Influence of Homogenization Heat Treatments on the Mechanical, Structural, Biodegradation, and Cavitation Behavior of Some Alloys in the ZnMg(Fe) System
Abstract
1. Introduction
2. Materials and Experimental Procedures
3. Experimental Results and Discussion and Interpretation of Results
3.1. Microstructural Characterization of the Experimental Alloys
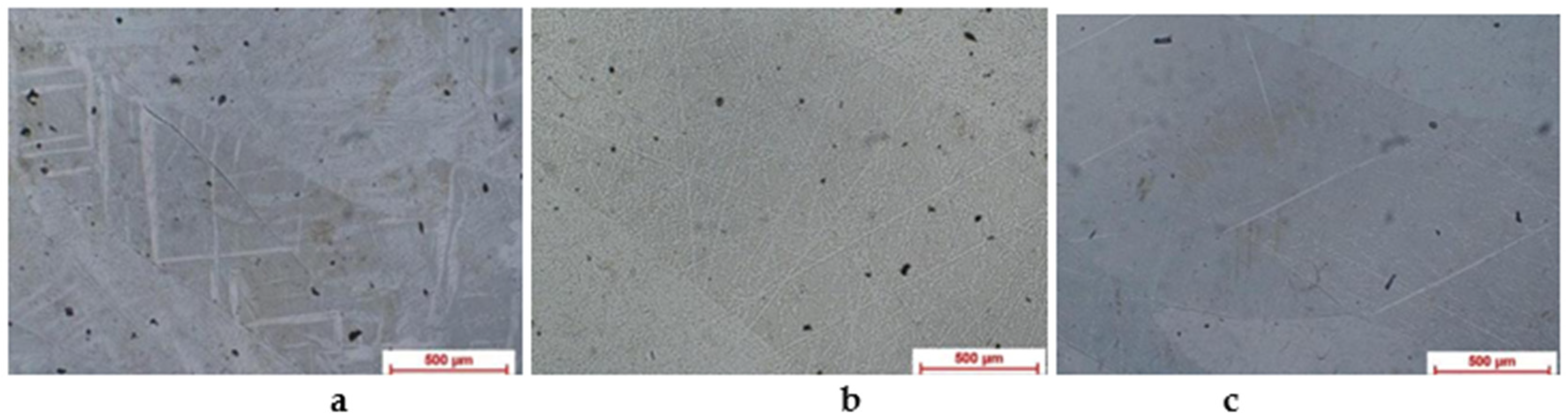
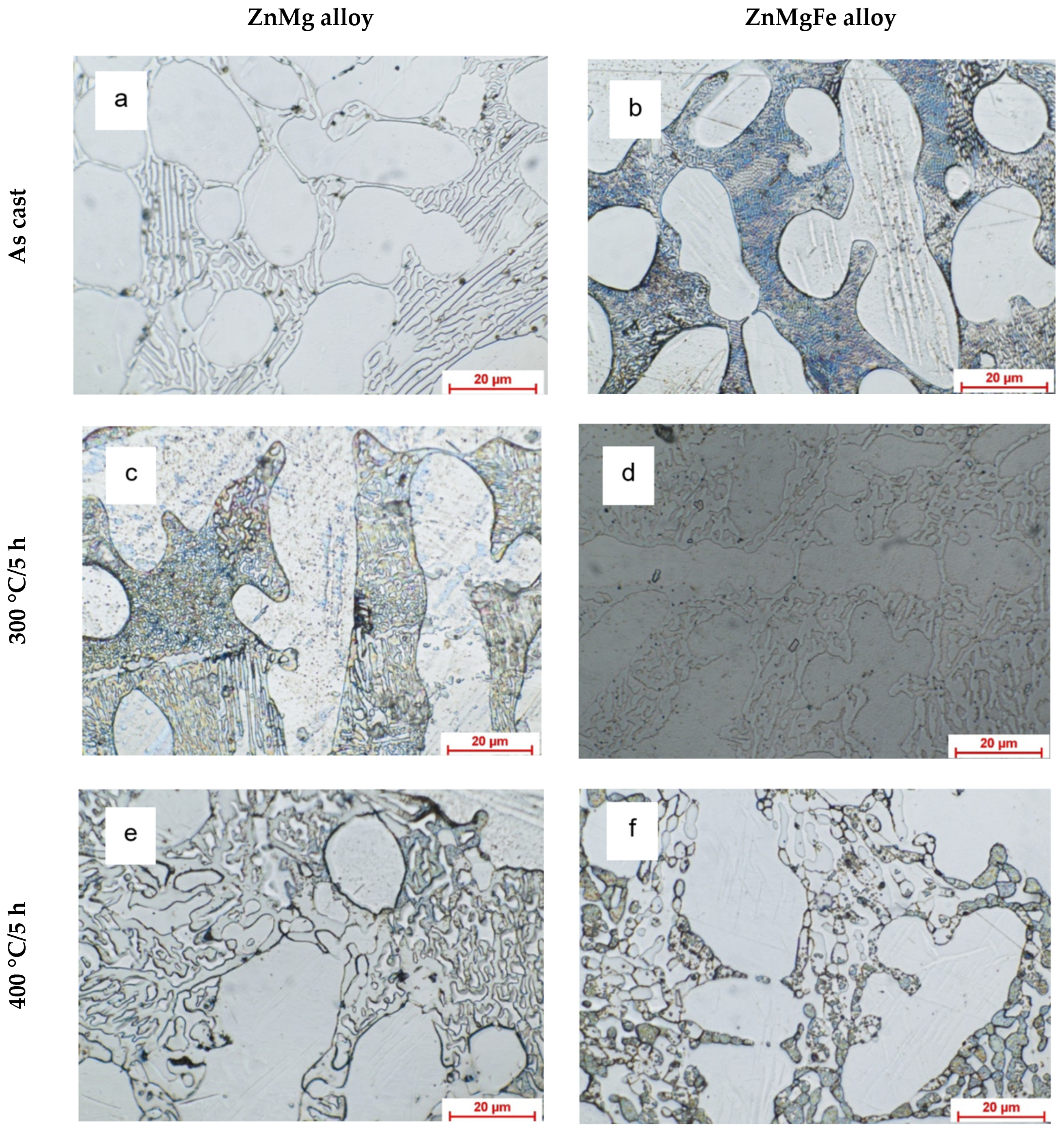
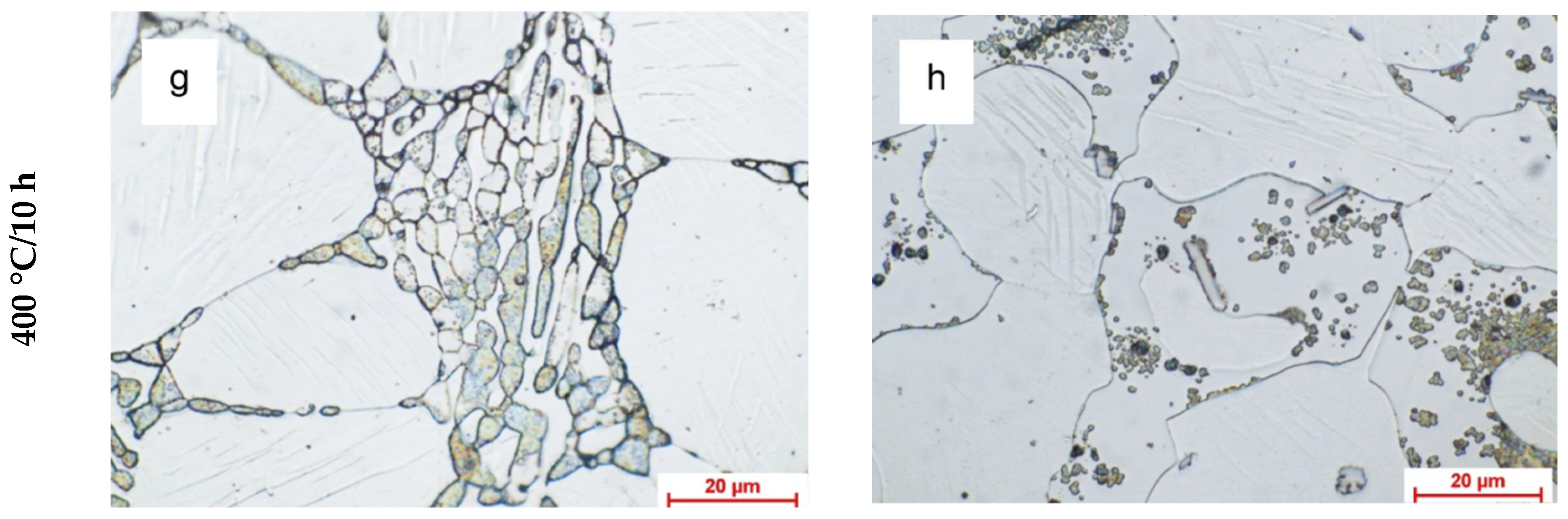
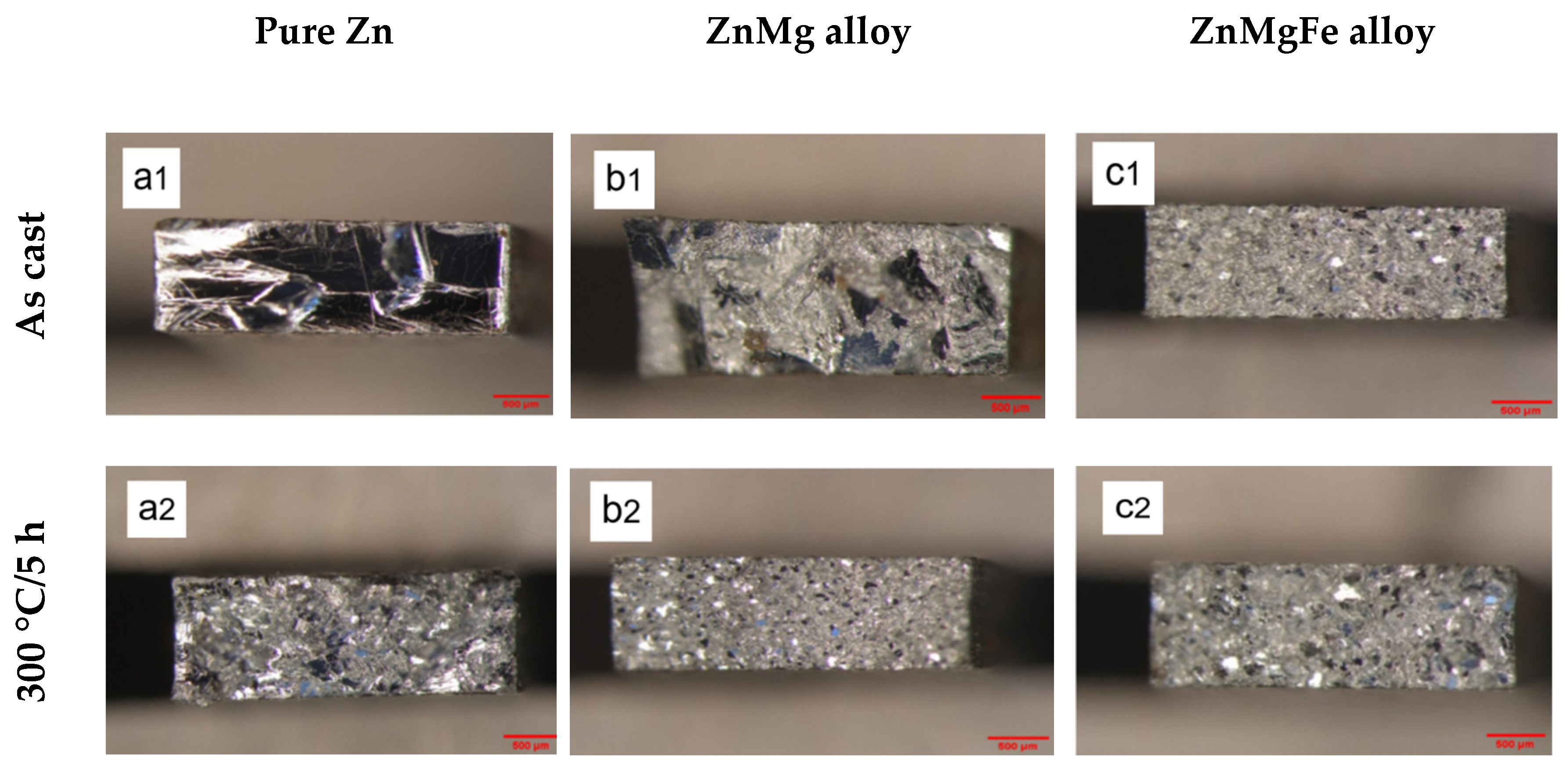
3.1.1. Optic Metallographic Analysis
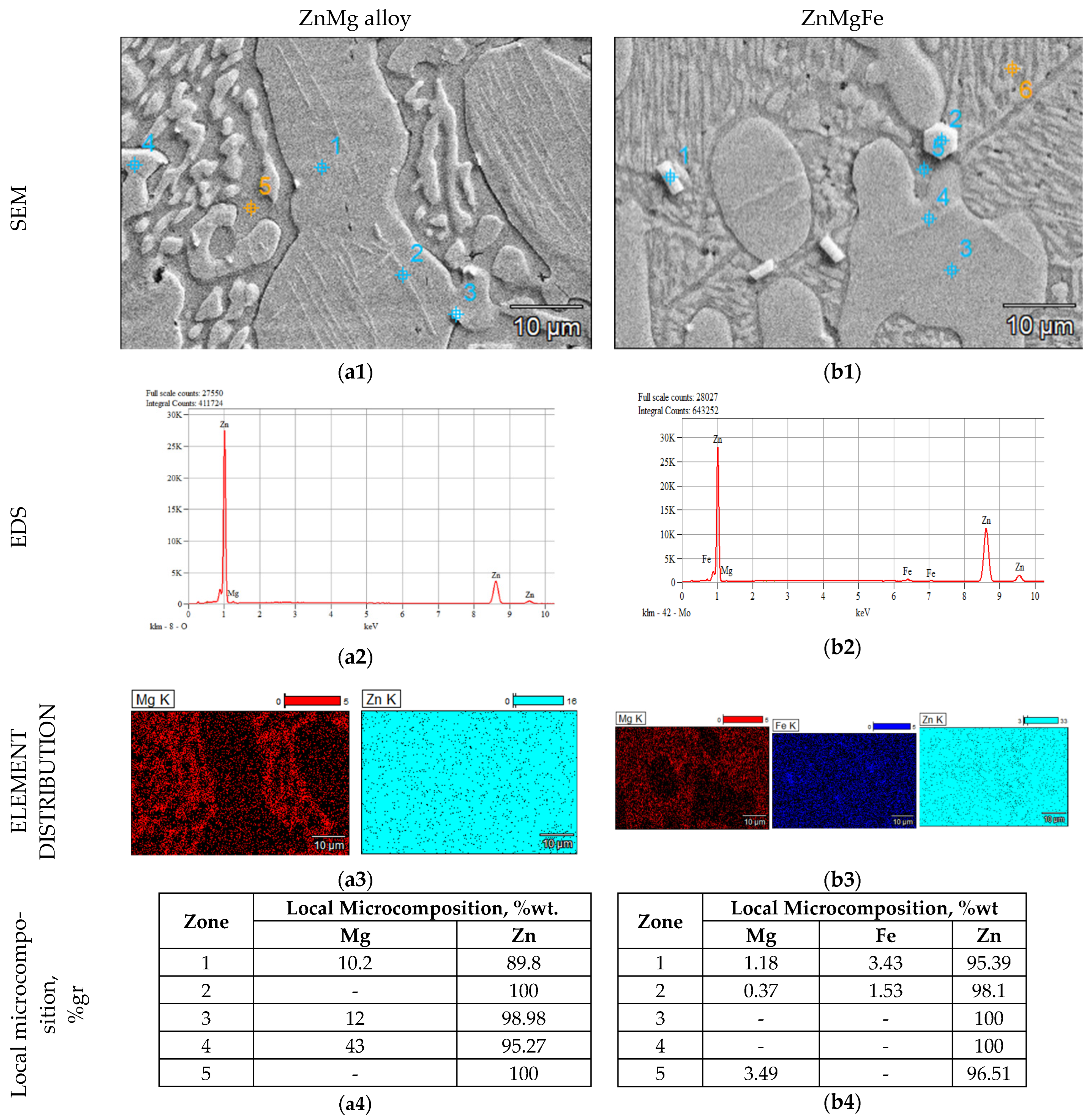
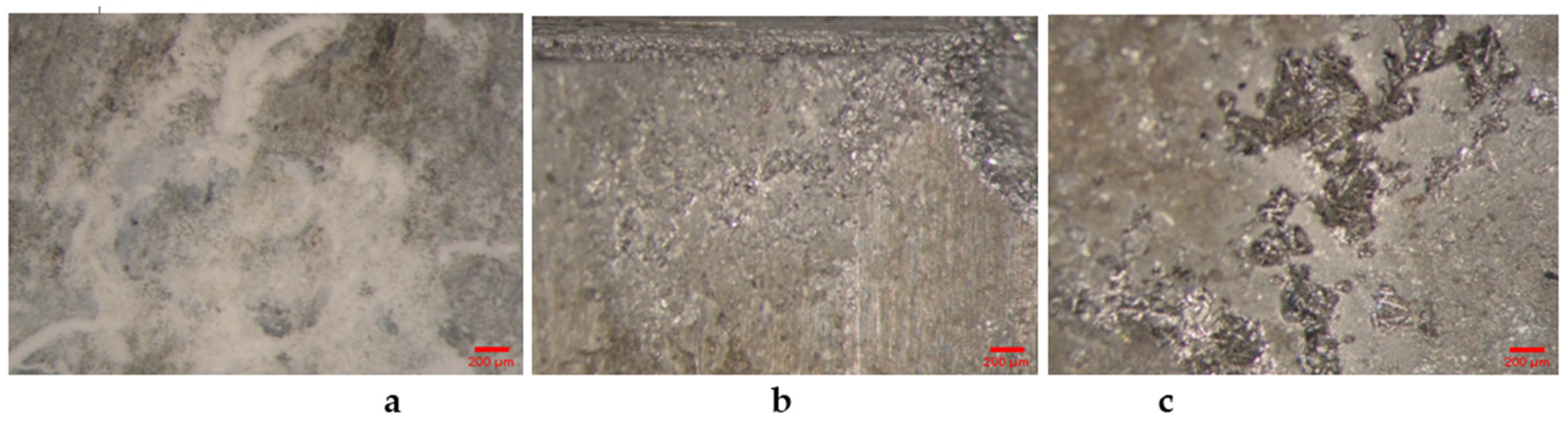
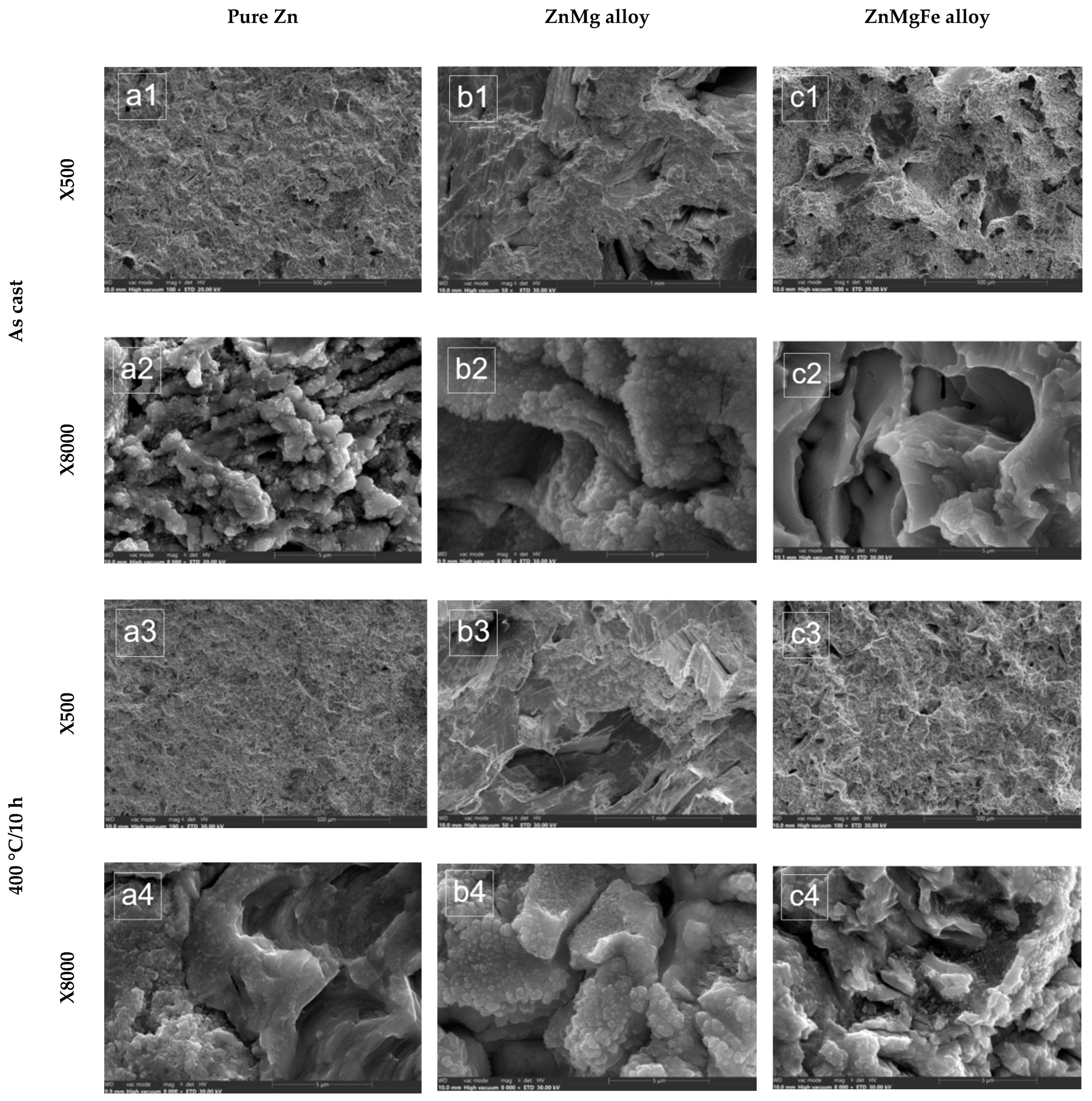
3.1.2. SEM Analysis of Alloys from the ZnMg(Fe) System
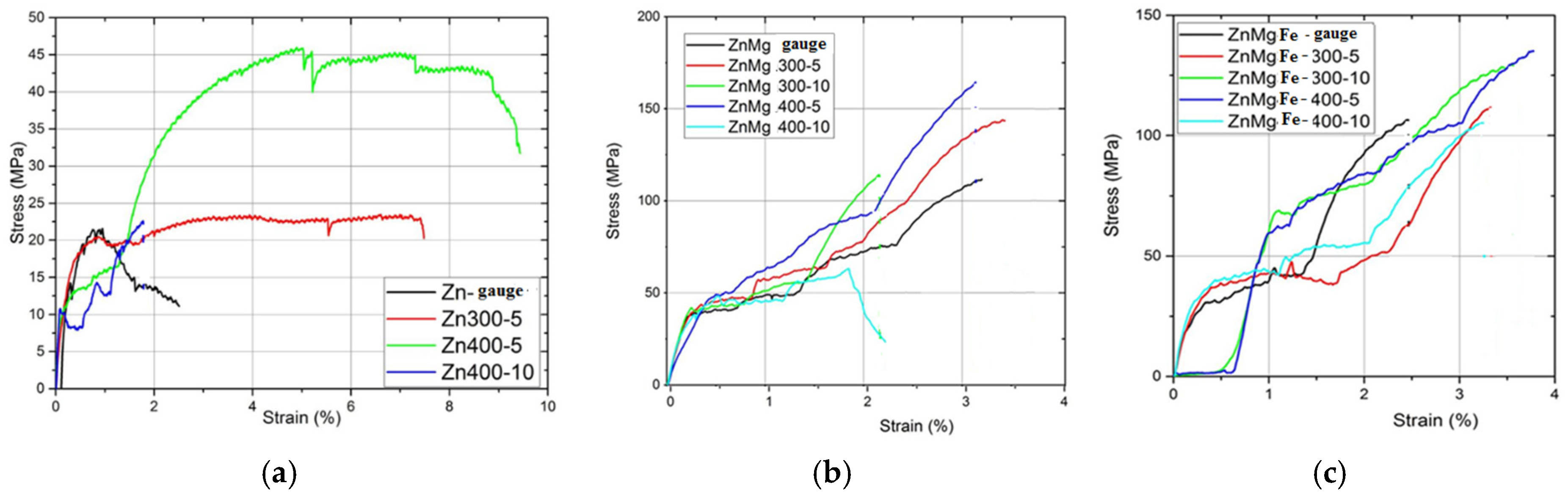
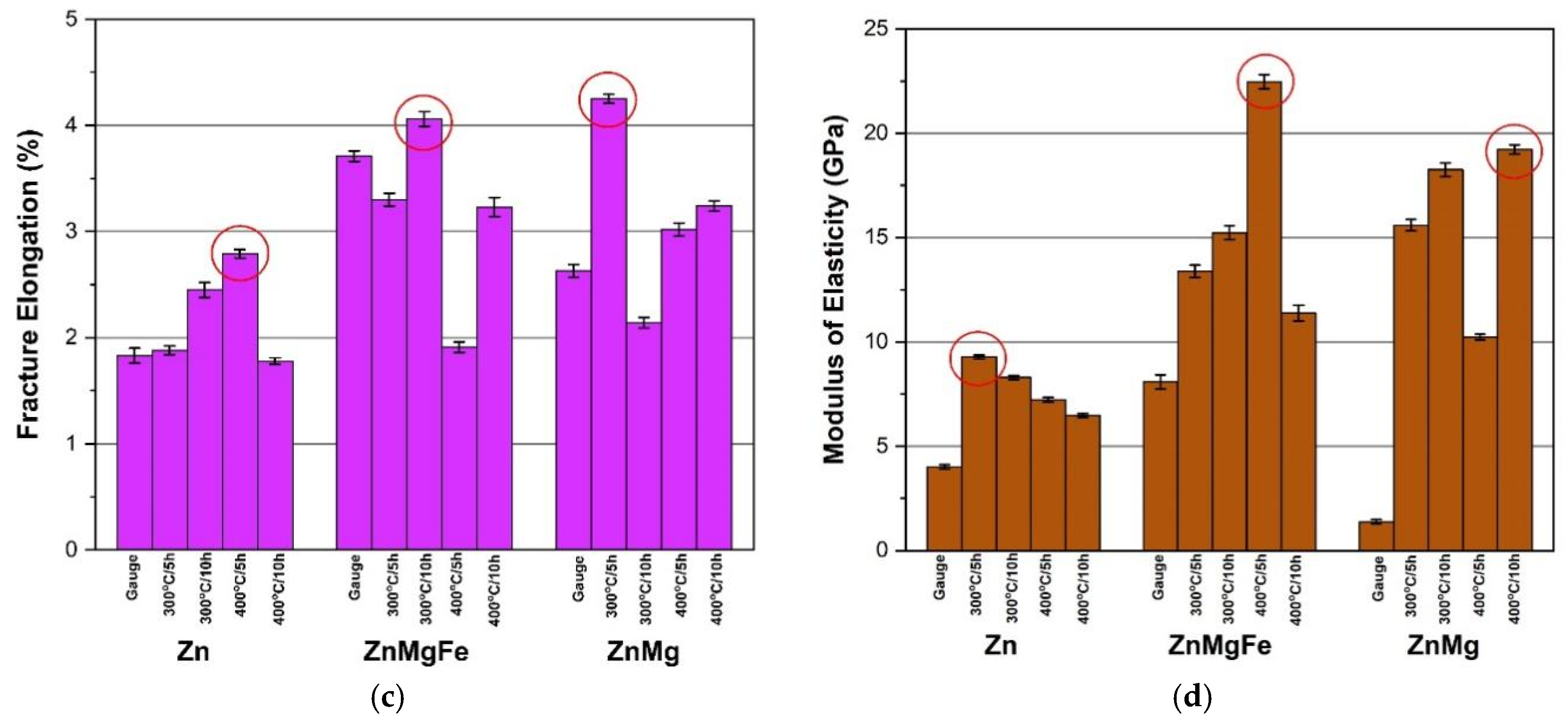
3.2. Physical–Mechanical Characterization of the Experimental Alloys
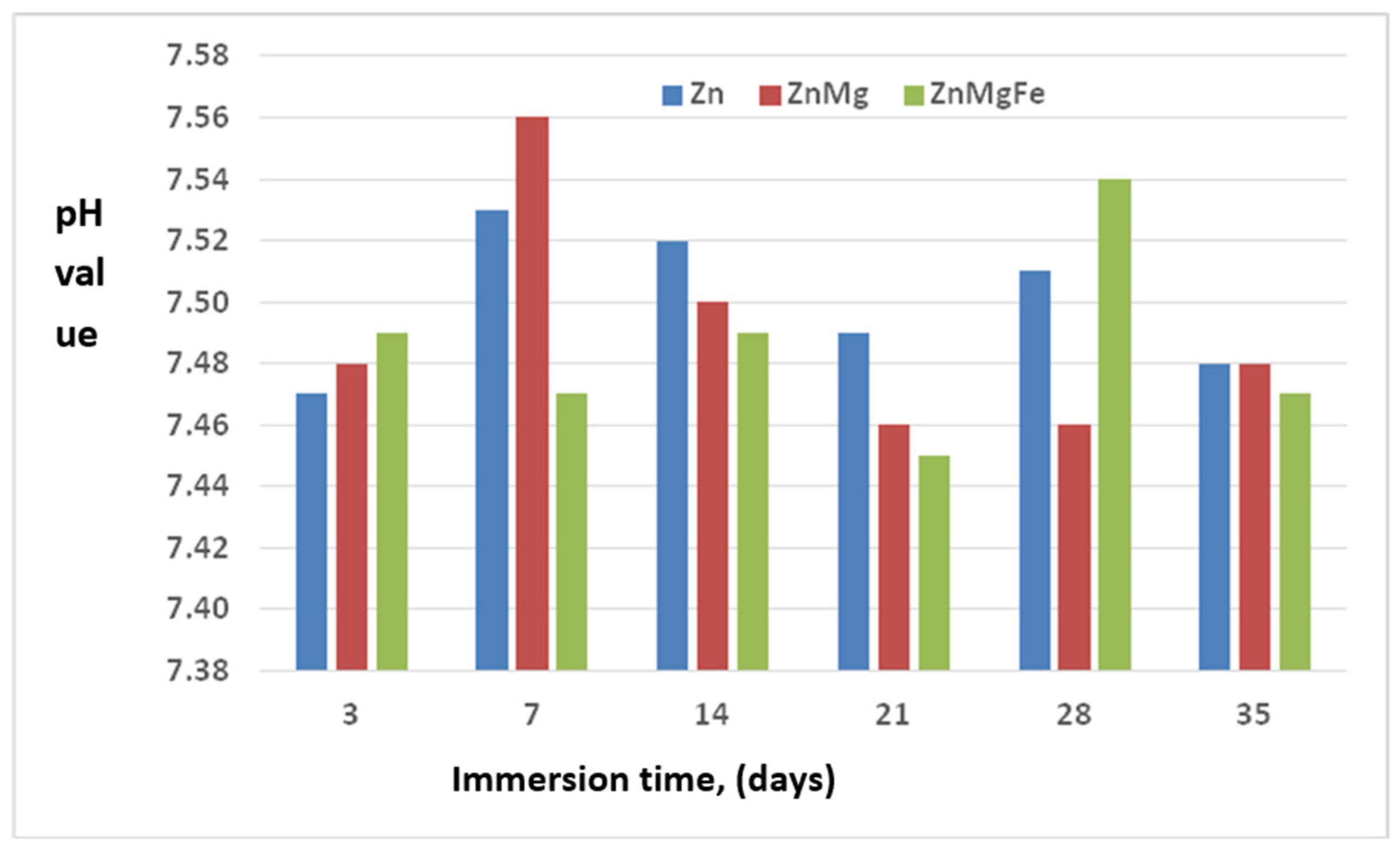
3.3. Characterization of the Biodegradability of the Experimental Alloys
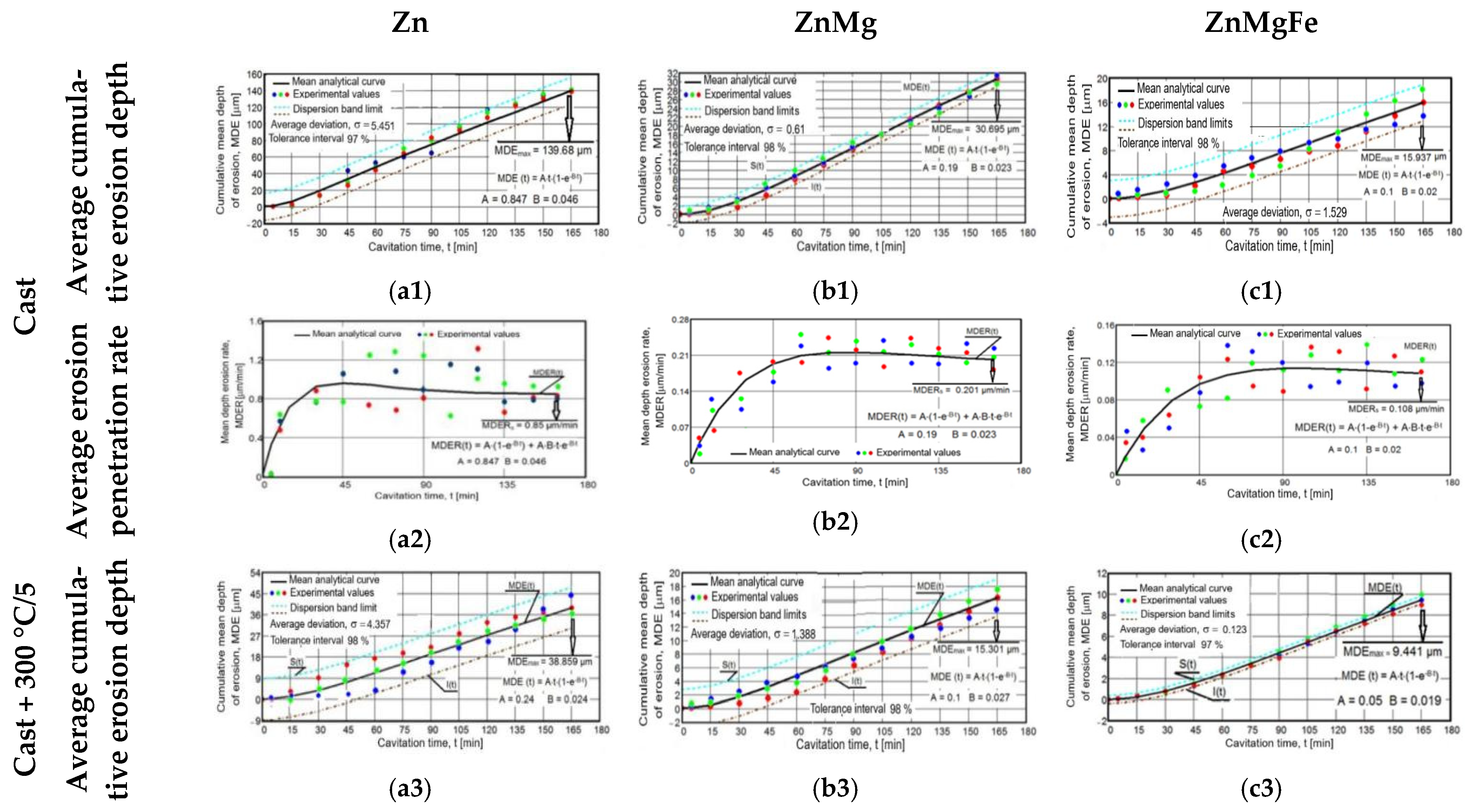
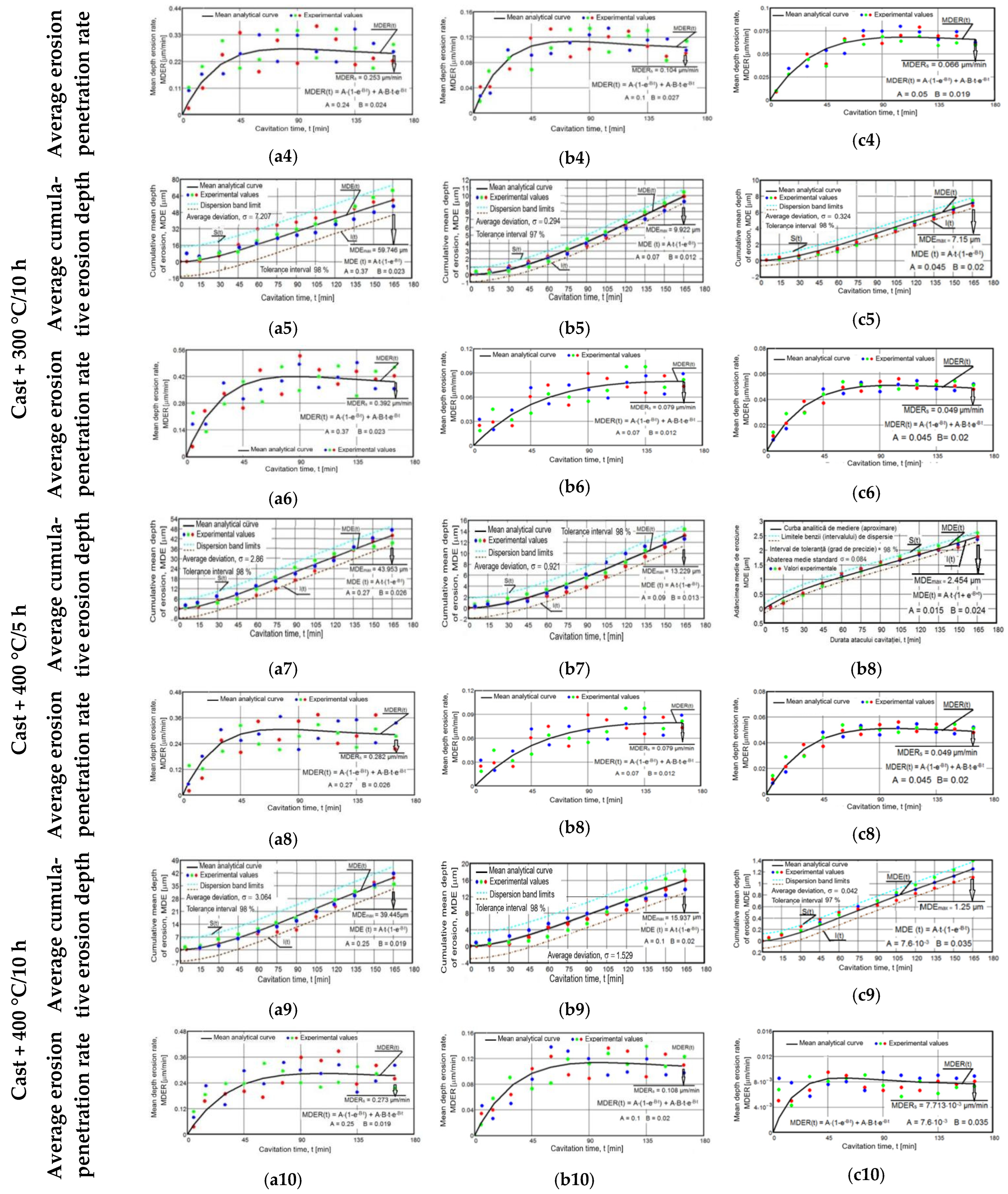
3.4. Characterization of the Erosion Cavitation Behavior of the Experimental Alloys
- -
- For the cumulative average of erosion depth,
- -
- For the average erosion rate,
- -
- The most significant material losses developed after 45–60 min, and after 120 min, they increased approximately linearly/constantly, with small differences between successive values, as is shown in the erosion depth diagrams (Figure 11(a1,a3,a5,a7,a9,b1,b3,b5,b7,b9,c1,c3,c5,c7,c9)).
- -
- There were large differences between the experimental values of the MDE and MDER parameters obtained for the three samples in the same state and after the same duration of exposure to cavitation attack. In addition, Figure 11(a2,a4,a6,a8,a10,b2,b4,b6,b8,b10,c2,c4,c6,c8,c10) show that there were increases and decreases in the values obtained for the erosion velocities (MDER) during cavitation. These developments led to an irregular dispersion from the averaging curve. These aspects were also clarified by the stereomacroscopic and SEM images in Figure 12; through the dimensions and shapes of the caverns, as well as through the connections between them, these figures show the dimensions and geometries of the ejected grains, as well as the types of breaks generated by the cyclic stress of cavitation fatigue, which was dependent on the type of microstructure and the value of the mechanical properties.
- -
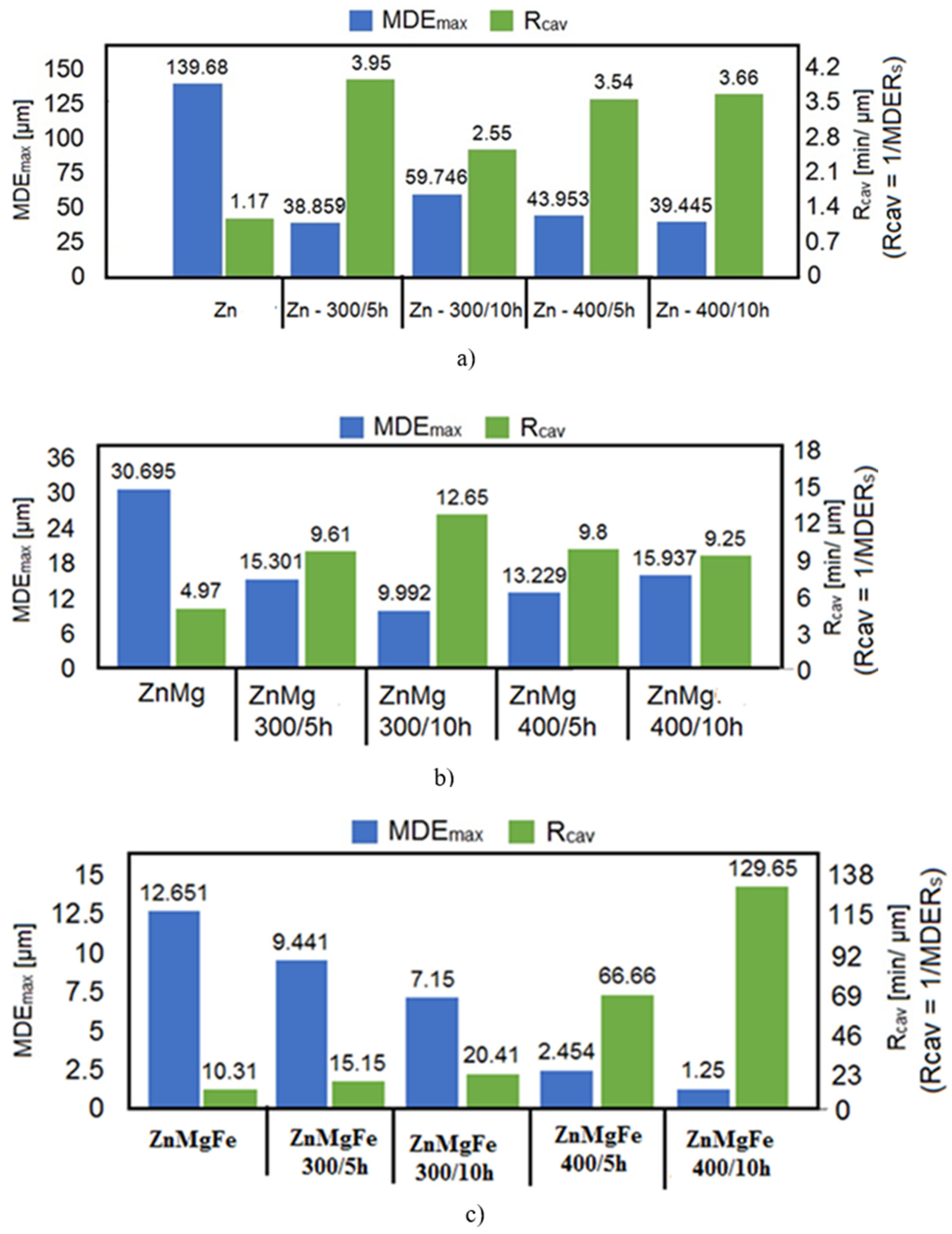
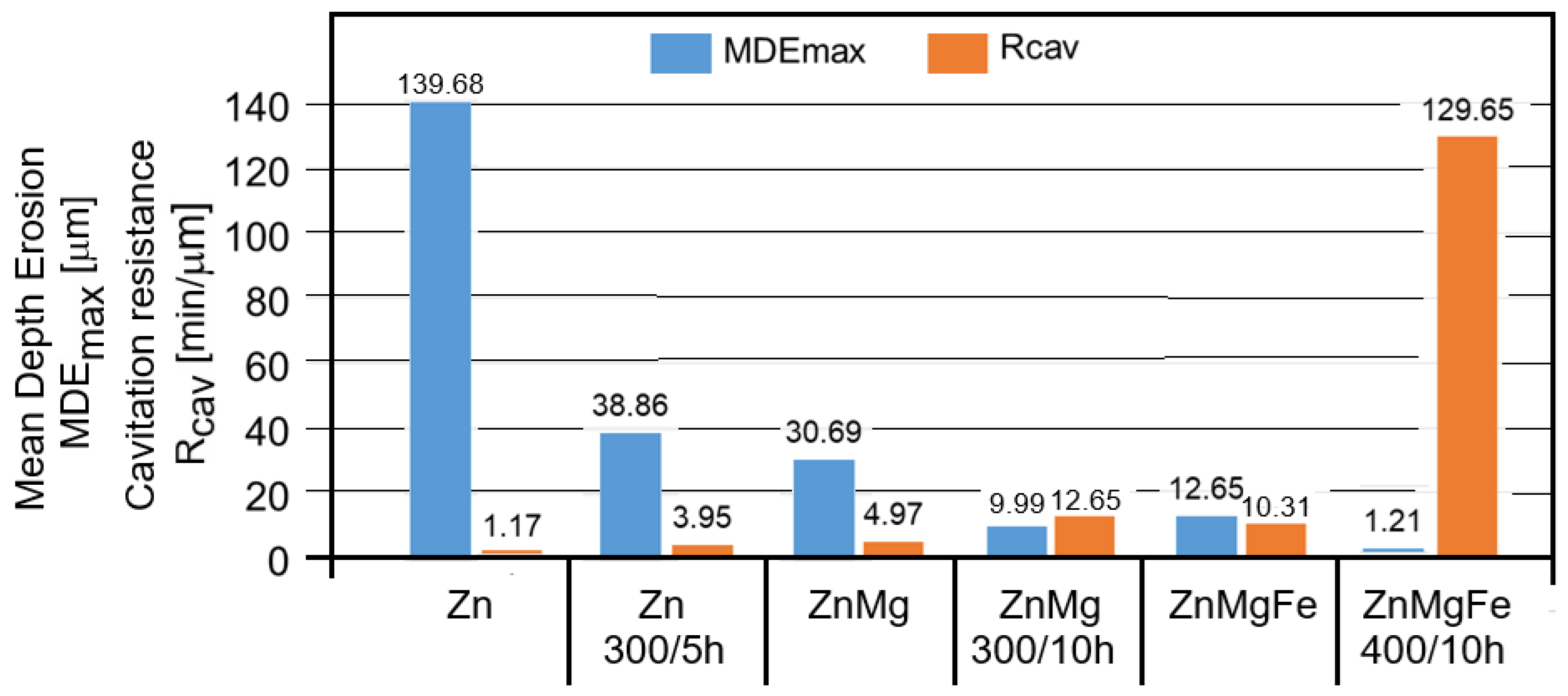
- The appearance of the asymptotic evolution of the MDE curves, with linearization for a certain duration of the cavitation attack, was similar in all specimens, with differences in terms of the final values. For the different structural states of zinc, the values of the maximum cumulative cavitational erosion penetration depth were the highest, at 139 μm in the end (for casting) and in the range of 40–60 μm (for homogenized specimens). In the ZnMg alloy, the maximum cavitational erosion depth was 30 μm and it reached about 13–15 μm in the homogenized specimens. In the ternary alloy, ZnMgFe, the maximum cumulative cavitational erosion penetration depth was 15 μm in the cast specimens, and it reached the lowest values in the homogenized specimens, in the range of 2–12 μm. This mode of evolution was specific to the alloy, but the differences in the slope of the linear area are given by the values of the mechanical properties. According to studies in this field [33,35,40,41], the decrease in the slope and the increase in the resistance of the structure to cavitation stresses are specific to the alloy state, with high values for the ultimate strength, yield strength, modulus of elasticity, and Brinell hardness and low values for elongation.
- -
- The stabilization of the MDER curves began very early in zinc (after 45 min; Figure 11(a2)); in the range of 45–60 min in homogenized zinc (Figure 11(a3–a10)); much later for ZnMg alloys, after 60 min (Figure 11(b3–b10)); and right from minute 90 in the ZnMgFe alloy (Figure 11(c3–c10)). This stabilization shows that the stressed layer was mechanically hardened by impact with shock waves and cavitational microjets, and that the pressure force was dampened by the water and air that penetrated into the formed caverns [33,35,40,41,42].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Love, B.J. Biomaterials: A Systems Approach to Engineering Concepts, 1st ed.; Academic Press: London, UK, 2017. [Google Scholar]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical stability and corrosion resistance of Ti–Mo alloys for biomedical application. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; Suyalatu; Takaichi, A.; Nomura, N.; Tsutsumi, Y.; Doi, H.; Kurosu, S.; Chiba, A.; Igarashi, Y.; Hanawa, T. Effects of chromium and nitrogen content on the microstructures and mechanical properties of as-cast Co–Cr–Mo alloys for dental applications. Acta Biomater. 2012, 8, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Manam, N.; Harun, W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.; Ibrahim, M. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Hermawan, H. Biodegradable Metals: From Concept to Applications; Springer Science & Business Media: Johor, Malaysia, 2012. [Google Scholar]
- Toong, D.W.Y.; Ng, J.C.K.; Huang, Y.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.S.; Ang, H.Y. Bioresorbable metals in cardiovascular stents: Material insights and progress. Materialia 2020, 12, 100727. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Murni, N.; Izman, S.; Kurniawan, D.; Froemming, G.; Hermawan, H. In vitro degradation and cell viability assessment of Zn–3Mg alloy for biodegradable bone implants. Proc. Inst. Mech. Eng. H J. Eng. Med. 2015, 229, 335–342. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The suitability of Zn-1.3%Fe alloy as a biodegradable implant material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.T.; Li, X.; Zheng, Y.F. In vitro evaluation of the feasibility of commercial Zn alloys as biodegradable metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Xu, X.; Lu, Y.; Chen, L.; Li, D.; Dai, Y.; Kang, Y.; Yu, K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn-0.8%Li-(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng. C 2019, 99, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshiad, H.R.; Hamzah, E.; Low, H.T.; Cho, M.H.; Kasirisgarani, M.; Farahany, S.; Mostafa, A.; Medraj, M. Thermal characteristics, mechanical properties, in vitro degradation and cytotoxicity of novel biodegradable Zn–Al–Mg and Zn–Al–Mg–xBi alloys. Acta Metall. Sin-Engl. 2017, 30, 201–211. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Levy, G.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants-a review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef]
- Han, K.; Ohnuma, I.; Okuda, K.; Kainuma, R. Experimental determination of phase diagram in the Zn-Fe binary system. J. Alloys Compd. 2018, 737, 490–504. [Google Scholar] [CrossRef]
- Yue, R.; Niu, J.; Li, Y.; Ke, G.; Huang, H.; Pei, J.; Ding, W.; Yuan, G. In vitro cytocompatibility, hemocompatibility and antibacterial properties of biodegradable Zn-Cu-Fe alloys for cardiovascular stents applications. Mater. Sci. Eng. C 2020, 113, 111007. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, mechanical properties and in vitro degradation behavior of novel Zn-Cu-Fe alloys. Mater. Char. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Gao, X.-X.; Chen, H.-T.; Liu, X.-F.; Li, A.; Zhang, H.-J.; Wang, L.-N. Enhance-ment in mechanical and corrosion resistance properties of a biodegradable Zn-Fe alloy through second phase refinement. Mater. Sci. Eng. C 2020, 116, 111197. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Linsley, C.S.; Pan, S.; DeBenedetto, C.; Liu, J.; Wu, B.M.; Li, X. Highly ductile Zn-2Fe-WC nanocomposite as biodegradable. Mater. Metall. Mater. Trans. A 2020, 51, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- ASTM, Standard G32; Standard Method of Vibratory Cavitation Erosion Test. ASTM: West Conshohocken, PE, USA, 2016.
- Bordeasu, I. Monografia Laboratorului de Cercetare a Eroziunii prin Cavitatie al Universitatii Politehnica Timisoara (1960–2020); Editura Politehnica: Timişoara, Romania, 2020. [Google Scholar]
- Bordeaşu, I.; Mitelea, I.; Salcianu, L.; Craciunescu, C.M. Cavitation Erosion Mechanisms of Solution Treated X5CrNi18-10 Stainless Steels. J. Tribol.-Trans. ASM 2016, 138, 031102. [Google Scholar] [CrossRef]
- Bordeasu, I.; Ghera, C.; Luca, A.-N.; Manea, A.-S.; Stroita, D.-C.; Salcianu, L.C.; Ghiban, B.; Micu, L.M. Investigation of Cavitation Resistance of Biocompatible Zinc-Based Alloys for Biomedical Applications. In International Conference on Machine and Industrial Design in Mechanical Engineering; Springer Nature: Cham, Switzerland, 2024; pp. 272–281. [Google Scholar]
- Bordeaşu, I.; Patrascoiu, C.; Badarau, R.; Sucitu, L.; Popoviciu, M.O.; Balasoiu, V. New contributions to cavitation erosion curves modeling. FME Trans. 2006, 34, 39–43. [Google Scholar]
- Ciungu, G.; Micu, L.M.; Bordeasu, I.; Iordache, C.M.; Ghiban, B.; Ghera, C. Research of the Cavitation Resistance of a Biodegradable Alloy Zn-Cu. U.P.B. Sci. Bull. Series B 2024, 86, 247–260. [Google Scholar]
- Iordache, C.M.; Luca, A.N.; Bordeasu, I.; Ciungu, G.; Ghiban, B. Cavitational Erosion Behavior of a Biodegradable Alloy from the Zn-Mg System for Biomedical Applications. Tribol. Ind. 2024, 46, 315–323. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.C.; Birjega, R.; Dinescu, M. Hydroxyapatite Coatings on Mg-Ca Alloy Prepared by Pulsed Laser Deposition: Properties and Corrosion Resistance in Simulated Body Fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Iordache (Gheorghe), C.M. Correlation Between Structure-Mechanical Characteristics-Biodegradability- Cavitation of Some Biomedical Alloys form the System ZnMg(Fe). Ph.D. Thesis, National University of Science and Technology Politehnica Bucharest, Bucharest, Romania, 2024. (in Romanian). [Google Scholar]
- Ciungu, G. Experimental Studies on the Cavitation and Biodegradation Behaviour of Some Alloys of the System ZnCu(Mg) System. Ph.D. Thesis, University Politehnica Bucharest, Bucharest, Romania, 2024; pp. 247–260. [Google Scholar]
- Ghiban, B.; Antoniac, A.; Bordeasu, I.; Antoniac, I.; Petre, G.; Rau, J.; Bordeasu, D.; Micu, L.M. Biodegradability and Cavitation Erosion Behavior of Some Zinc Alloys from the System ZnCuMg. Metals 2025, 15, 161. [Google Scholar] [CrossRef]
- Cerbu, C.; Ursache, S.; Botis, M.F.; Hadar, A. Simulation of the Hybrid Carbon-Aramid Composite Materials Based on Mechanical Characterization by Digital Image Correlation Method. Polymers 2021, 13, 4148. [Google Scholar] [CrossRef]
- Ursache, S.; Cerbu, C.; Hadar, A. Characteristics of Carbon and Kevlar Fibres, Their Composites and Structural Applications in Civil Engineering-A Review. Polymers 2024, 16, 127. [Google Scholar] [CrossRef]
- Gheorghe, A.; Hadar, A.; Apostolescu, Z.; Amza, C.G.; Anton, L. Determination through numerical computation of the designed and functional parameters of ultra acustic systems for ultrasonic welding of intelligent composite materials, 2007. Mat. Plast. 2007, 44, 121–128. [Google Scholar]
- Cormos, R.; Petrescu, H.; Hadar, A.; Gheorghiu, H. Finite Element Analysis of the Multilayered Honeycomb Composite Material Subjected to Impact Loading. Mat. Plast. 2017, 54, 180–185. [Google Scholar] [CrossRef]
- ASTM STP 408; Experience with a 20—KC Cavitations Erosion Test, Erosion by Cavitations or Impingement. ASTM: Atlantic City, NJ, USA, 1960.
- ASTM STP 408; Corelation of Cavitation Damage with Other Material and Fluid Properties, Erosion by Cavitation or Impingement. ASTM: Atlantic City, NJ, USA, 1966.
- Franc, J.P.; Kueny, J.L.; Karimi, A.; Fruman, D.H.; Fréchou, D.; Briançon-Marjollet, L.; Yves Billard, J.Y.; Belahadji, B.; Avellan, F.; Michel, J.M. La Cavitation. Mécanismes Physiques et Aspects Industriels; Press Universitaires de Grenoble: Grenoble, France, 1995. [Google Scholar]






| Alloy | Chemical Composition, %wt | ||||||
|---|---|---|---|---|---|---|---|
| Mg | Fe | S | P | Si | Ni | Zn | |
| Zn | - | - | - | 0.019 | 0.45 | 0.009 | Rest |
| ZnMg | 3.30 | - | 0.36 | 0.019 | 1.06 | 0.02 | Rest |
| ZnMgFe | 3.61 | 1,01 | 0.3 | - | 0.72 | 0.01 | Rest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiban, B.; Bordeasu, I.; Antoniac, A.; Antoniac, I.; Gheorghe, C.M.; Bordeasu, D.; Micu, L.M.; Ghera, C.; Salcianu, L.C.; Florea, B.; et al. Influence of Homogenization Heat Treatments on the Mechanical, Structural, Biodegradation, and Cavitation Behavior of Some Alloys in the ZnMg(Fe) System. Crystals 2025, 15, 458. https://doi.org/10.3390/cryst15050458
Ghiban B, Bordeasu I, Antoniac A, Antoniac I, Gheorghe CM, Bordeasu D, Micu LM, Ghera C, Salcianu LC, Florea B, et al. Influence of Homogenization Heat Treatments on the Mechanical, Structural, Biodegradation, and Cavitation Behavior of Some Alloys in the ZnMg(Fe) System. Crystals. 2025; 15(5):458. https://doi.org/10.3390/cryst15050458
Chicago/Turabian StyleGhiban, Brandușa, Ilare Bordeasu, Aurora Antoniac, Iulian Antoniac, Cristina Maria Gheorghe, Dorin Bordeasu, Lavinia Madalina Micu, Cristian Ghera, Laura Cornelia Salcianu, Bogdan Florea, and et al. 2025. "Influence of Homogenization Heat Treatments on the Mechanical, Structural, Biodegradation, and Cavitation Behavior of Some Alloys in the ZnMg(Fe) System" Crystals 15, no. 5: 458. https://doi.org/10.3390/cryst15050458
APA StyleGhiban, B., Bordeasu, I., Antoniac, A., Antoniac, I., Gheorghe, C. M., Bordeasu, D., Micu, L. M., Ghera, C., Salcianu, L. C., Florea, B., Ostoia, D., & Fratila, A. M. (2025). Influence of Homogenization Heat Treatments on the Mechanical, Structural, Biodegradation, and Cavitation Behavior of Some Alloys in the ZnMg(Fe) System. Crystals, 15(5), 458. https://doi.org/10.3390/cryst15050458








