Study of the Process of Sorption of Iron and Copper from Sulfuric Acid in Their Joint Presence by Natural Zeolite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
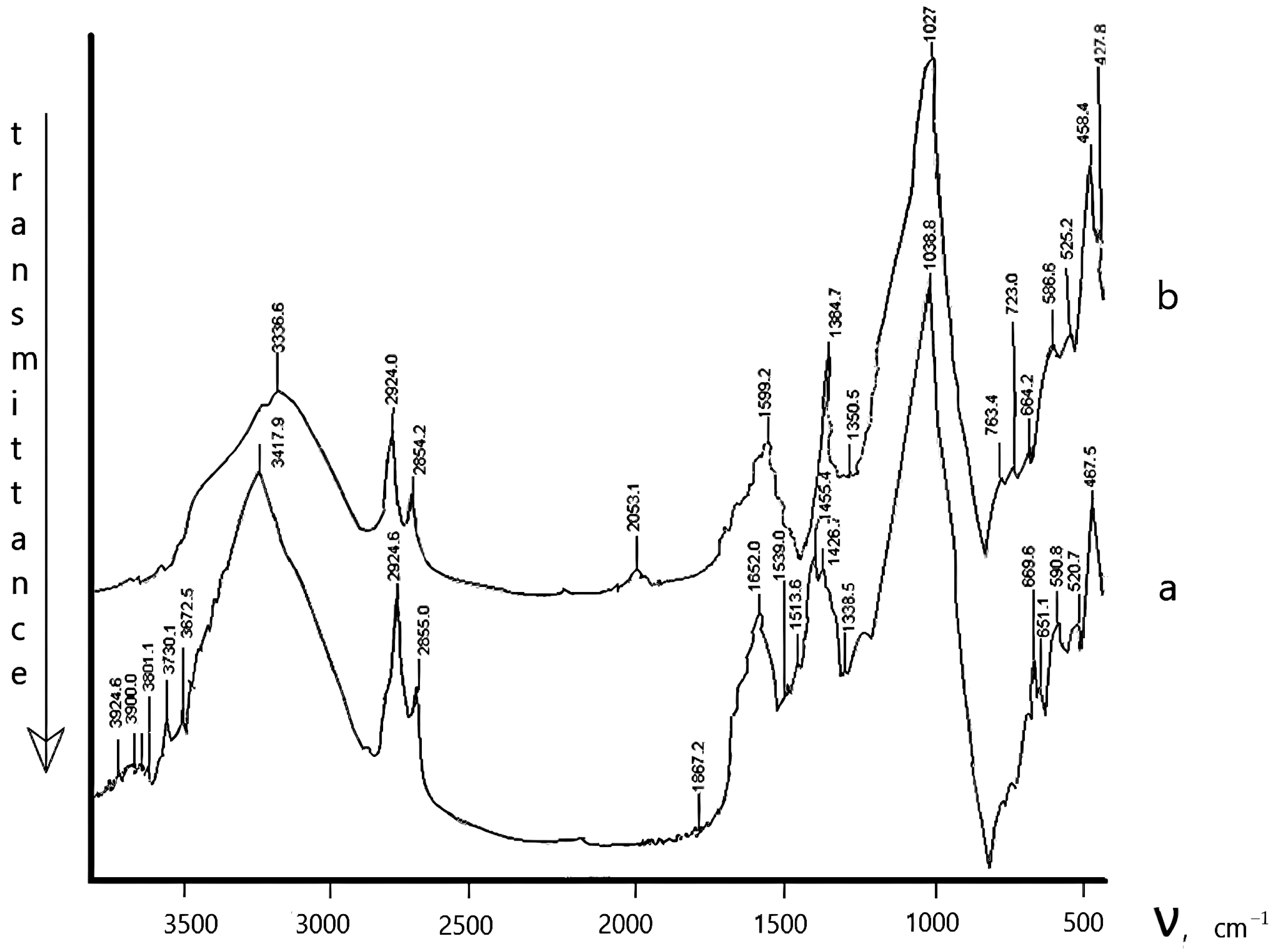
The Physicochemical Study of the Natural Zeolite from the Shankanai Deposit and Treated in Reactive Sulfuric Acid
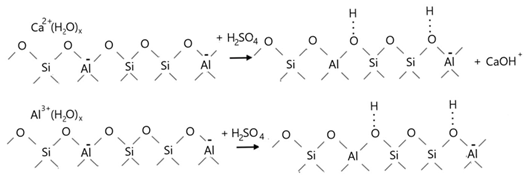
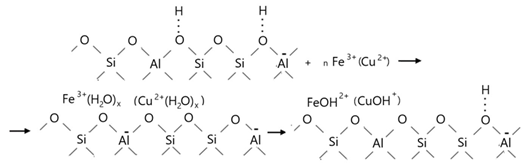
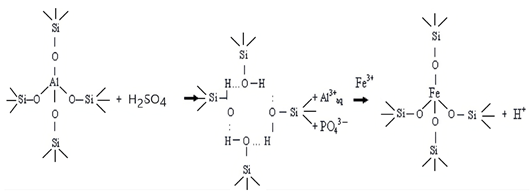
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Zhang, D.-F.; Liu, Z.-F.; Ren, B.-Z. The Purification Methods for Waste Sulfuric Acid in Chemical Industry. 2014. Available online: https://www.researchgate.net/publication/291799602_Purification_methods_for_waste_sulfuric_acid_in_chemical_industry (accessed on 3 March 2025).
- Electronic Resource. Available online: https://www.dedietrich.com/en/solutions-and-products/mineral-acid-treatment/sulfuric-acid-treatment (accessed on 17 January 2025).
- GOST 667-73; Battery Sulfuric Acid. (ST SEV 3856-82) Technical Conditions—Introduction 01.01.75.—M.: USSR Gosstandart. Publishing House of Standards: Cincinnati, OH, USA, 1989; Volume 7, p. 3.
- Kassikov, A.G.; Areshina, N.S.; Kudryakov, M.V.; Khomchenko, O.A. Complex processing of washing sulfuric acid of the copper-nickel production by the extraction method. Chem. Technol. 2004, 6, 25–31. [Google Scholar]
- Kassikov, A.G.; Areshina, N.S.; Bagrova, E.G.; Dyakova, L.V.; Kasikova, N.I.; Okorochkova, E.A.; Petrova, A.M.; Khomchenko, O.A. Processing of the copper-nickel production waste, using the liquid extraction processes. In Proceedings of the Scientific Foundations and Technologies for Processing the Complex Raw Materials and Synthesis of the Functional Materials on Their Basis: Collection. Reports. All-Russian Scientific Conference, Apatity, Russia, 8–11 April 2008; pp. 92–95. [Google Scholar]
- Anarbayev, A.A.; Moldabekov, S.h.M.; Zhantassov, K.T. Method for Purification of Sulfuric Acid. Pre-Patent RK No. 5250. Published 15 October 1997. Available online: https://kz.patents.su/0-pp5250-sposob-ochistki-sernojj-kisloty.html (accessed on 13 January 2022).
- Khachatryan, S.V. Heavy metal adsorption by the Armenian natural zeolite from natural aqueous solutions. Chem. Biol. 2014, 35, 31–35. [Google Scholar]
- Prokhorenko, V.T.; Kravchenko, A.I.; Tretyak, E.V.; Toshinskiy, V.I.; Yakushev, V.I.; Avalyich, D.; Kuznetsov, A.A.; Kondratenko, A.A.; Vladimir, S.; Vorobyeva, I.P.; et al. Authorship Certificate 1805095. Method for Regenerating Waste Sulfuric acid. SU 1805095 Publ. 30.03. 1993, Bulletin No. 12. 3p. Available online: https://rusneb.ru/catalog/000224_000128_0001805095_19930330_A1_SU/ (accessed on 15 January 2025).
- Lu, N.; Dyusembayeva, S.E.; Ashikhina, T. Purification of technical sulfuric acid from iron. Ind. Kazakhstan 2007, 5, 72–73. [Google Scholar]
- Kim, P.P.; Petrovskiy, A.M.; Peretrutov, A.A.; Komarov, V.A.; Chubenko, M.N. Regeneration of waste sulfuric acid from the production of energy-saturated materials with a comprehensive solution for the environmental protection and human safety. Mod. High-Tech Technol. 2015, 7, 48–52. Available online: https://www.elibrary.ru/item.asp?edn=ulebqr (accessed on 10 February 2025).
- Volovicheva, N.A.; Korolkova, S.V.; Vesentsev, A.I. Sorption of ions of Cu2+ AND Fe3+ By the clay containing montmorillonite of maslova pristan field at individual and joint presence in water solutions. Sci. News Ser. Nat. Sci. 2016, 25, 63–69. Available online: https://cyberleninka.ru/article/n/sorbtsiya-ionov-cu2-i-fe3-montmorillonit-soderzhaschey-glinoy-maslovopristanskogo-mestorozhdeniya-pri-individualnom-i-sovmestnom/viewer (accessed on 10 February 2025).
- Martirosyan, V.A.; Lisovskaya, Y.u.O.; Sasuntsyan, M.E. Extraction of Copper from Solutions of Sulfuric Acid Leaching of Gold-copper Sulfide Concentrates of Drmbona by the Extraction Method. Bull. GIUA Ser. Chem. Environ. Technol. 2014, 17, 1–8. Available online: https://www.researchgate.net/publication/303939039 (accessed on 25 March 2025).
- Safiullina, A.M.; Krylova, L.N.; Krylovabtsev, D.A.; Panin, V.V. Method of Extraction of Copper from Sulfate Solutions Containing Ferrous Iron. Ions. Patent RU(11)2339714(13), 27 November 2008. Available online: https://patents.google.com/patent/RU2339714C1/ru (accessed on 11 February 2025).
- Ebook: An Analysis of the Production of Sulfuric Acid and Phosphate Fertilizers. Review; NIUIF: Moscow, Russia, 1983; 120p, Available online: https://thelib.net/2269352-analiz-proizvodstva-sernoj-kisloty-fosforsoderzhaschih-udobrenij-i-kormovyh-fosfatov-za-1983-god.html (accessed on 31 January 2024).
- Blyum, G.Z.; Golub, A.E.; Efremov, A.A.; Zagorets, P.A.; Krapchatov, V.P.; Polevoy, P.S.; Ryabenko, E.A.; Khachaturov-Tavrizyan, A.E.; Shilin, S.A. Authorship Certificate 1527144 Method for Purifying Sulfuric Acid. Publication date: 12 July 1989. Available online: https://www.elibrary.ru/item.asp?id=40953979 (accessed on 5 February 2025).
- Aleksandrova, V.S.; Zykova, O.T.; Markiyev, E.Y. The ion-exchange properties and IR spectra of the natural clinoptilolite, modified with titanium hydroxophosphates. J. Appl. Chem. 2004, 77, 32–36. Available online: https://www.elibrary.ru/item.asp?id=17781570 (accessed on 13 January 2025). [CrossRef]
- Katz, E.M.; Nikashina, V.A.; Bychkova, Y.a.V. Sorption of heavy metals Ni, Cd, Cr, Zn, Cu from surface water on natural and modified clinoptilolites. Sorpt. Chromatogr. Process. 2013, 13, 808–815. Available online: https://www.elibrary.ru/item.asp?edn=rpvzbz/ (accessed on 11 February 2025).
- Davila-Rangel, J.I.; Solache, R.; Hos, M. Sorption of cobalt by two Mexican clinoptilolite rich tuffs zeolitic rocks and kaolinite. J. Radioanal. Nucl. Chem. 2006, 270, 465–471. Available online: https://www.academia.edu/48505708/Sorption_of_cobalt_by_two_Mexican_clinoptilolite_rich_tuffs_zeolitic_rocks_and_kaolinite (accessed on 4 March 2025). [CrossRef]
- Sprynskiy, M.; Buszewski, B.; Terzyk, A.; Namiesnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Panayotova, M.I. Kinetics and thermodynamics of the copper ions removal from wastewater by use of zeolite. Waste Manag. 2001, 21, 671–676. Available online: https://pubmed.ncbi.nlm.nih.gov/11530923/ (accessed on 8 February 2025). [CrossRef] [PubMed]
- Belova, T.P.; Ratchina, T.I.; Gavrilenko, Y.S. Adsorption of copper, nickel and cobalt by the natural zeolite from aqueous solutions. Mining information and analysis. Bull. (Sci. Tech. J.) 2014, 6, 76–80. Available online: https://www.elibrary.ru/item.asp?id=22471424 (accessed on 11 March 2025).
- Belova, T.P.; Gavrilenko, Y.S.; Ershova, L.S. Adsorption of copper, nickel, cobalt and iron by the natural zeolite from aqueous solutions in dynamic mode. Min. Inf. Anal. Bull. (Sci. Tech. J.) 2014, 300–305. Available online: https://www.elibrary.ru/item.asp?id=23057991/ (accessed on 14 February 2025).
- Zharmenov, A.A.; Naguman, P.N.; Tokayeva, Z.M.; Dyussembayeva, S.E. Purification of Technical Sulfuric Acid. Patent 13891RK 11208 RK. Publ. 16.07.07; Bull. No. 7. p. 6. Available online: https://kz.patents.su/6-13891-sposob-ochistki-tehnicheskojj-sernojj-kisloty.html (accessed on 11 December 2024).
- Vanderpool, C.D.; Hoffman, T.J. Method for Purifying Sulfuric Acid Solutions. U.S. Patent 5015458, 14 May 1991. Available online: https://patents.google.com/patent/US5015458A/en (accessed on 19 December 2024).
- Fakhrtdinova, O.A.; Nazarenko, O.B.; Martemyanov, D.V.; Putenpurakalchira, M.V. Study of properties of modified Shivirtuy zeolite. Section 5 power engineering: Efficiency, reliability, safety. In Proceedings of the TPU Conference, National Research Tomsk Polytechnic University, Tomsk, Russia, 2014; Section 5. pp. 114–116. Available online: https://earchive.tpu.ru/bitstream/11683/25623/1/conference_tpu-2014-C15-V2-039.pdf (accessed on 14 February 2025).
- Drago, R. Physical Methods in Chemistry; Mir: Moscow, Russia, 1981; Volume 2, 456p, Available online: https://bigenc.ru/b/fizicheskie-metody-v-khimii-62eeba (accessed on 3 January 2025).
- Kadirbekov, K.; Zhambakin, D.; Kadirbekov, A.; Imanbekov, K. Acid Activation of Natural Zeolite with High Content of Iron Oxides in Creation of Selective Sorbents and Catalysts. MATEC Web Conf. 2017, 96, 2. [Google Scholar] [CrossRef][Green Version]
- Gettuko, R.; Babuko, G.; Narasimhulu, K. Beneficiation of Natural Zeolite through Flash Calcination for Its Use as a Mineral Admixture in Concrete. J. Mater. Civ. Eng. 2014, 26, 24–33. [Google Scholar] [CrossRef]
- Seitzhanova, M.A.; Doszhanov, E.O.; Kuldeev, E.I.; Mansurov, Z.A.; Taju, K.; Tanirbergenova, S.K.; Kanzharkan, E.; Tazhkenova, G.K. Effect of heat treatment on the sorption characteristics of zeolite used in water purification. Combust. Plasma Chem. 2023, 21, 173–179. [Google Scholar] [CrossRef]
- Sultanbayeva, G.; Kaiynbayeva, R.; Chernyakova, R.; Temel, H.; Jussipbekov, U.; Tassibekov, K. Sustainable Chromium Remediation: Sorption of Chromium from Leaching Solutions of Refined. Sustainability 2025, 17, 2726. [Google Scholar] [CrossRef]
- Cook, C. Applications of the Mössbauer Spectroscopy in Industry. Hyper-Fine Interact. 2002, 141–142, 21–34. [Google Scholar] [CrossRef]
- Tarassevich, Y.I. Natural Sorbents in the Water Purification Processes; Nauka: Kyiv, Ukraine, 1985; pp. 546–552. [Google Scholar]
- Saba, S. The Mossbauer Spectroscopy: Fundamentals and Applications. 2023. Available online: https://psiberg.com/mossbauer-spectroscopy/ (accessed on 8 February 2025).
- Goldanskiy, V.I. Chemical Applications of the Mossbauer Spectroscopy; Mir: Moscow, Russia, 1970; 502p. [Google Scholar]
- Makatun, V.N.; Shchegrov, L.N. State of water in inorganic crystal hydrates and features of their dehydration reaction. Russ. Chem. Rev. 1972, 41, 905–918. [Google Scholar] [CrossRef]
- Ivanovskaya, M.I.; Tolstik, A.I.; Kotikov, D.A.; Pankov, V.V. Structural features of Zn–Mn ferrite synthesized by spray pyrolysis. Russ. J. Phys. Chem. 2009, 83, 2283–2288. Available online: https://elib.bsu.by/bitstream/123456789/9977/1/FKH2283.pdf (accessed on 3 January 2025). [CrossRef]
- Breck, W. Donald Zeolite Molecular Sieves. Translated from English by Klyachko, A.L. “World”, M. 1976. 768p. Available online: http://chemteq.ru/library/inorganic/2011.html (accessed on 3 January 2025).
- Sultanbayeva, G.; Agatayeva, A.; Kaiynbayeva, R.; Kozhabekova, N.; Chernyakova, R.; Jussipbekov, U. Study of the Sorption Properties of Natural Zeolite in Relation to Indium(III) and Gallium(III) Cations on the Model Systems. Crystals 2022, 12, 1220. [Google Scholar] [CrossRef]
- Mitrakova, T.N.; Lukyanchikova, O.N.; Lozinskaya, E.F. Sorption of the copper (ii) ions by the natural materials. Condens. Media Interfaces 2016, 18, 72–80. Available online: http://www.kcmf.vsu.ru/resources/t_18_1_2016_008.pdf (accessed on 4 March 2025).
- Natural Zeolites; Chemistry: Moscow, Russia, 1985; 222p, Available online: https://rusneb.ru/catalog/010003_000061_3e4f536d8e96949d5730865851c11560/ (accessed on 4 March 2025).
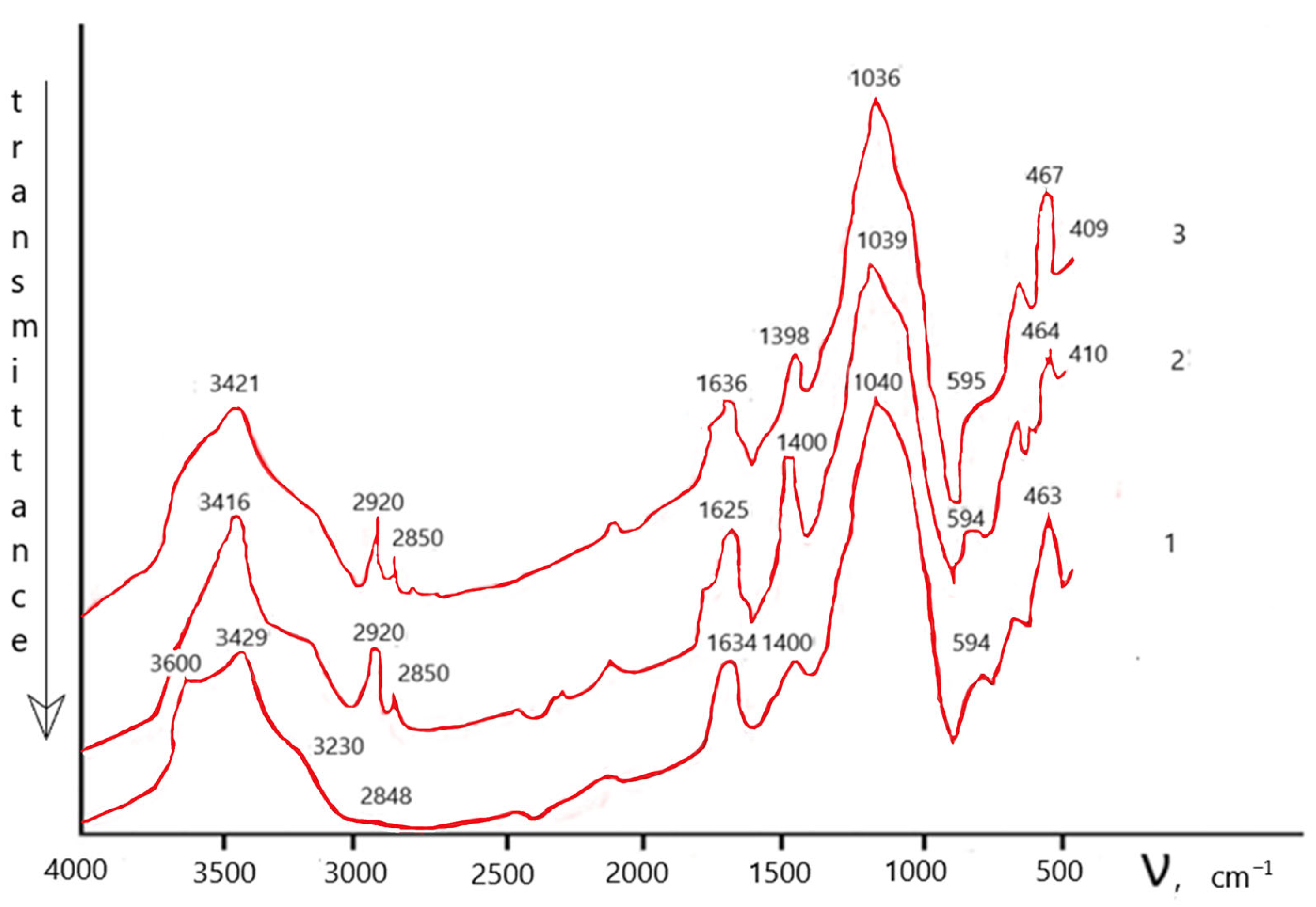
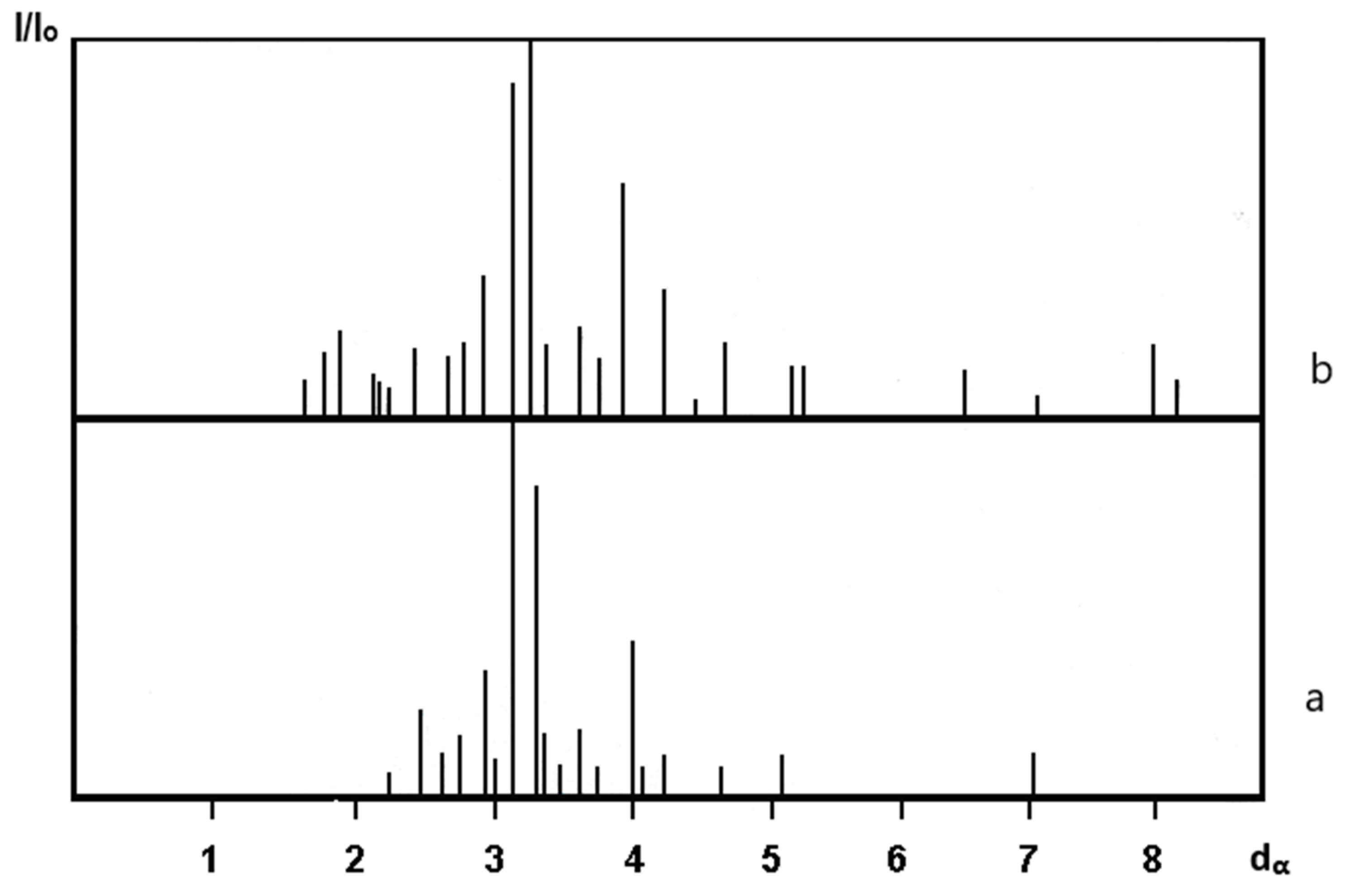
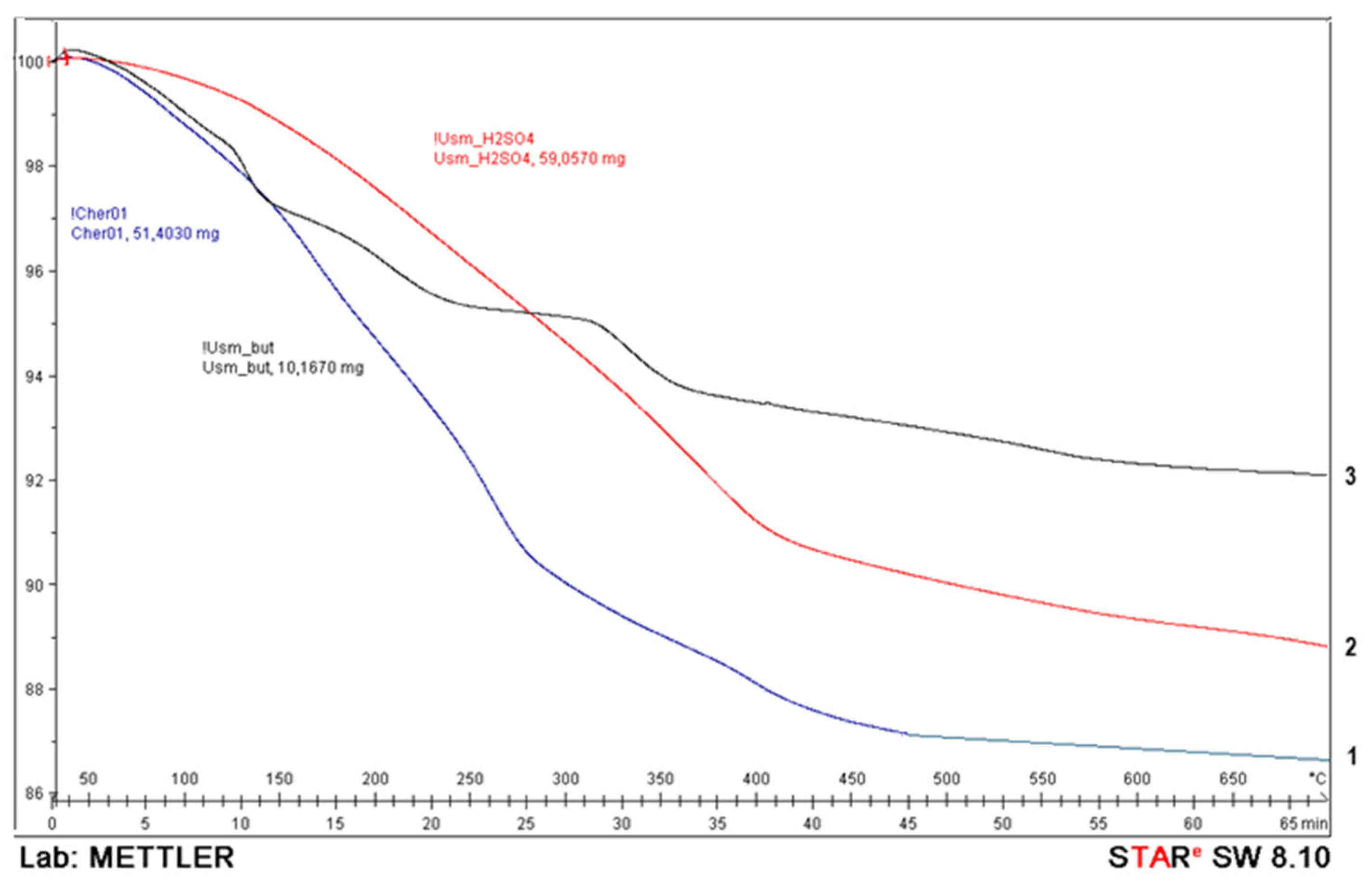
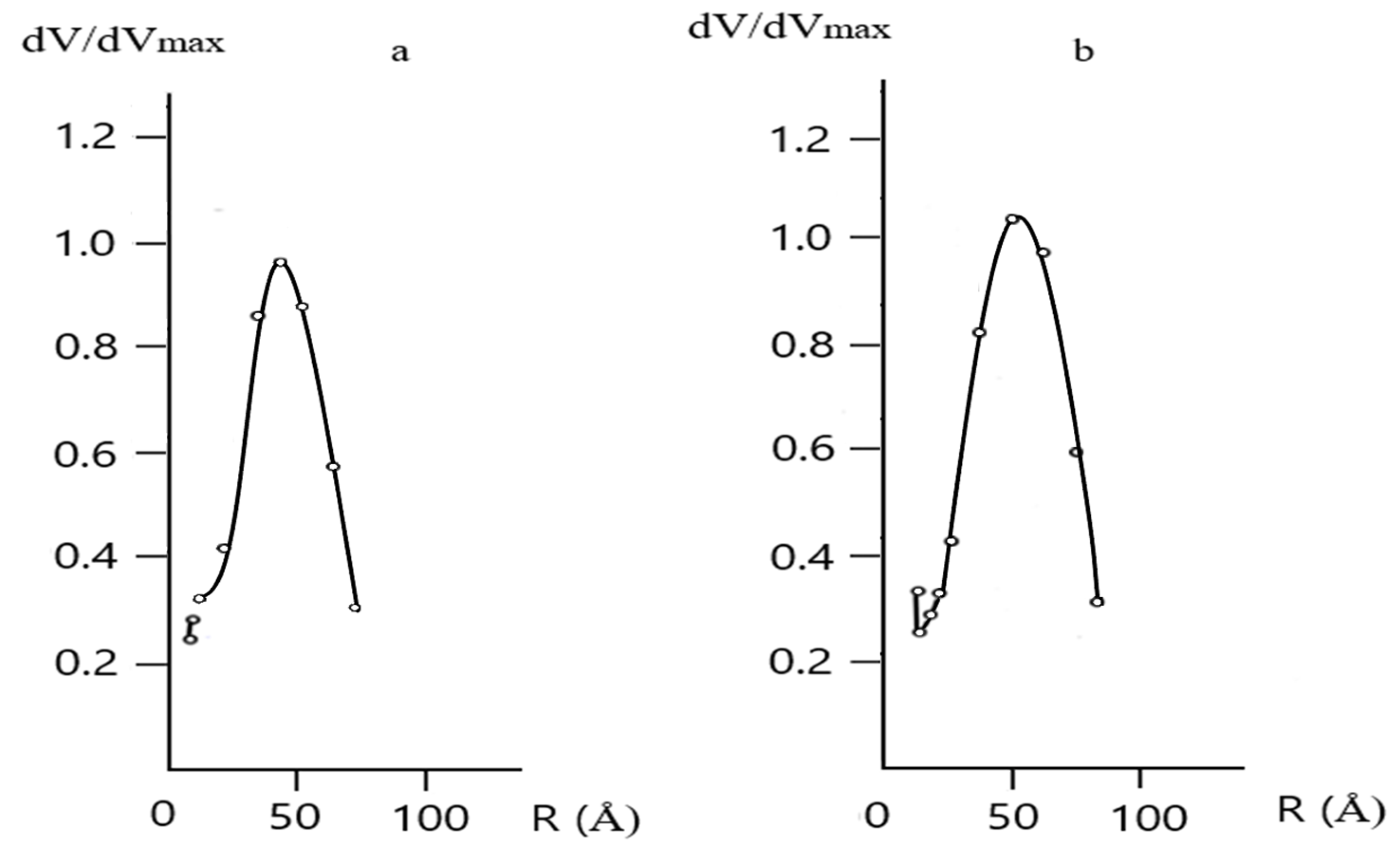
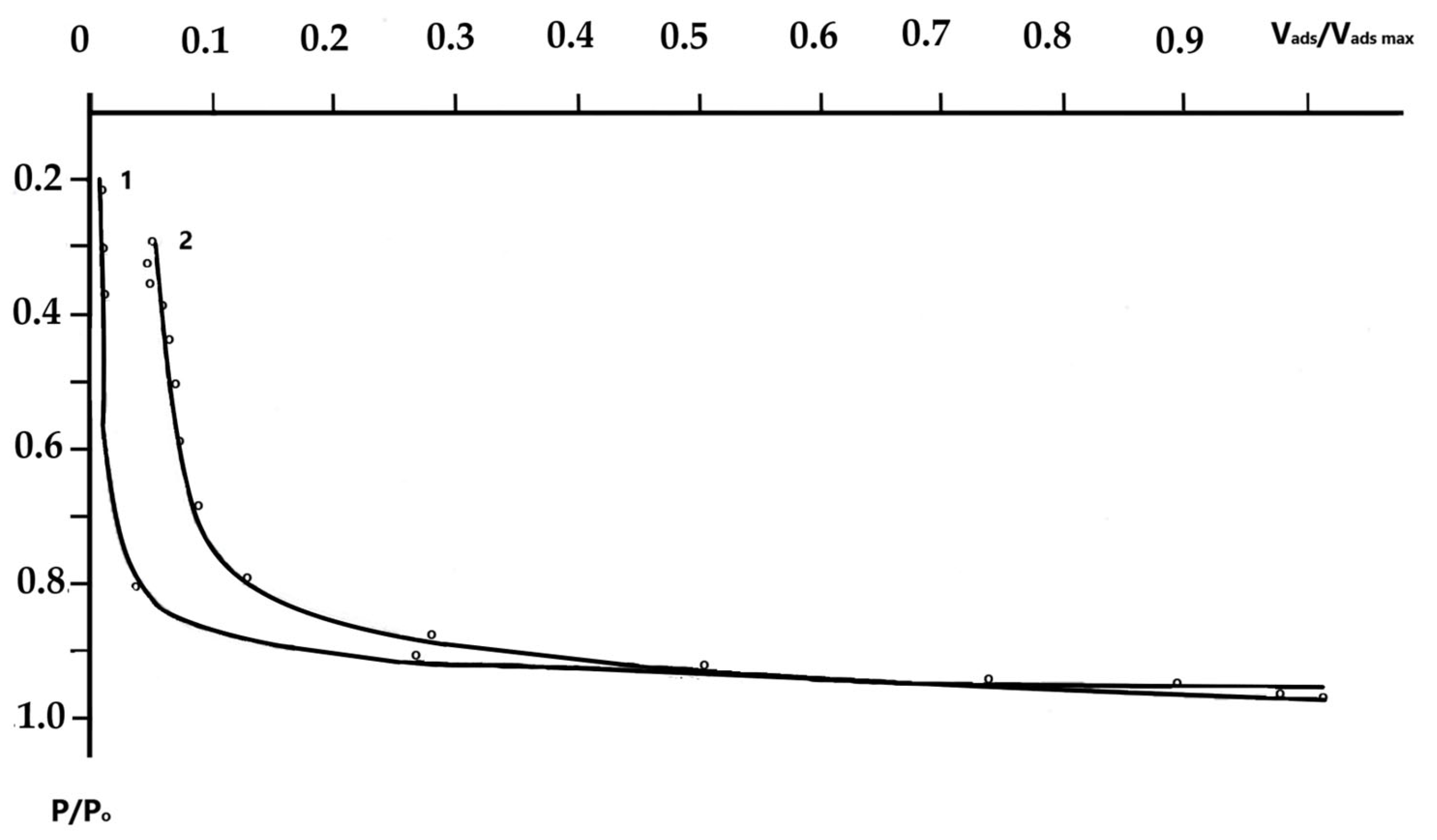
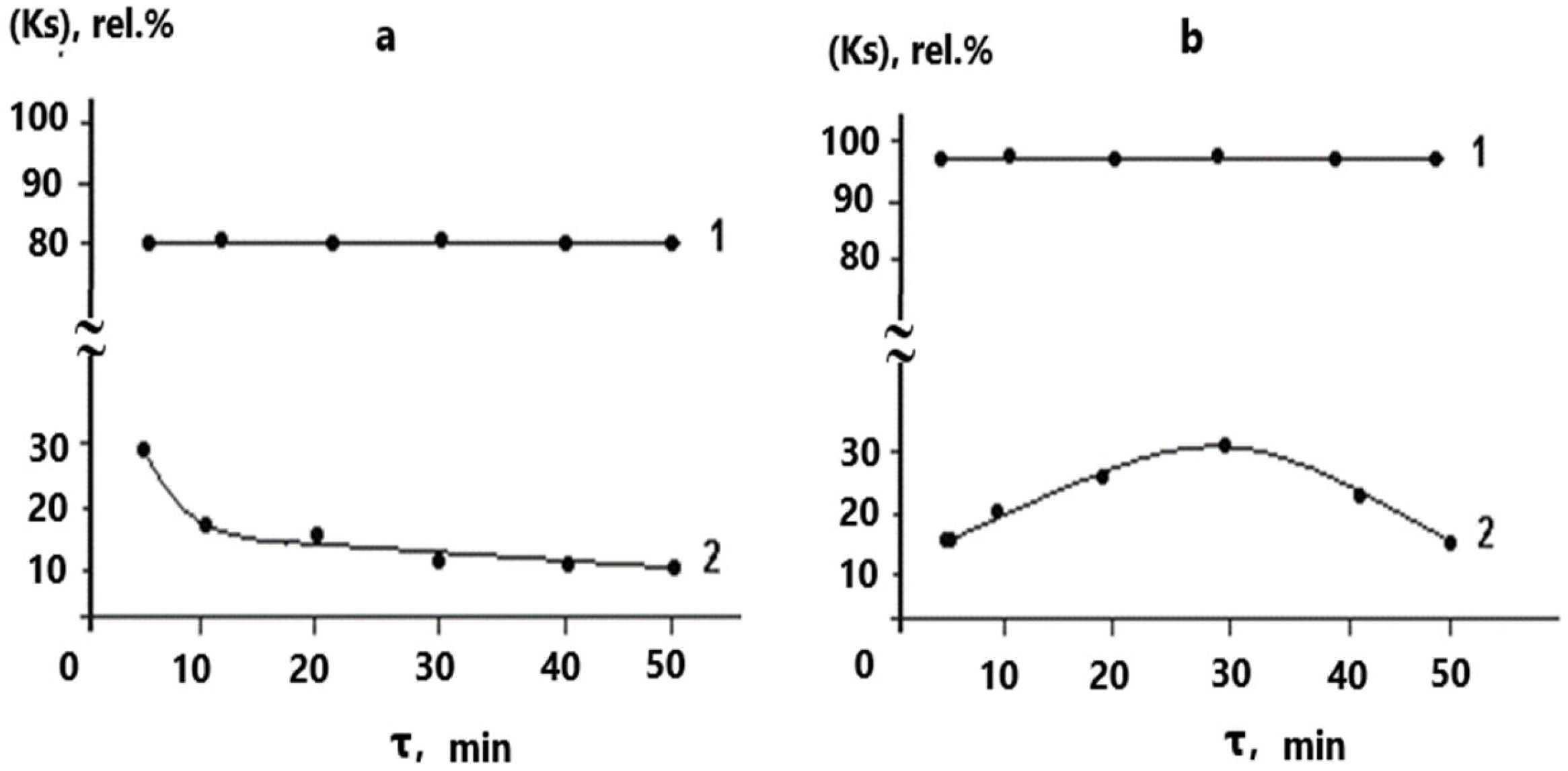
| Time, min. | Fe | Cu | ||
|---|---|---|---|---|
| Residual Content in Acid, mg/L | Sorption Degree (Ks), Rel.% | Residual Content in Acid, mg/L | Sorption Degree (Ks), Rel.% | |
| CFe = 0.002% (36.234 mg/L); CCu = 0.001% (18.119 mg/L) | ||||
| 5 | 1.811 | 95.00 | 11.754 | 35.13 |
| 10 | 1.811 | 95.00 | 12.578 | 30.58 |
| 20 | 3.623 | 90.00 | 13.187 | 27.22 |
| 30 | 3.623 | 90.00 | 12.883 | 28.90 |
| 40 | 3.623 | 90.00 | 12.900 | 28.81 |
| 50 | 3.623 | 90.00 | 13.172 | 27.30 |
| CFe = 0.01% (183.125 mg/L); CCu = 0.001% (18.110 mg/L) | ||||
| 5 | 1.831 | 99.00 | 12.619 | 30.32 |
| 10 | 1.831 | 99.00 | 12.107 | 33.15 |
| 20 | 1.831 | 99.00 | 11.764 | 35.04 |
| 30 | 1.831 | 98.80 | 13.483 | 25.55 |
| 40 | 1.831 | 99.00 | 13.689 | 24.41 |
| 50 | 1.831 | 99.00 | 14.482 | 20.03 |
| Time, min. | Fe | Cu | ||
|---|---|---|---|---|
| Residual Content in Acid, mg/L | Sorption Degree (Ks), Rel.% | Residual Content in Acid, mg/L | Sorption Degree (Ks), Rel.% | |
| CFe = 0.002% (36.190 mg/L); CCu = 0.002% (36.315 mg/L) | ||||
| 5 | 7.2199 | 80.05 | 25.5621 | 29.81 |
| 10 | 7.2163 | 80.06 | 28.5726 | 21.32 |
| 20 | 7.1837 | 80.15 | 29.1857 | 19.41 |
| 30 | 7.1946 | 80.12 | 32.0516 | 11.74 |
| 40 | 7.2561 | 79.95 | 32.0516 | 10.15 |
| 50 | 7.2452 | 79.98 | 32.8614 | 9.51 |
| CFe = 0.01% (183.240 mg/L); CCu = 0.01% (183.167 mg/L) | ||||
| 5 | 14.5676 | 92.05 | 180.7126 | 1.34 |
| 10 | 14.6409 | 92.01 | 173.4591 | 5.30 |
| 20 | 18.1408 | 90.10 | 165.7478 | 9.51 |
| 30 | 19.8815 | 89.15 | 165.1617 | 9.83 |
| 40 | 18.3240 | 90.00 | 165.7295 | 9.52 |
| Time, min | Initial Concentration of the Fe and Cu Cations in Acid | |||||
|---|---|---|---|---|---|---|
| 0.002% (36 mg/L) | 0.005% (90 mg/L) | 0.01% (183 mg/L) | ||||
| Amount of the Sorbed Cations, mg/L | ||||||
| Fe | Cu | Fe | Cu | Fe | Cu | |
| 5 | 28.9701 | 10.7529 | 85.7851 | 13.7628 | 168.6724 | 2.4544 |
| 10 | 28.9737 | 7.7424 | 85.8753 | 18.3625 | 168.5991 | 9.7079 |
| 20 | 29.0063 | 7.1293 | 85.7851 | 22.4823 | 165.0992 | 17.4192 |
| 30 | 28.9954 | 4.2634 | 85.7851 | 27.3808 | 163.3585 | 18.0053 |
| 40 | 28.9339 | 4.2634 | 85.8753 | 16.9772 | 164.916 | 17.4375 |
| 50 | 28.9448 | 3.4536 | 85.8753 | 8.4388 | 152.4740 | 16.1187 |
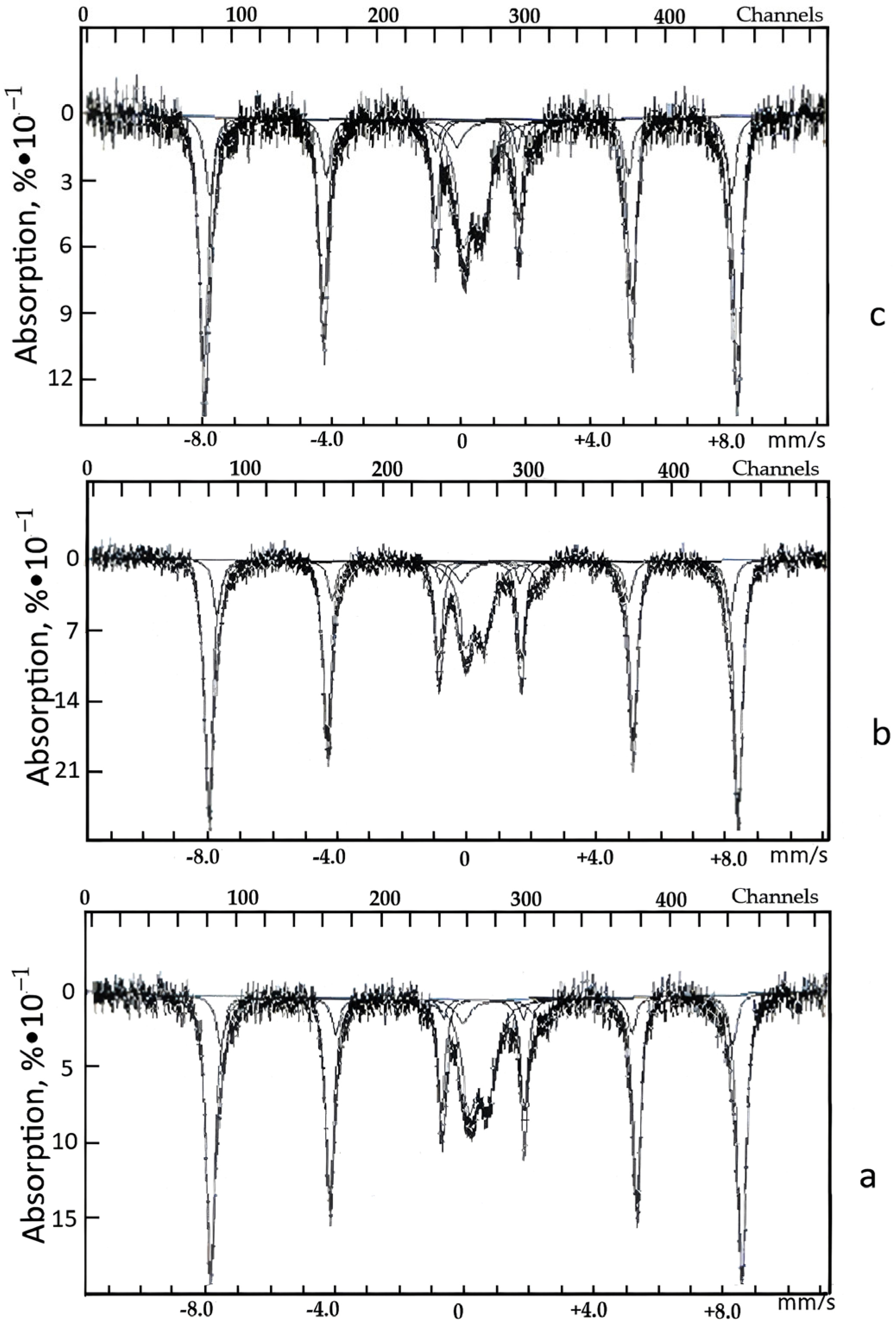
| Sample | δE1, mm/s | ΔEQ, mm/s | Heff. ke. | S, % | Note |
|---|---|---|---|---|---|
| Natural zeolite | 0.33 | 0.66 | − | 17 | Fe3+ |
| 1.18 | 2.35 | − | 6 | Fe2+ | |
| 0.37 | −0.21 | 512 | 68 | αFe2O3 | |
| 0.38 | −0.16 | 491 | 9 | Unknown form | |
| Acid-treated zeolite | 0.34 | 0.59 | − | 16 | Fe3+ |
| 1.11 | 2.42 | − | 5 | Fe2+ | |
| 0.37 | −0.22 | 510 | 68 | αFe2O3 | |
| 0.38 | −0.21 | 490 | 11 | Unknown form | |
| Zeolite after the sorption CFe = 0.014% | 0.34 | 0.59 | − | 20 | Fe3+ |
| 1.07 | 2.31 | − | 6 | Fe2+ | |
| 0.37 | −0.21 | 510 | 66 | αFe2O3 | |
| 0.37 | −0.23 | 486 | 8 | Unknown form |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiynbayeva, R.; Chernyakova, R.; Sultanbayeva, G.; Kozhabekova, N.; Jussipbekov, U.; Tussupkaliyev, E. Study of the Process of Sorption of Iron and Copper from Sulfuric Acid in Their Joint Presence by Natural Zeolite. Crystals 2025, 15, 494. https://doi.org/10.3390/cryst15060494
Kaiynbayeva R, Chernyakova R, Sultanbayeva G, Kozhabekova N, Jussipbekov U, Tussupkaliyev E. Study of the Process of Sorption of Iron and Copper from Sulfuric Acid in Their Joint Presence by Natural Zeolite. Crystals. 2025; 15(6):494. https://doi.org/10.3390/cryst15060494
Chicago/Turabian StyleKaiynbayeva, Raushan, Raissa Chernyakova, Gita Sultanbayeva, Nazym Kozhabekova, Umirzak Jussipbekov, and Ersin Tussupkaliyev. 2025. "Study of the Process of Sorption of Iron and Copper from Sulfuric Acid in Their Joint Presence by Natural Zeolite" Crystals 15, no. 6: 494. https://doi.org/10.3390/cryst15060494
APA StyleKaiynbayeva, R., Chernyakova, R., Sultanbayeva, G., Kozhabekova, N., Jussipbekov, U., & Tussupkaliyev, E. (2025). Study of the Process of Sorption of Iron and Copper from Sulfuric Acid in Their Joint Presence by Natural Zeolite. Crystals, 15(6), 494. https://doi.org/10.3390/cryst15060494






