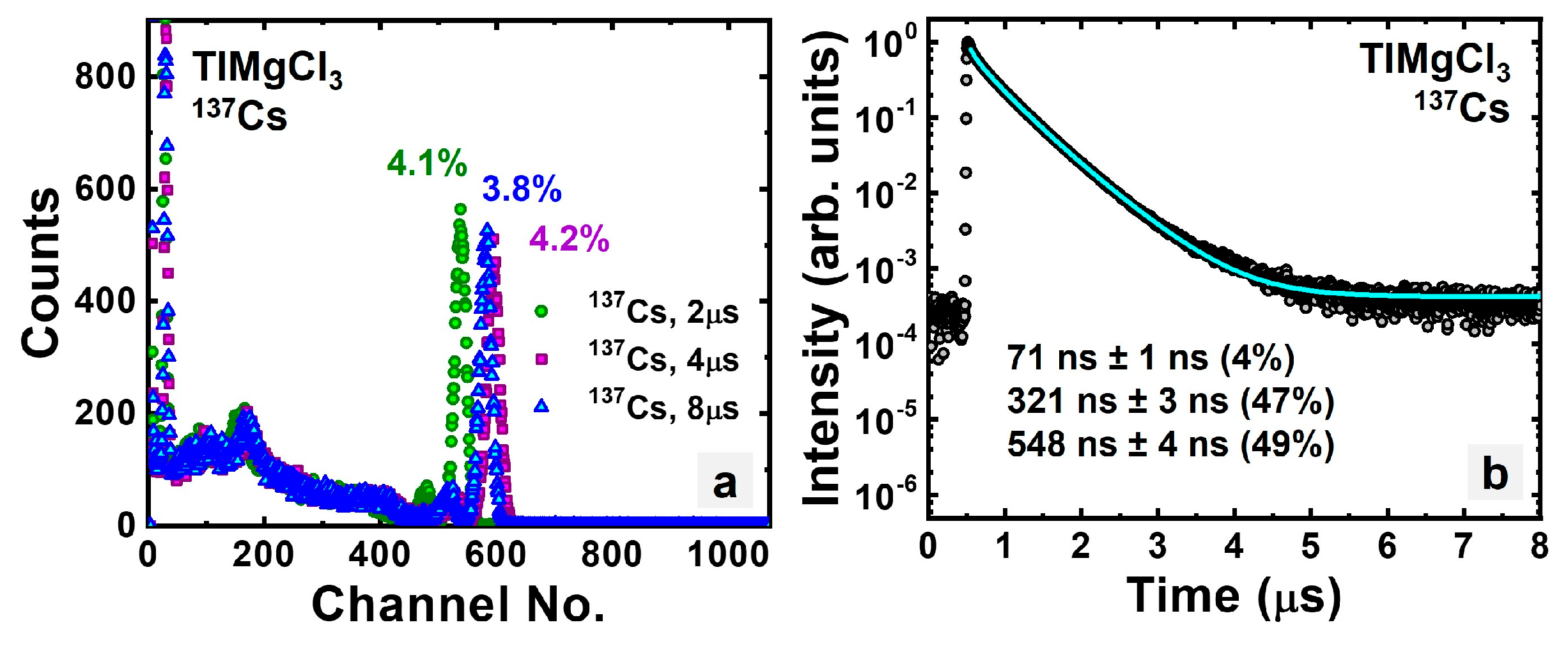
3.1.1. Intrinsic TlMgCl3
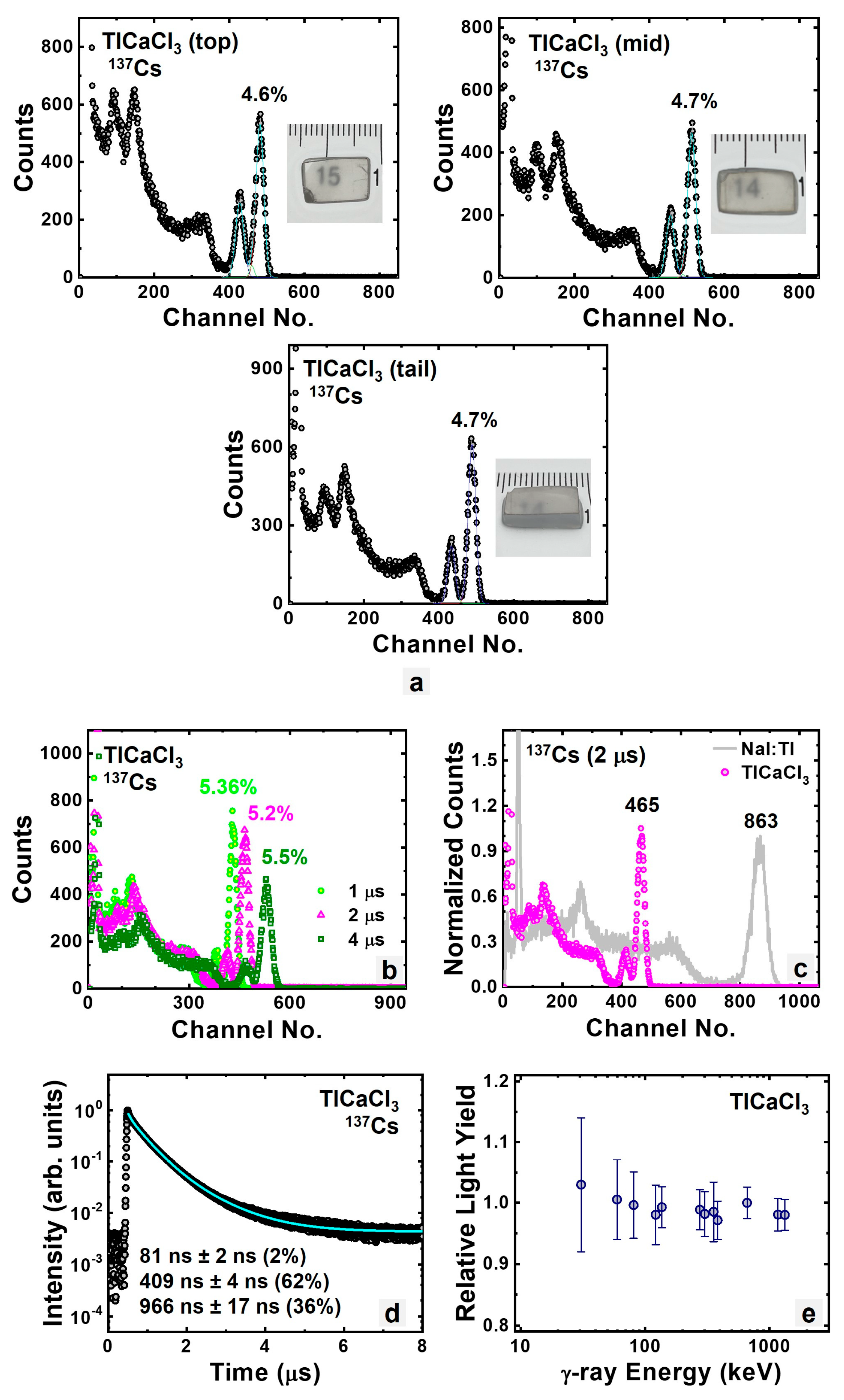
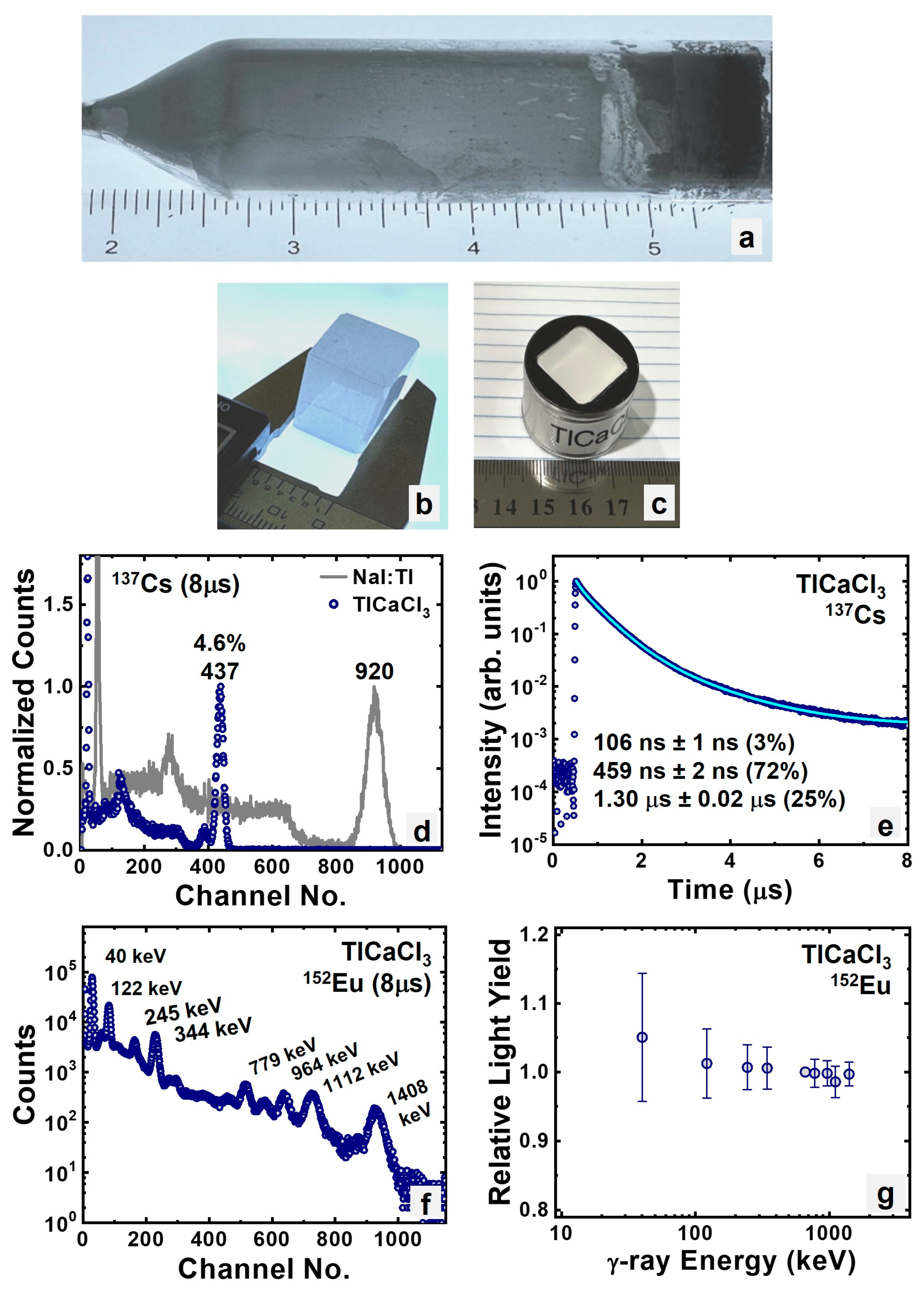
3.1.2. Intrinsic TlCaCl3
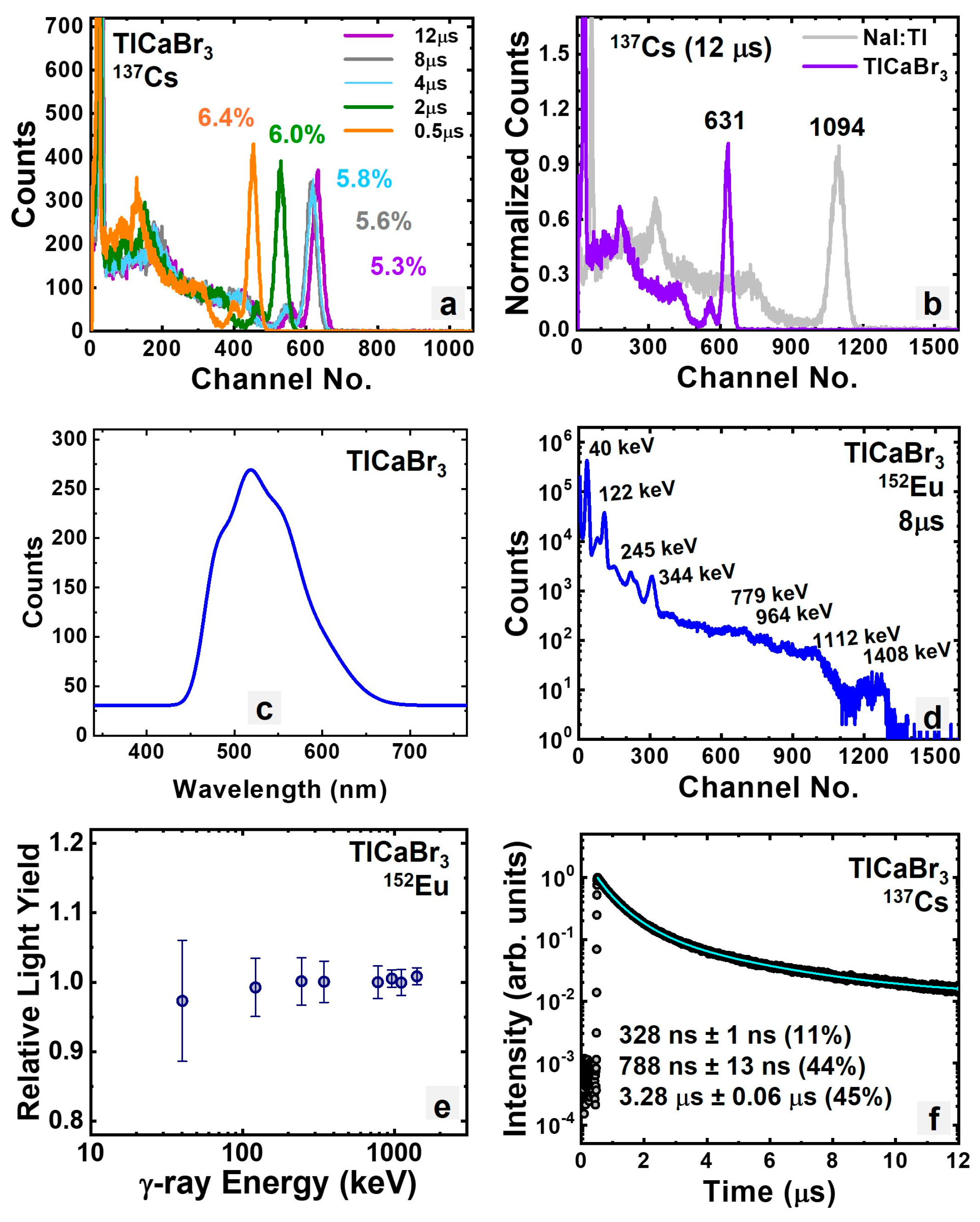
3.1.3. Intrinsic TlCaBr3
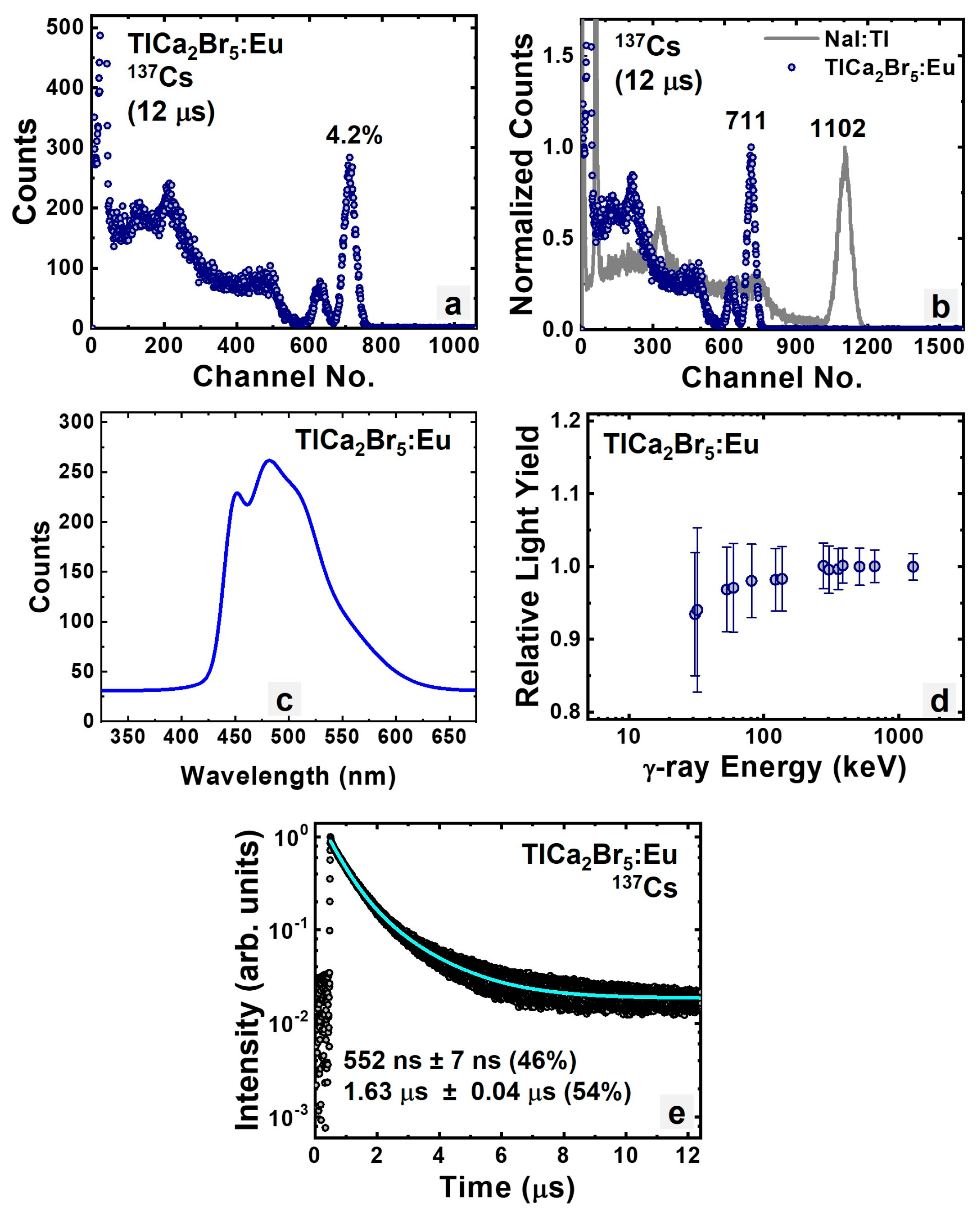
3.1.4. Intrinsic and Eu-Doped TlCa2Br5
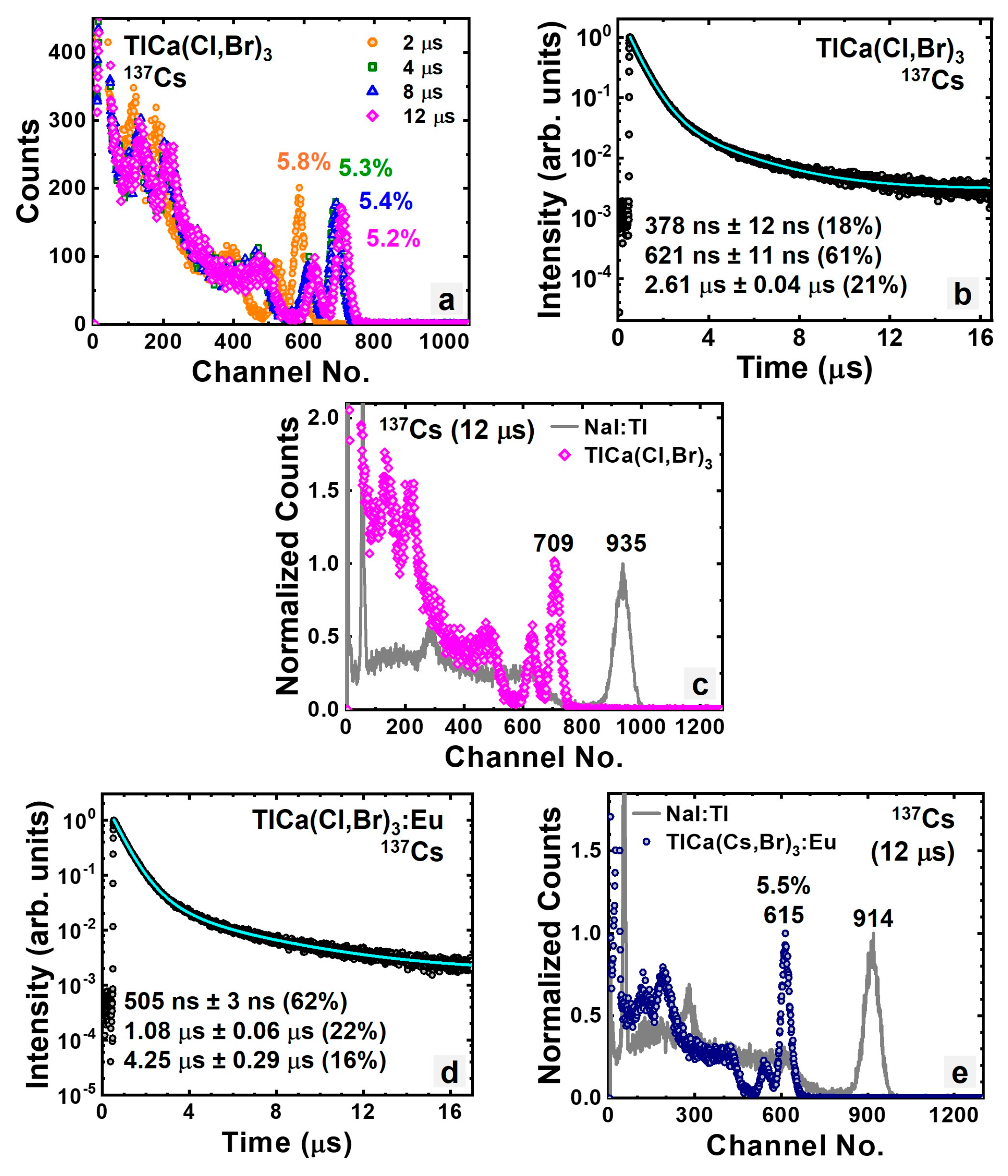
3.1.5. Intrinsic and Eu-Doped Mixed Halide TlCa(Cl,Br)3
3.2. 1-Inch-Diameter Crystal Growth
3.2.1. Intrinsic TlMgCl3
3.2.2. Intrinsic TlCaCl3
3.2.3. Eu-Doped TlSr2I5
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brau, J.E.; Jaros, J.A.; Ma, H. Advances in Calorimetry. Annu. Rev. Nucl. Part. Sci. 2010, 60, 615–644. [Google Scholar] [CrossRef]
- Inorganic Scintillator Library. Available online: https://scintillator.lbl.gov/inorganic-scintillator-library (accessed on 28 March 2024).
- Kim, H.J.; Rooh, G.; Kim, S. Tl2LaCl5 (Ce3+): New fast and efficient scintillator for X- and γ-ray detection. J. Lumin. 2017, 186, 219–222. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Wei, H.; Finkelstein, J.; Glodo, J.; Shah, K.S. Tl2LaCl5:Ce, high performance scintillator for gamma-ray detectors. Nucl. Instrum. Meth. A 2017, 869, 107–109. [Google Scholar] [CrossRef]
- Shirwadkar, U.; Loyd, M.; Du, M.-H.; van Loef, E.; Ciampi, G.; Pandian, L.S.; Stand, L.; Koschan, M.; Zhuravleva, M.; Melcher, C.; et al. Thallium-based scintillators for high-resolution gamma-ray spectroscopy: Ce3+-doped Tl2LaCl5 and Tl2LaBr5. Nucl. Instrum. Meth. A 2020, 962, 163684. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Burger, A.; Parkhe, H. Latest updates in growth and performance of Ce-doped Tl2LaCl5 and Tl2GdBr5 and Eu-doped TlCa2Br5 and TlSr2I5. Opt. Mater. 2021, 121, 111495. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Wei, H.; Finkelstein, J.; Glodo, J.; Shah, K.S. Intrinsic scintillators: TlMgCl3 and TlCaI3. J. Cryst. Growth 2017, 475, 216–219. [Google Scholar] [CrossRef]
- van Loef, E.; Pandian, L.; Kaneshige, N.; Ciampi, G.; Stand, L.; Rutstrom, D.; Tratsiak, Y.; Zhuravleva, M.; Melcher, C.; Shah, K. Crystal Growth, Density Functional Theory, and Scintillation Properties of TlCaX3 (X = Cl, Br, I). IEEE Trans. Nucl. Sci. 2023, 70, 1378–1383. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Saeki, K.; Nakauchi, S.; Yanagida, T.; Koshimizu, M.; Asai, K. New Intrinsic Scintillator with Large Effective Atomic Number: Tl2HfCl6 and Tl2ZrCl6 Crystals for X-ray and Gamma-ray Detections. Sens. Mater. 2018, 30, 1577–1583. [Google Scholar] [CrossRef]
- Vuong, P.Q.; Tyagi, M.; Kim, S.H.; Kim, H.J. Crystal growth of a novel and efficient Tl2HfCl6 scintillator with improved scintillation characteristics. Cryst. Eng. Comm. 2019, 21, 5898–5904. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Buliga, V.; Motakef, S.; Burger, A. Latest Progress on Advanced Bridgman Method-Grown K2PtCl6 Cubic Structure Scintillator Crystals. IEEE Trans. Nucl. Sci. 2020, 67, 1020–1026. [Google Scholar] [CrossRef]
- Phan, Q.V.; Kim, H.J.; Rooh, G.; Kim, S.H. Tl2ZrCl6 crystal: Efficient scintillator for X- and γ-ray spectroscopies. J. Alloys Compd. 2018, 766, 326–330. [Google Scholar] [CrossRef]
- Kim, H.J.; Rooh, G.; Khan, A.; Kim, S. New Tl2LaBr5: Ce3+ crystal scintillator for γ-rays detection. Nucl. Instrum. Meth. A 2017, 849, 72–75. [Google Scholar] [CrossRef]
- Moretti, F.; Perrodin, D.; Szornel, J.; Bourret, E.D. Full Ce substitution on La in Tl2LaCl5: Impact and performance. Mater. Adv. 2024, 5, 3858–3862. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Wei, H.; Finkelstein, J.; Glodo, J.; Shah, K. Tl2LiYCl6: Large Diameter, High Performing Dual Mode Scintillator. Crys. Growth Des. 2017, 17, 3960–3964. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Buliga, V.; Burger, A. Thallium strontium iodide: A high efficiency scintillator for gamma-ray detection. Opt. Mater. 2020, 100, 109624. [Google Scholar] [CrossRef]
- Feigelson, R.S. Crystal Growth Through the Ages: A Historical Perspective. In Handbook of Crystal Growth; Nishinaga, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1A, pp. 1–83. [Google Scholar]
- Table of Isotopes CD-ROM. Available online: https://application.wiley-vch.de/books/info/0-471-35633-6/toi99/toi.htm (accessed on 1 June 2018).
- Table of Isotopes CD-ROM: Energy and Intensity Standards. Available online: https://application.wiley-vch.de/books/info/0-471-35633-6/toi99/www/decay/eandi.pdf (accessed on 1 June 2018).
- FAQ-937 What’s the Difference Between SE and SD of Fitted Parameters in Curve Fitting? Available online: https://www.originlab.com/doc/Quick-Help/SE-SD-Diff-in-Fitting (accessed on 5 January 2025).
- Photomultiplier Tubes and Assemblies for Scintillation Counting & High Energy Physics. Available online: https://www.hamamatsu.com/content/dam/hamamatsu-photonics/sites/documents/99_SALES_LIBRARY/etd/High_energy_PMT_TPMZ0003E.pdf (accessed on 17 May 2018).
- NaI(Tl) Scintillation Crystal|Sodium Iodide. Available online: https://www.luxiumsolutions.com/radiation-detection-scintillators/crystal-scintillators/naitl-scintillation-crystals/ (accessed on 8 July 2024).


| Compound | ||
|---|---|---|
| TlMgCl3 | ∅16 mm | ∅1-inch |
| TlCaCl3 | ∅16 mm | ∅1-inch |
| TlCaBr3 | ∅14 mm | |
| TlCa2Br5 | ∅16 mm | |
| TlCa2Br5:Eu | ∅16 mm | |
| TlCa(Cl,Br)3 | ∅16 mm | |
| TlCa(Cl,Br)3:Eu | ∅16 mm | |
| TlSr2I5:Eu | ∅16 mm | ∅1-inch |
| ∅ 16 mm Crystals | ||||
|---|---|---|---|---|
| Compound | Zeff | ER | Tprimary (ns) | Light Yield (Photons/MeV) |
| TlMgCl3 | 69.7 | 4.6% | 338, 572 | 28,000 |
| TlCaCl3 | 68.9 | 5.2% | 409 | 22,000 |
| TlCaBr3 | 64.3 | 5.3% | 788, 3.28 μs | 54,000 |
| TlCa2Br5:Eu | 66.2 | 4.2% | 1.63 μs | 42,000 |
| TlCa(Cl,Br)3 | 64.3 | 5.2% | 621 | 71,000 |
| TlCa(Cl,Br)3:Eu | 66.3 | 5.5% | 505 | 63,000 |
| ∅ 1-Inch Crystals | ||||
| Compound | Zeff | ER | Tprimary (ns) | Light Yield (Photons/MeV) |
| TlMgCl3 | 69.7 | 3.8% | 321, 548 | 28,000 |
| TlCaCl3 | 68.9 | 4.6% | 459 | 19,000 |
| TlSr2I5:Eu | 68.9 | 3.5% | 395 | 72,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrami, R.; Ariesanti, E.; Sabet, H. Large-Diameter Bulk Crystal Growth and Scintillation Characterization of Thallium-Based Ternary Halide Crystals for Detection and Imaging. Crystals 2025, 15, 502. https://doi.org/10.3390/cryst15060502
Hawrami R, Ariesanti E, Sabet H. Large-Diameter Bulk Crystal Growth and Scintillation Characterization of Thallium-Based Ternary Halide Crystals for Detection and Imaging. Crystals. 2025; 15(6):502. https://doi.org/10.3390/cryst15060502
Chicago/Turabian StyleHawrami, Rastgo, Elsa Ariesanti, and Hamid Sabet. 2025. "Large-Diameter Bulk Crystal Growth and Scintillation Characterization of Thallium-Based Ternary Halide Crystals for Detection and Imaging" Crystals 15, no. 6: 502. https://doi.org/10.3390/cryst15060502
APA StyleHawrami, R., Ariesanti, E., & Sabet, H. (2025). Large-Diameter Bulk Crystal Growth and Scintillation Characterization of Thallium-Based Ternary Halide Crystals for Detection and Imaging. Crystals, 15(6), 502. https://doi.org/10.3390/cryst15060502







