Synthesis, Morphology, and Luminescent Properties of Nanocrystalline KYF4:Eu3+ Phosphors
Abstract
1. Introduction
2. Materials and Methods
3. Results
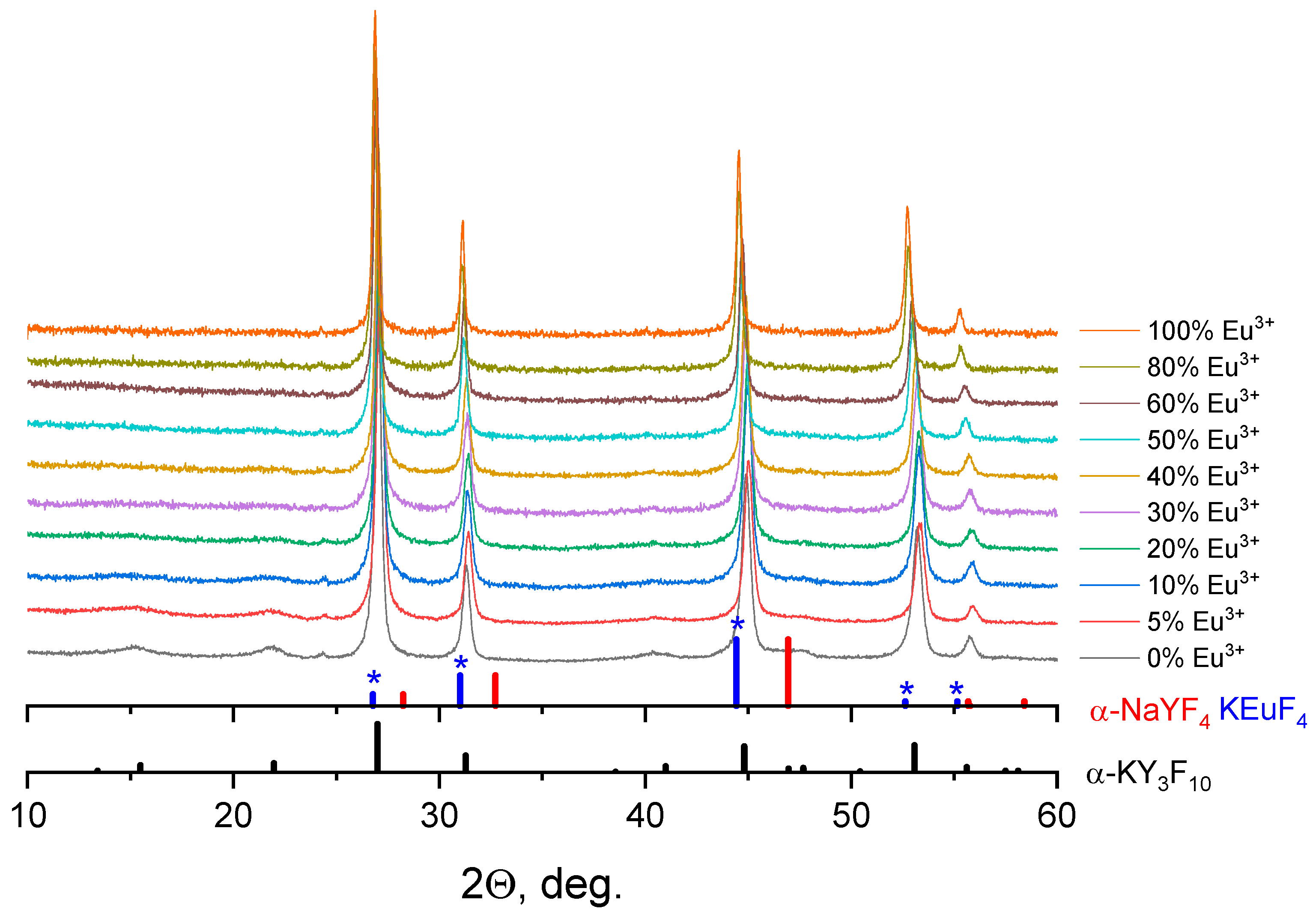
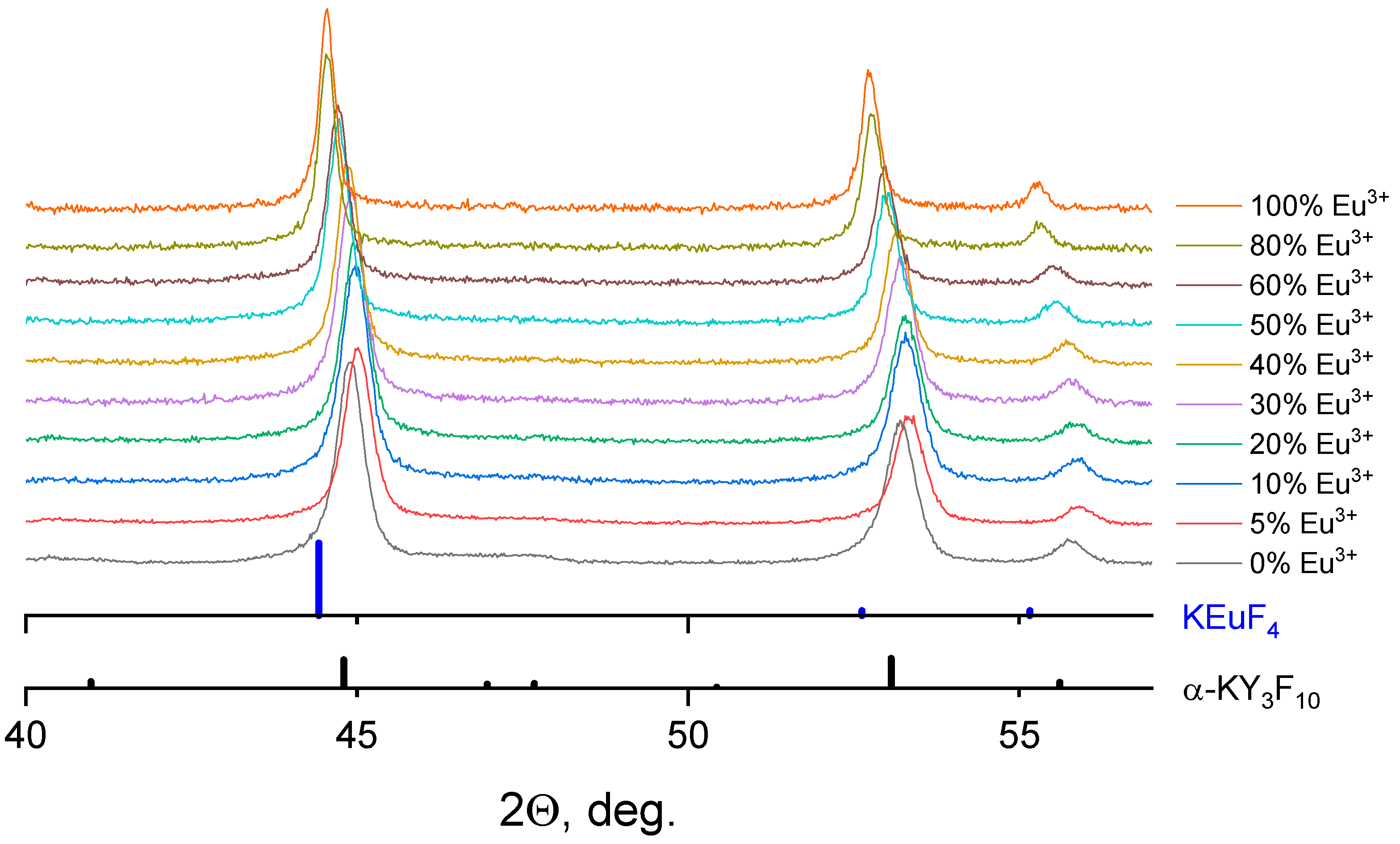
3.1. Crystal Structure and Morphology
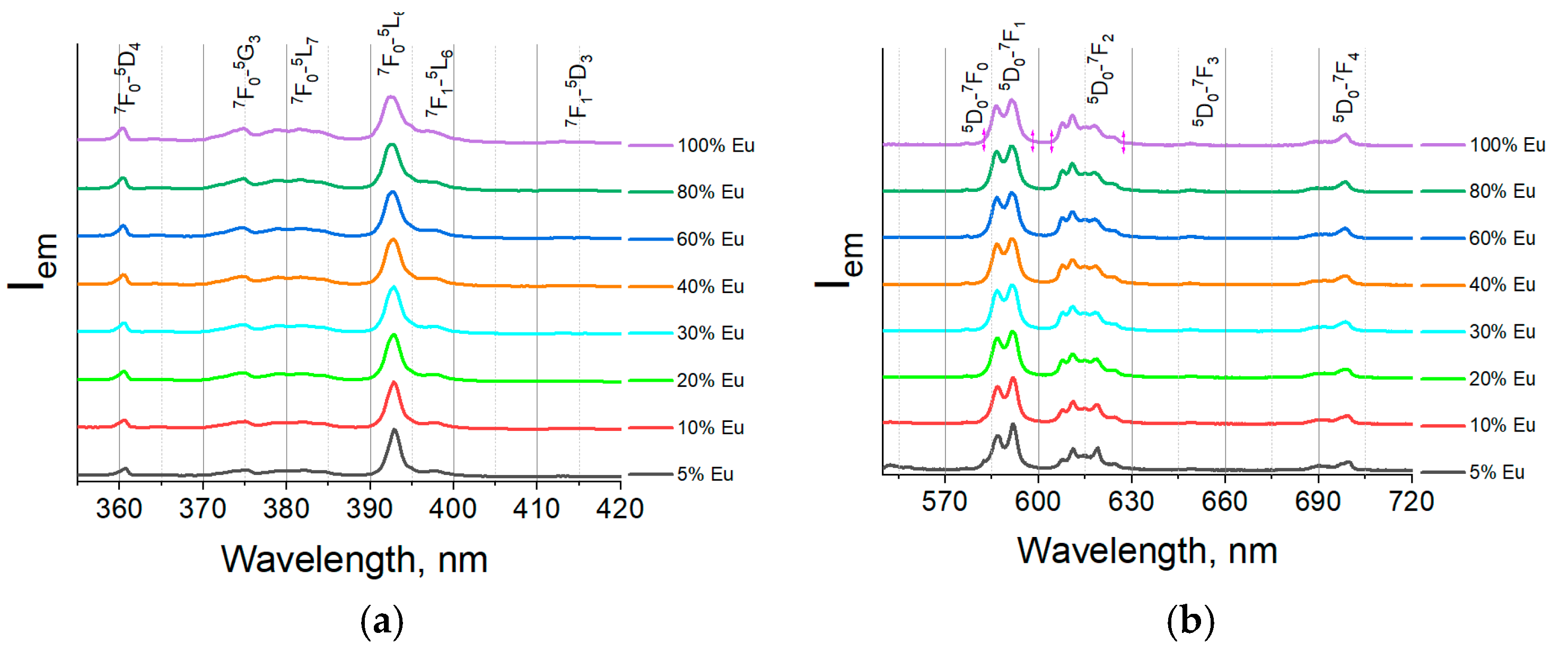
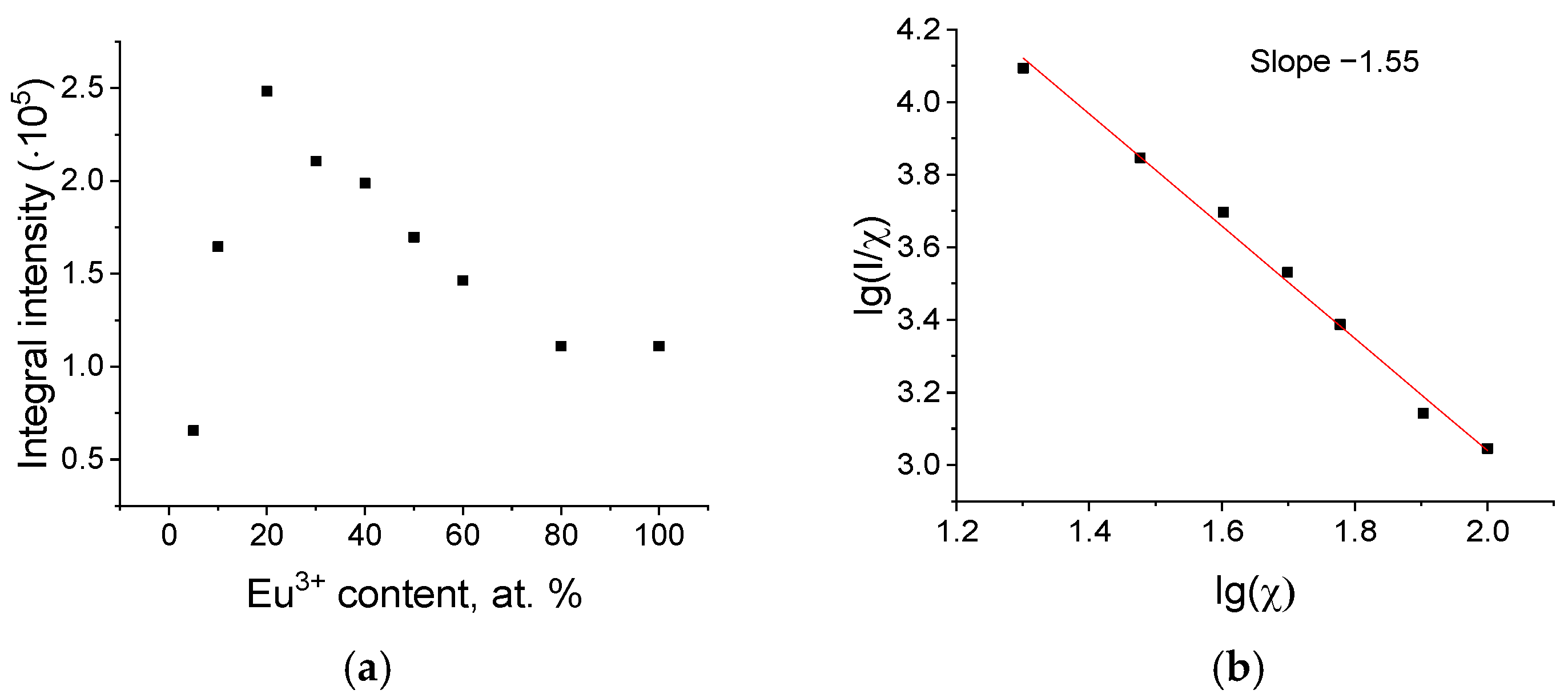
3.2. Luminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Swieten, T.P.; Yu, D.; Yu, T.; Vonk, S.J.W.; Suta, M.; Zhang, Q.; Meijerink, A.; Rabouw, F.T. A Ho3+ -Based Luminescent Thermometer for Sensitive Sensing over a Wide Temperature Range. Adv. Opt. Mater. 2021, 9, 2001518. [Google Scholar] [CrossRef]
- Ćirić, A.; Stojadinović, S.; Dramićanin, M.D. Luminescence Temperature Sensing Using Thin-Films of Undoped Gd2O3 and Doped with Ho3+, Eu3+ and Er3+ Prepared by Plasma Electrolytic Oxidation. Ceram. Int. 2020, 46, 23223–23231. [Google Scholar] [CrossRef]
- Pominova, D.; Proydakova, V.; Romanishkin, I.; Ryabova, A.; Kuznetsov, S.; Uvarov, O.; Fedorov, P.; Loschenov, V. Temperature Sensing in the Short-Wave Infrared Spectral Region Using Core-Shell NaGdF4:Yb3+, Ho3+, Er3+@NaYF4 Nanothermometers. Nanomaterials 2020, 10, 1992. [Google Scholar] [CrossRef]
- Tou, M.; Mei, Y.; Bai, S.; Luo, Z.; Zhang, Y.; Li, Z. Depositing CdS Nanoclusters on Carbon-Modified NaYF4:Yb,Tm Upconversion Nanocrystals for NIR-Light Enhanced Photocatalysis. Nanoscale 2016, 8, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, R.; Fan, R.; Huang, Z.; Liu, Y.; Guo, G.; Fu, H. Enhanced Luminescence in Yb3+ Doped Core-Shell Upconversion Nanoparticles for Sensitive Doxorubicin Detection. J. Lumin. 2020, 217, 116812. [Google Scholar] [CrossRef]
- Schulze, T.F.; Schmidt, T.W. Photochemical Upconversion: Present Status and Prospects for Its Application to Solar Energy Conversion. Energy Environ. Sci. 2015, 8, 103–125. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.; Liu, R.; Li, G. Screen Printing of Multi-Mode Emission NaYF4:Yb,Er(Tm)/NaYF4:Ce,Mn Composites for Anti-Counterfeiting Applications. New J. Chem. 2021, 45, 9818–9828. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion Luminescence Properties of NaYF4:Yb:Er Nanoparticles Codoped with Gd3+. J. Phys. Chem. C 2015, 119, 3363–3373. [Google Scholar] [CrossRef]
- Lu, E.; Pichaandi, J.; Arnett, L.P.; Tong, L.; Winnik, M.A. Influence of Lu3+ Doping on the Crystal Structure of Uniform Small (5 and 13 Nm) NaLnF4 Upconverting Nanocrystals. J. Phys. Chem. C 2017, 121, 18178–18185. [Google Scholar] [CrossRef]
- Hill, T.K.; Mohs, A.M. Image-guided Tumor Surgery: Will There Be a Role for Fluorescent Nanoparticles? WIREs Nanomed. Nanobiotechnology 2016, 8, 498–511. [Google Scholar] [CrossRef]
- Sun, J.-X.; Xu, J.-Z.; An, Y.; Ma, S.-Y.; Liu, C.-Q.; Zhang, S.-H.; Luan, Y.; Wang, S.-G.; Xia, Q.-D. Future in Precise Surgery: Fluorescence-Guided Surgery Using EVs Derived Fluorescence Contrast Agent. J. Control. Release 2023, 353, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Quan, Z.; Yang, P.; Kong, D.; Lin, J. Different Microstructures of β-NaYF4 Fabricated by Hydrothermal Process: Effects of PH Values and Fluoride Sources. Chem. Mater. 2007, 19, 4933–4942. [Google Scholar] [CrossRef]
- Li, S.; Ye, S.; Chen, X.; Liu, T.; Guo, Z.; Wang, D. OH− Ions-Controlled Synthesis and Upconversion Luminescence Properties of NaYF4:Yb3+,Er3+ Nanocrystals via Oleic Acid-Assisted Hydrothermal Process. J. Rare Earths 2017, 35, 753–760. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Tian, L.; Yu, Y.; Kong, X.; Zhao, J.; Zhang, H. Controlled Synthesis and Morphology Dependent Upconversion Luminescence of NaYF4:Yb, Er Nanocrystals. Nanotechnology 2007, 18, 275609. [Google Scholar] [CrossRef]
- Sun, C.; Schäferling, M.; Resch-Genger, U.; Gradzielski, M. Solvothermal Synthesis of Lanthanide-doped NaYF4 Upconversion Crystals with Size and Shape Control: Particle Properties and Growth Mechanism. ChemNanoMat 2021, 7, 174–183. [Google Scholar] [CrossRef]
- Guo, X.; Li, P.; Yin, S.; Qin, W. Effect of NaF/RE (RE=Yb, Tm) Molar Ratio on the Morphologies and Upconversion Properties of NaYbF4:Tm3+ Microrods. J. Fluor. Chem. 2017, 200, 91–95. [Google Scholar] [CrossRef]
- Zhuang, J.; Liang, L.; Sung, H.H.Y.; Yang, X.; Wu, M.; Williams, I.D.; Feng, S.; Su, Q. Controlled Hydrothermal Growth and Up-Conversion Emission of NaLnF4 (Ln = Y, Dy−Yb). Inorg. Chem. 2007, 46, 5404–5410. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, F.; Yao, L.; Cai, W.; Li, X. Selected Synthesis of Cubic and Hexagonal NaYF4 Crystals via a Complex-Assisted Hydrothermal Route. J. Cryst. Growth 2006, 290, 296–300. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Chen, B.; Zhao, F.; Xu, X.; Ren, K.; Lu, Y.; Qiao, X.; Qian, G.; Fan, X. Phase and Morphology Evolution of Luminescent NaLnF4 (Ln = La to Yb) Micro-Crystals: Understanding the Ionic Radii and Surface Energy-Dependent Solution Growth Mechanism. CrystEngComm 2019, 21, 6652–6658. [Google Scholar] [CrossRef]
- Ladol, J.; Khajuria, H.; Khajuria, S.; Sheikh, H.N. Hydrothermal Synthesis, Characterization and Luminescent Properties of Lanthanide-Doped NaLaF4 Nanoparticles. Bull. Mater. Sci. 2016, 39, 943–952. [Google Scholar] [CrossRef][Green Version]
- Kolesnikov, I.E.; Vidyakina, A.A.; Vasileva, M.S.; Nosov, V.G.; Bogachev, N.A.; Sosnovsky, V.B.; Skripkin, M.Y.; Tumkin, I.I.; Lähderanta, E.; Mereshchenko, A.S. The Effect of Eu3+ and Gd3+ Co-Doping on the Morphology and Luminescence of NaYF4:Eu3+, Gd3+ Phosphors. New J. Chem. 2021, 45, 10599–10607. [Google Scholar] [CrossRef]
- Vidyakina, A.A.; Kolesnikov, I.E.; Bogachev, N.A.; Skripkin, M.Y.; Tumkin, I.I.; Lähderanta, E.; Mereshchenko, A.S. Gd3+-Doping Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials 2020, 13, 3397. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous Phase and Size Control of Upconversion Nanocrystals through Lanthanide Doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef]
- Vidyakina, A.A.; Zheglov, D.A.; Oleinik, A.V.; Freinkman, O.V.; Kolesnikov, I.E.; Bogachev, N.A.; Skripkin, M.Y.; Mereshchenko, A.S. Microcrystalline Anti-Stokes Luminophores NaYF4 Doped with Ytterbium, Erbium, and Lutetium Ions. Russ. J. Gen. Chem. 2021, 91, 844–849. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, K.; Verma, S.; Chaudhary, B.; Som, S.; Sharma, V.; Kumar, V.; Swart, H.C. Recent Advances in Enhanced Luminescence Upconversion of Lanthanide-Doped NaYF4 Phosphors. Phys. B Condens. Matter 2018, 535, 278–286. [Google Scholar] [CrossRef]
- Cressoni, C.; Vurro, F.; Milan, E.; Muccilli, M.; Mazzer, F.; Gerosa, M.; Boschi, F.; Spinelli, A.E.; Badocco, D.; Pastore, P.; et al. From Nanothermometry to Bioimaging: Lanthanide-Activated KY 3 F 10 Nanostructures as Biocompatible Multifunctional Tools for Nanomedicine. ACS Appl. Mater. Interfaces 2023, 15, 12171–12188. [Google Scholar] [CrossRef] [PubMed]
- Serna-Gallén, P. Fantastic Photons and Where to Excite Them: Revolutionizing Upconversion with KY3F10-Based Compounds. Crystals 2024, 14, 762. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, M.; Zhao, L.; Feng, W.; Li, F.; Huang, C. Visible-Light-Excited and Europium-Emissive Nanoparticles for Highly-Luminescent Bioimaging in Vivo. Biomaterials 2014, 35, 5830–5839. [Google Scholar] [CrossRef]
- McNemar, C.W.; Horrocks, W. Dew. Europium(III) Ion Luminescence as a Structural Probe of Parvalbumin Isotypes. Biochim. Biophys. Acta (BBA)–Protein Struct. Mol. Enzymol. 1990, 1040, 229–236. [Google Scholar] [CrossRef]
- Das, D.; Gupta, S.K.; Sudarshan, K. Europium Luminescence as a Structural Probe to Understand Defect Evolution in CeO2/Eu3+, M3+ (M = Y and La): Contrasting Role of Codopant Ionic Size. J. Mater. Sci. 2021, 56, 17205–17220. [Google Scholar] [CrossRef]
- Cao, C.; Yang, H.K.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, K.H. Hydrothermal Synthesis, Phase Evolution, and Optical Properties of Eu3+ -Doped KF–YF 3 System Materials. J. Mater. Res. 2012, 27, 2988–2995. [Google Scholar] [CrossRef]
- Bartholomew, J.G.; Zhang, Z.; Di Lieto, A.; Tonelli, M.; Goldner, P. High Resolution Spectroscopy of the 7F0↔5D0 Transition in Eu3+:KYF4. J. Lumin. 2016, 171, 221–225. [Google Scholar] [CrossRef]
- Bajaj, R.; Rao, A.S.; Prakash, G.V. Linear and Nonlinear Photoluminescence from Thermally Stable KYF4:Eu3+ Cubic Nanocrystals. J. Alloys Compd. 2021, 885, 160893. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xiao, Q.; Zhu, H.; Li, R.; Chen, X. Eu3+ Doped KYF4 Nanocrystals: Synthesis, Electronic Structure, and Optical Properties. Nanoscale 2011, 3, 3164. [Google Scholar] [CrossRef]
- Andraud, C.; Denis, J.P.; Blanzat, B.; Vedrine, A. Luminescent Properties of Eu3+ and Tb3+ in KY3F10. Chem. Phys. Lett. 1983, 101, 357–360. [Google Scholar] [CrossRef]
- Serna-Gallén, P.; Beltrán-Mir, H.; Cordoncillo, E.; Balda, R.; Fernández, J. A Site-Selective Fluorescence Spectroscopy Study of the Crystal Phases of KY3F10: Leveraging the Optical Response of Eu3+ Ions. J. Alloys Compd. 2023, 953, 170020. [Google Scholar] [CrossRef]
- Gaume-Mahn, F.; Boulon, G.; Gâcon, J.C.; Sériot, J.; Védrine, A. Spectra-Structure Relations in KY3F10 Isotypic Compounds Doped with Eu2+ or Eu3+. In The Rare Earths in Modern Science and Technology; Springer: Boston, MA, USA, 1978; pp. 547–552. [Google Scholar]
- Porcher, P.; Caro, P. Crystal Field Parameters for Eu3+ in KY3F10. J. Chem. Phys. 1976, 65, 89–94. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, J.; Cao, X. Sonochemical Synthesis of Monodispersed KY3F10:Eu3+ Nanospheres with Bimodal Size Distribution. Mater. Lett. 2008, 62, 3007–3009. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Szefczyk, B.; Roszak, R.; Roszak, S. Structure of the Hexagonal NaYF4 Phase from First-Principles Molecular Dynamics. RSC Adv. 2014, 4, 22526. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Povolotskiy, A.V.; Mamonova, D.V.; Kurochkin, E.Y.; Kurochkin, A.V.; Lahderanta, E.; Mikhailov, M.D. Asymmetry ratio as a parameter of Eu3+ local environment in phosphors. J. Rare Earths 2018, 36, 474–481. [Google Scholar] [CrossRef]
- Honma, T.; Toda, K.; Ye, Z.-G.; Sato, M. Concentration quenching of the eu3+-activated luminescence in some layered perovskites with two-dimensional arrangement. J. Phys. Chem. Solids 1998, 59, 1187–1193. [Google Scholar] [CrossRef]
- Naik, R.; Nagabhushana, H.; Sharma, S.C.; Nagabhushana, B.M.; Nagaswarupa, H.P.; Premkumar, H.B. Low temperature synthesis and photoluminescence properties of red emitting Mg2SiO4:Eu3⁺ nanophosphor for near UV light emitting diodes. Sens. Actuators B Chem. 2014, 195, 140–149. [Google Scholar] [CrossRef]
- Van Uitert, L.G. Characterization of energy transfer interactions between rare earth ions. J. Electrochem. Soc. 1967, 114, 1048–1053. [Google Scholar] [CrossRef]
- Miao, J.; Su, J.; Wen, Y.; Rao, W. Preparation, Characterization and Photoluminescence of Sm3+ Doped NaGdF4 Nanoparticles. J. Alloys Compd. 2015, 636, 8–11. [Google Scholar] [CrossRef]
- Singh, N.S.; Ningthoujam, R.S.; Luwang, M.N.; Singh, S.D.; Vatsa, R.K. Luminescence, Lifetime and Quantum Yield Studies of YVO4:Ln3+ (Ln3+ = Dy3+, Eu3+) Nanoparticles: Concentration and Annealing Effects. Chem. Phys. Lett. 2009, 480, 237–242. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, S.; Shao, W.; Zhang, X.; Xu, J.; Yu, L.; Chen, K. Enhanced Up-Conversion Luminescence from NaYF4:Yb,Er Nanocrystals by Gd3+ Ions Induced Phase Transformation and Plasmonic Au Nanosphere Arrays. RSC Adv. 2016, 6, 102869–102874. [Google Scholar] [CrossRef]
- Shi, F.; Zhao, Y. Sub-10 Nm and Monodisperse β-NaYF4:Yb,Tm,Gd Nanocrystals with Intense Ultraviolet Upconversion Luminescence. J. Mater. Chem. C 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Kolokolov, D.S.; Kurochkin, A.V.; Voznesenskiy, M.A.; Osmolowsky, M.G.; Lahderanta, E.; Osmolovskaya, O.M. Morphology and doping concentration effect on the luminescence properties of SnO2:Eu3+ nanoparticles. J. Alloys Compd. 2020, 824, 153640–153648. [Google Scholar] [CrossRef]


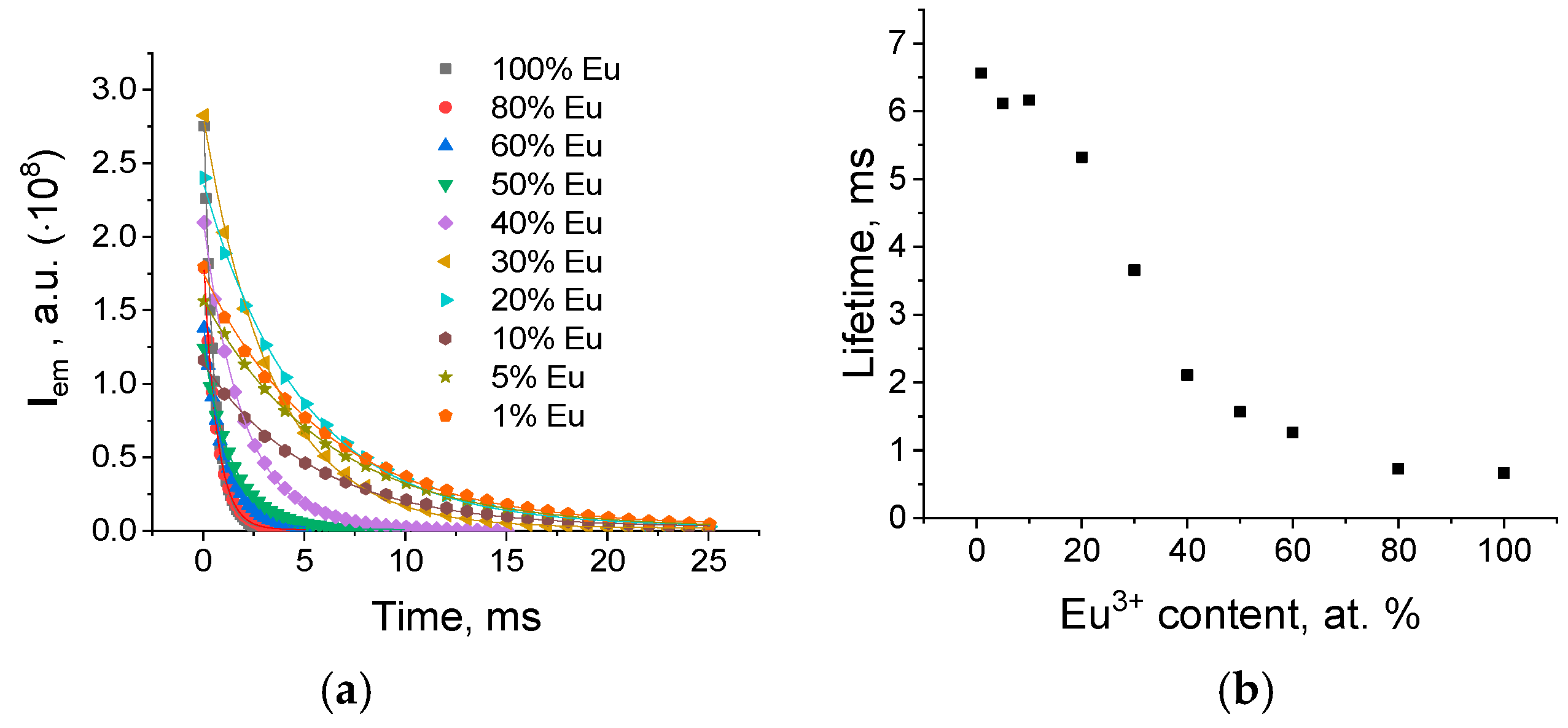
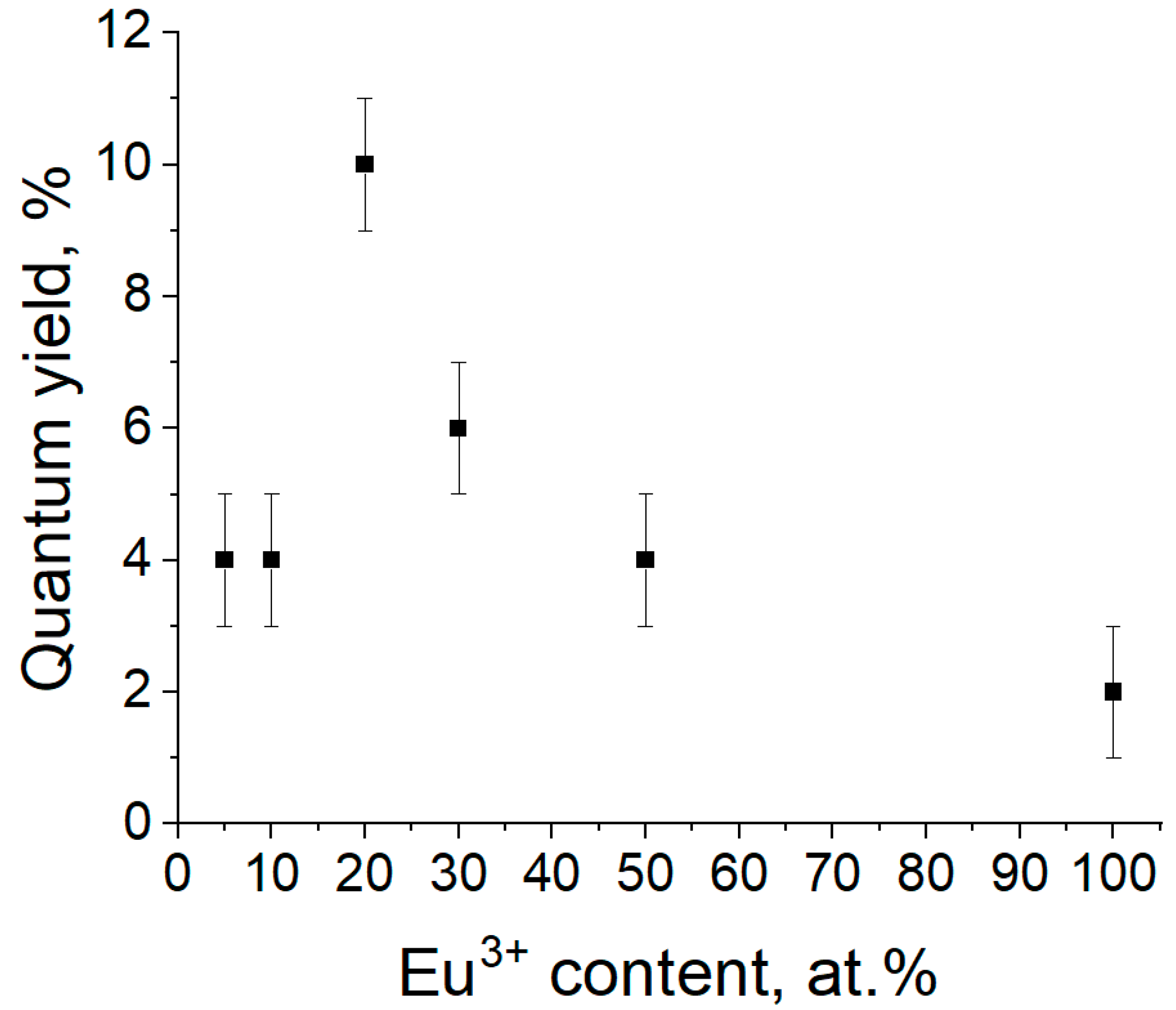
| ΧEu (at.%) | Atotal(s−1) | Arad(s−1) | Anrad(s−1) | η (%) |
|---|---|---|---|---|
| 20 | 188 | 101 | 87 | 54 |
| 30 | 273 | 103 | 170 | 38 |
| 40 | 474 | 103 | 371 | 22 |
| 50 | 637 | 104 | 534 | 16 |
| 60 | 794 | 104 | 689 | 13 |
| 80 | 1369 | 106 | 1262 | 8 |
| 100 | 1504 | 109 | 1395 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prichisly, K.S.; Betina, A.A.; Petrova, A.L.; Bulatova, T.S.; Orlov, S.N.; Kolesnikov, I.E.; Bogachev, N.A.; Skripkin, M.Y.; Mereshchenko, A.S. Synthesis, Morphology, and Luminescent Properties of Nanocrystalline KYF4:Eu3+ Phosphors. Crystals 2025, 15, 500. https://doi.org/10.3390/cryst15060500
Prichisly KS, Betina AA, Petrova AL, Bulatova TS, Orlov SN, Kolesnikov IE, Bogachev NA, Skripkin MY, Mereshchenko AS. Synthesis, Morphology, and Luminescent Properties of Nanocrystalline KYF4:Eu3+ Phosphors. Crystals. 2025; 15(6):500. https://doi.org/10.3390/cryst15060500
Chicago/Turabian StylePrichisly, Kirill S., Anna A. Betina, Anastasia L. Petrova, Tatyana S. Bulatova, Sergey N. Orlov, Ilya E. Kolesnikov, Nikita A. Bogachev, Mikhail Yu. Skripkin, and Andrey S. Mereshchenko. 2025. "Synthesis, Morphology, and Luminescent Properties of Nanocrystalline KYF4:Eu3+ Phosphors" Crystals 15, no. 6: 500. https://doi.org/10.3390/cryst15060500
APA StylePrichisly, K. S., Betina, A. A., Petrova, A. L., Bulatova, T. S., Orlov, S. N., Kolesnikov, I. E., Bogachev, N. A., Skripkin, M. Y., & Mereshchenko, A. S. (2025). Synthesis, Morphology, and Luminescent Properties of Nanocrystalline KYF4:Eu3+ Phosphors. Crystals, 15(6), 500. https://doi.org/10.3390/cryst15060500







