1. Introduction
Austenitic stainless steels are widely used in industries that require high corrosion resistance and good mechanical properties, such as biomedicine, shipbuilding, and the aerospace industry [
1,
2]. Nitriding significantly improves the mechanical properties of austenitic stainless steels. This effect is due to the formation of nitrogen-containing austenite (S-phase) and other hard phases that strengthen the surface of the material. The introduction of nitrogen atoms into the steel structure leads to the formation of iron and chromium nitrides [
3,
4]. The authors of studies [
5,
6,
7,
8] found that nitriding promotes the formation of stronger surface layers without significantly altering the fundamental properties of the steel. Study [
9] showed that low-temperature nitriding of austenitic steels forms a dense protective layer that prevents the penetration of aggressive substances such as chlorides. Study [
10] established that increasing the nitriding time promotes the formation of a thicker nitride film, which reduces the coefficient of friction and enhances surface durability.
There are several nitriding methods used to improve the properties of stainless steels. Gas nitriding—performed at high temperatures in an ammonia atmosphere—creates a nitride layer that increases hardness but may reduce corrosion resistance [
11]. Ion (plasma) nitriding uses an ion discharge to introduce nitrogen into the steel surface, providing high hardness and wear resistance, although it requires complex equipment [
12]. Low-temperature nitriding is especially useful for medical and biomedical applications, as it prevents the formation of brittle phases [
13]. Electrolytic plasma nitriding (EPN) is an innovative method that uses electrolytic plasma for rapid nitrogen diffusion, distinguishing it from other steel surface treatment methods. The study by the authors [
14] showed that EPN provides a higher nitrogen saturation rate on the surface compared to traditional methods. Unlike traditional gas nitriding, EPN allows avoiding high temperatures that can cause thermal distortion of the steel structure [
15].
Electric discharges occurring in the vapor-gas envelope surrounding the treated surface play a key role in the implementation of the EPN process. It is these discharges that provide localized heating, surface activation, and the generation of active nitrogen species capable of effectively diffusing into the metal. The type, intensity, and stability of the discharges significantly affect the microstructure, phase composition, and properties of the formed layer. Unstable and intense discharges can lead to overheating, micro-melting, and the formation of defects, whereas controlled glow discharges contribute to the formation of a uniform and dense hardened layer. Discharges can be divided into three main categories: discharges occurring directly within the liquid [
16], discharges in the gas phase above the liquid surface, including cases where the liquid itself acts as a conductive electrode [
17], and discharges in multiphase systems—within bubbles, in vapor-gas mixtures, aerosol environments, and foams [
18]. Plasma in a liquid environment is studied using visual observations and optical emission spectroscopy [
19]. These methods make it possible to determine key plasma characteristics such as the type and shape of plasma formations, its composition, temperature, and the density of its constituent components [
20,
21].
The aim of this work is to investigate the spectral characteristics of the electric discharge during the electrolytic plasma nitriding of austenitic stainless steel and its structural-phase transformations. Special attention is given to identifying the active plasma components responsible for the formation of the nitrided layer, as well as the nitrogen distribution with depth in the material.
2. Materials and Methods
For the EPN process, austenitic stainless steel grade 12Kh18N10T was selected, which is equivalent to AISI 321 steel. The chemical composition of 12Kh18N10T steel according to GOST 5632-72 is as follows: iron (Fe) up to 67%, chromium (Cr) 17–19%, nickel (Ni) 9–11%, manganese (Mn) up to 2%, titanium (Ti) 0.4–1%, silicon (Si) up to 0.8%, copper (Cu) up to 0.3%, carbon (C) up to 0.12%, phosphorus (P) up to 0.035%, and sulfur (S) up to 0.02%. The specimens were prepared in the form of cubes with dimensions of 20 × 20 × 20 mm. Before the process, the specimen surfaces were prepared using a HYMP-2 grinding and polishing machine, achieving a surface roughness level of Ra 1.0 ± 0.1 µm. After polishing, the specimens were thoroughly rinsed with running water to remove any remaining abrasive materials and contaminants. During the experiment, a water-based electrolyte was used, containing 10% sodium carbonate, 22% carbamide, 3% ammonium chloride, and 60% distilled water.
The experiment was conducted using an EPN setup equipped with a 40 kW DC power rectifier and a specialized treatment chamber containing a plasma torch, as shown in
Figure 1. The nitriding process of the samples was carried out as follows: the working bath (5) was prefilled with the electrolyte. Using a pump (4) installed at the bottom of the bath, the electrolyte was directed through pipelines (8) to the plasma torch zone (3), which was located inside the processing chamber (2). After passing through the plasma torch, the electrolyte returned to the bath through drain openings, ensuring continuous fluid circulation. The electrolyte flow rate was maintained in the range of 4–7 L/min. To stabilize the electrolyte temperature within the range of 40–50 °C, a heat exchanger was used, into which cooling water was supplied at a flow rate of 3–6 L/min. The sample (6) was fixed in the holder in such a way that the treated surface was positioned at a distance of 2–3 mm from the opening of the conical partition. Through this opening, the electrolyte was supplied as a jet, simultaneously cooling the sample surface and helping to maintain the desired temperature. The anode (7) was connected to the positive terminal of the power supply (1), while the sample, serving as the cathode, was connected to the negative terminal. To rapidly heat the surface to the saturation temperature, a voltage of 320 V was applied to the sample for 10 s, after which the voltage was reduced to 180 V, and the treatment continued for 10 min. The sample temperature during the process was maintained within the range of 500–550 °C by regulating the electrolyte flow rate [
22]. The selection of these parameters is based on preliminary studies and literature data [
14,
15], which indicate that a temperature range of 500–550 °C enables the formation of nitride layers without the brittle delta phase. However, nitriding was also carried out at temperatures of 650 and 750 °C to compare the changes in the characteristics of the nitrided layer depending on the temperature. Reducing the voltage from 320 V to 180 V after a short pulse ensures that the saturation temperature is reached and the plasma is stabilized, thereby preventing thermal damage to the surface.
The analysis of the spectral characteristics of the electrolytic plasma nitrocarburizing process was carried out using an ultra-high-resolution optical spectrometer ATP3030 (Optosky, Jimei, Xiamen, China). The identification of spectral lines was performed by comparing the obtained spectra with data from the National Institute of Standards and Technology (NIST) database [
23]. X-ray phase analysis was performed using an X’Pert PRO diffractometer (PANalytical, Almelo, The Netherlands) equipped with a copper anode tube operated at 40 kV and 30 mA. Cu-Kα radiation (λ = 1.541 Å) was recorded in the 2θ range from 30° to 100°, with a step size of 0.02° and a counting time of 0.5 s per step. The processing and interpretation of the diffraction patterns were carried out using HighScore Plus software, version 3.0e. The microstructure of the nitrided samples was examined using a TESCAN MIRA3 LMH scanning electron microscope (TESCAN, Brno, Czech Republic) after electrochemical etching in a solution consisting of 70% hydrochloric acid, 25% distilled water, and 5% hydrogen peroxide (30%).
Surface roughness of the samples was measured using a Surtronic S-100 Series profilometer (Taylor Hobson Ltd, Leicester, UK). Hardness was measured using a FISCHERSCOPE HM2000 microhardness tester under a load of 100 mN (Helmut Fischer GmbH, Sindelfingen, Germany). The determination of the friction coefficient was carried out on a universal tribological tester TRB3 (Anton Paar, Graz, Austria) using the ball-on-disk method, in accordance with ASTM G133 standard. The tests were conducted under dry sliding conditions at an ambient temperature of 25 ± 1 °C and a sliding speed of 0.05 m/s. The samples were brought into contact with a 100Cr6 steel counterbody under a vertical load of 10 N. Friction coefficient values were recorded after the sample completed a sliding distance of 60 m.
3. Results and Discussion
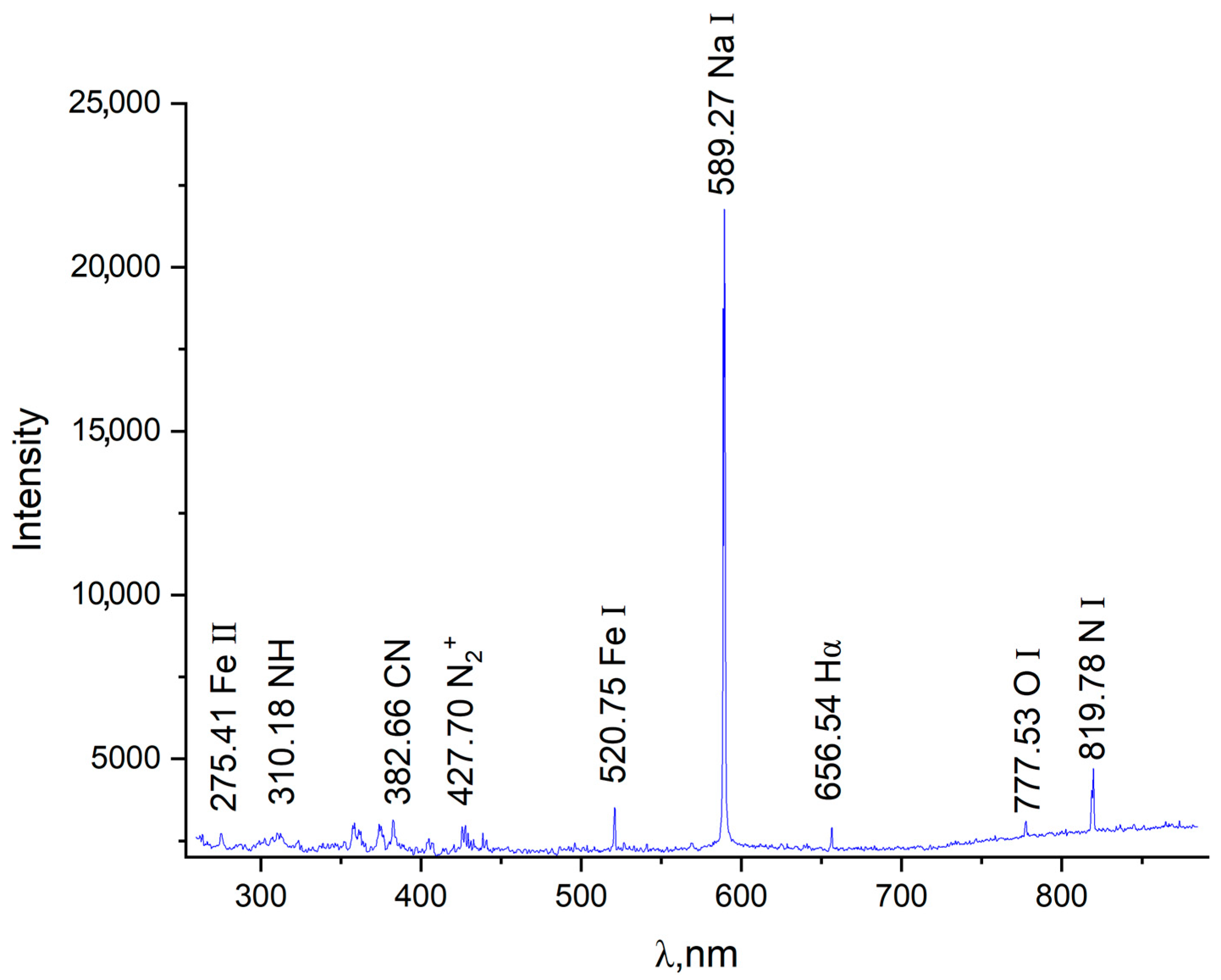
The obtained emission spectrum shown in
Figure 2 allows for a qualitative analysis of the active species formed in the near-electrode region. The identification of Na I (589.27 nm), Fe I/II, NH, CN lines, as well as molecular ions N
2+ and atomic nitrogen N I, confirms the presence of both ionized and excited neutral species in the plasma. Special attention should be paid to the intense peak at the wavelength of 589.27 nm, which corresponds to the resonance D-line of neutral sodium. This is due to the fact that the Na I 589.0/589.6 nm line is weakly sensitive to self-absorption [
20,
21]. The surge at 427.70 nm (N
2+) indicates the presence of molecular ions characteristic of excited nitrogen plasma, which plays a key role in the formation of the nitride layer. The presence of CN and NH radicals indicates the involvement of carbon- and hydrogen-containing components, originating from carbamide and ammonium in the electrolyte, in the complex chemical kinetics of the nitriding process.
Taking into account the characteristics of the equipment used, the broadening of spectral lines caused by pressure and the linear Stark effect was calculated using the full width at half maximum (FWHM) of the Lorentzian profile, Δ
λL, according to the formula [
24]:
where Δ
λG is the FWHM of the Gaussian profile (instrumental broadening of the Optosky ATP3030 spectrometer = 1.0 nm), Δ
λF is the FWHM of the Voigt profile (1.12 nm), and Δ
λL is the FWHM of the Lorentzian profile. As a result of the calculation, the FWHM of the Lorentzian profile was 0.496 nm.
To calculate the electron density
ne, an empirical formula relating
ne to the full width at half maximum (FWHM) of the Hα line was used [
25]:
where Δ
λL is the FWHM of the Lorentzian profile, and
Cn(
T) is taken from [
24] for the corresponding hydrogen Balmer series line. The coefficients used for calculating the electron density from the emission spectra of hydrogen Balmer series lines are:
C0 = 671.4,
C1 = −227.5,
C2 = 44.72,
C3 = −2.325. The calculated electron density of the plasma is approximately
ne ≈ 8.5 × 10
18 cm
−3. A similar study [
19] reported comparable levels of electron density (
ne = 6.1 × 10
16–7.9 × 10
16 cm
−3) for atmospheric discharges in an electrolyte consisting of a 5% NaCl solution in purified tap water.
Electrolytic plasma nitriding was carried out at temperatures of 550 °C, 650 °C, and 750 °C. After electrolytic plasma nitriding, an increase in surface roughness was observed with rising treatment temperature: for the sample treated at 550 °C, the roughness was 1.22 μm; at 650 °C, it was 3.56 μm; and at 750 °C, it was 5.2 μm. The increase in surface roughness indicates enhanced intensity of electrical discharges on the surface, as elevated temperatures lead to higher current density and energy of individual discharges. This results in more aggressive surface bombardment and partial melting of micro-protrusions, which enhances the surface microrelief and, consequently, increases the roughness parameters. This effect is also supported by several studies, which report an increase in the energy of plasma micro-explosions with rising voltage and temperature during EPN [
26,
27].
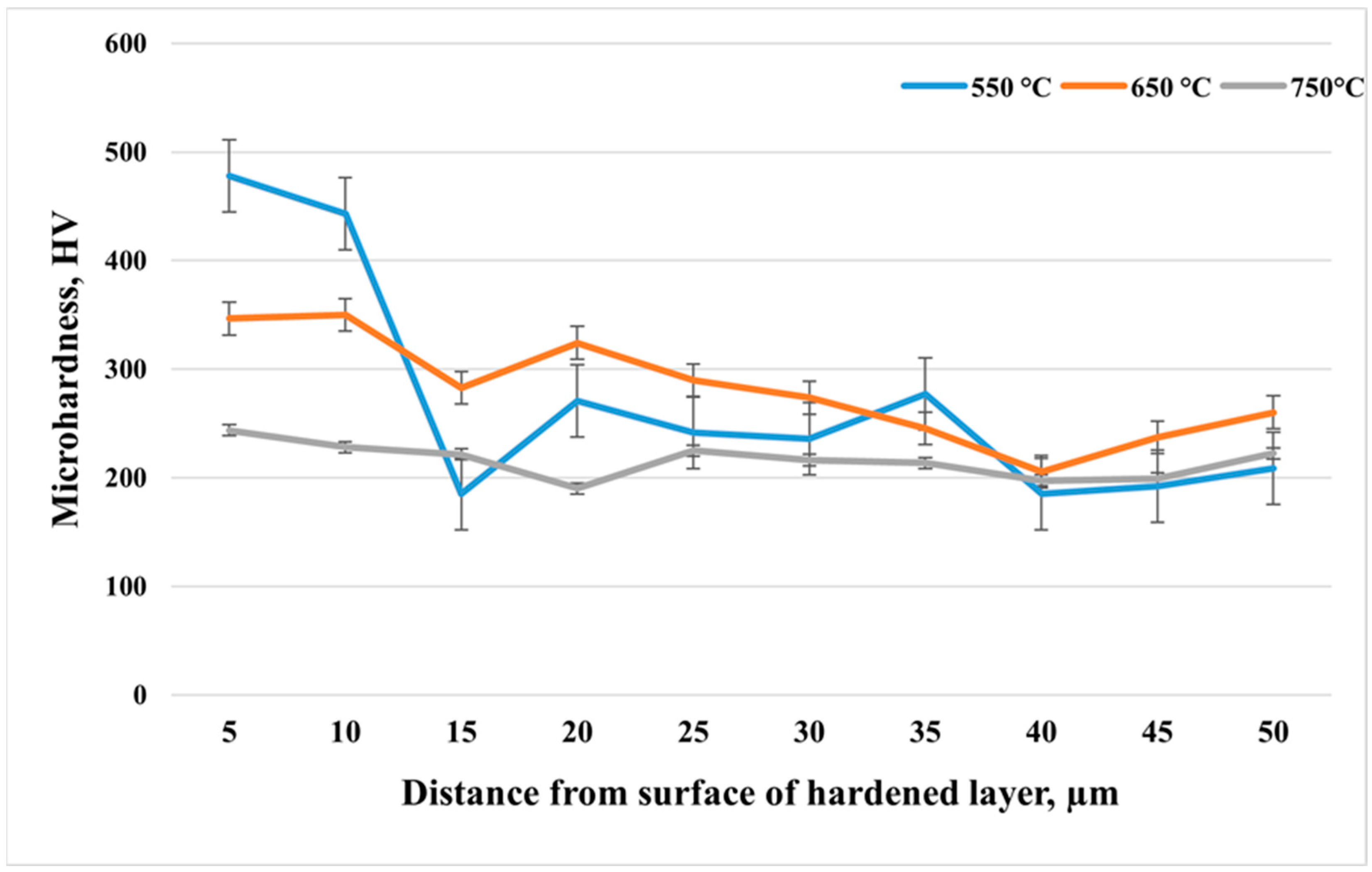
Figure 3 shows the microhardness distribution with depth from the surface of 12Kh18N10T stainless steel after cathodic electrolytic plasma nitriding at different temperatures: 550 °C, 650 °C, and 750 °C. In all cases, the treatment duration was 10 min. At a temperature of 550 °C, the maximum microhardness—up to 480 HV near the surface—was observed, indicating intensive nitrogen saturation and the formation of nitride phases in the surface zone. However, at a depth of about 15 μm, a sharp decrease in hardness to approximately 180 HV is observed, likely corresponding to the boundary between the nitrided layer and the unsaturated matrix. Beyond this point, the microhardness stabilizes at around 220–250 HV, which corresponds to the original austenitic structure of the steel. At a temperature of 650 °C, a more uniform hardness profile is achieved without sharp drops. The surface value is about 350–360 HV, and an elevated hardness level is maintained throughout the entire depth, which may indicate deeper and more uniform nitrogen diffusion, but with less pronounced formation of high-hardness nitride phases compared to 550 °C. In the case of 750 °C, the maximum surface hardness is approximately 250 HV and decreases to around 200 HV with depth. This behavior can be explained by the possible dissolution of nitrides and the reversion to the austenitic phase due to overheating, as well as enhanced diffusion and recrystallization processes that reduce the strengthening effect.
After measuring the surface roughness and microhardness depth profiles of the samples treated at different temperatures, the sample treated at 550 °C was selected for further investigation. This sample exhibited the highest microhardness and the lowest surface roughness among the treated specimens.
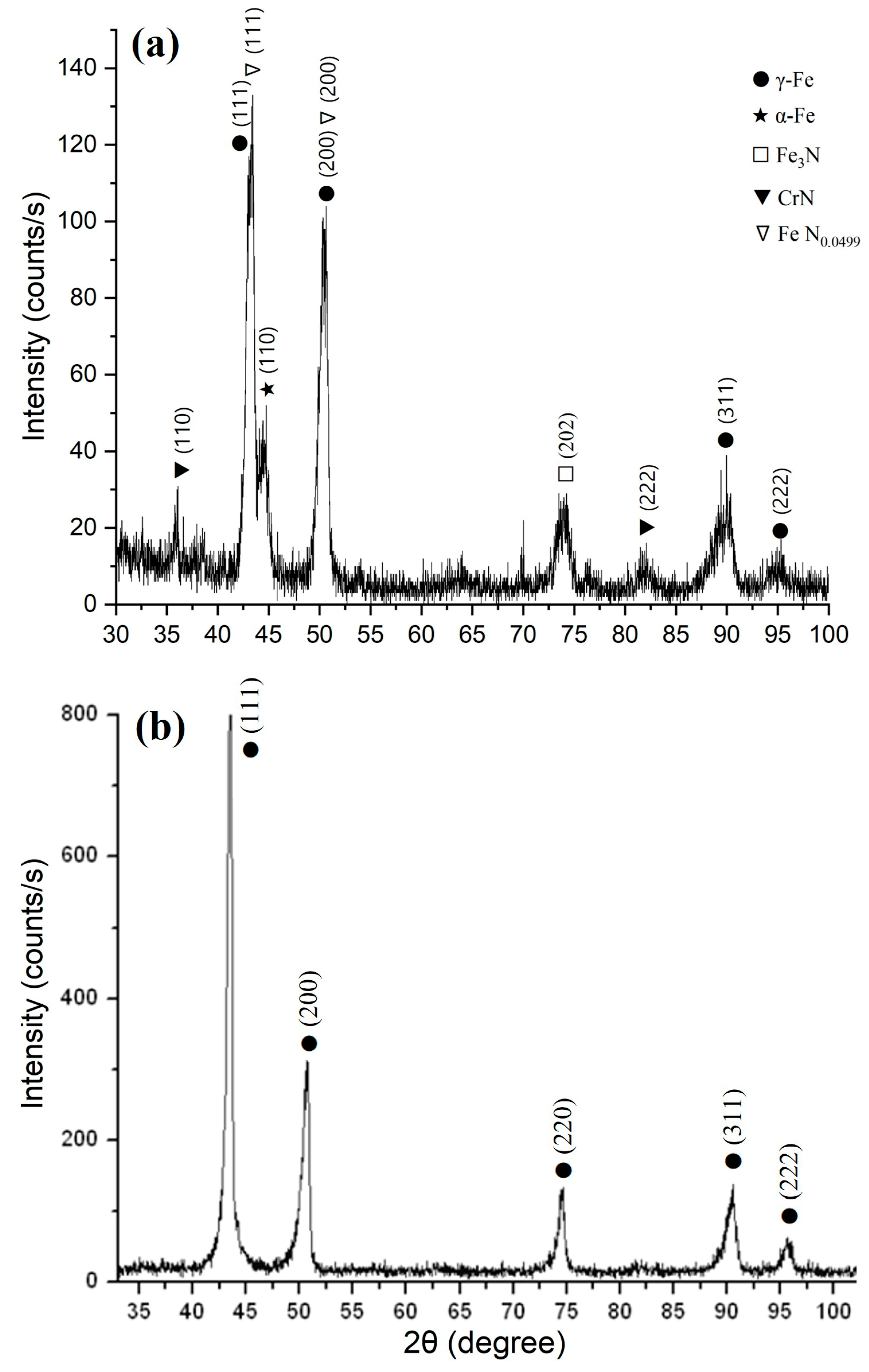
X-ray structural analysis of the 12Kh18N10T steel surface before and after electrolytic plasma nitriding (
Figure 4) revealed significant changes in the phase composition. In the initial state, intense diffraction reflections corresponding to the γ-phase (austenite) along the (111), (200), (220), (311), and (222) planes were observed, confirming the stability of the austenitic lattice and the structural homogeneity of the material prior to treatment (
Figure 4b). After nitriding, the main γ-Fe peaks remained, but additional reflections were identified corresponding to the Fe
3N phase (202 reflection), CrN phase (111 and 200 reflections), α-Fe phase (110 reflection), as well as a solid solution of nitrogen FeN
0.0499 in γ-Fe (111 and 200 reflections) (
Figure 4a). The presence of nitride phases indicates successful surface saturation with active nitrogen species, while the detection of the α-phase may suggest local destabilization of the austenitic structure due to the thermal effect of the discharge. The broad and low-intensity reflections of the nitrogen solid solution indicate the initial stage of nitrogen incorporation into the austenitic lattice. The reduced intensity of γ-phase reflections compared to the initial state further confirms the partial redistribution of the crystalline structure. The obtained results are consistent with emission spectroscopy data, which recorded active nitrogen species in the plasma (N
2+ at λ = 427.7 nm and N I at λ = 819.8 nm), providing a high nitriding potential under the investigated conditions [
28].
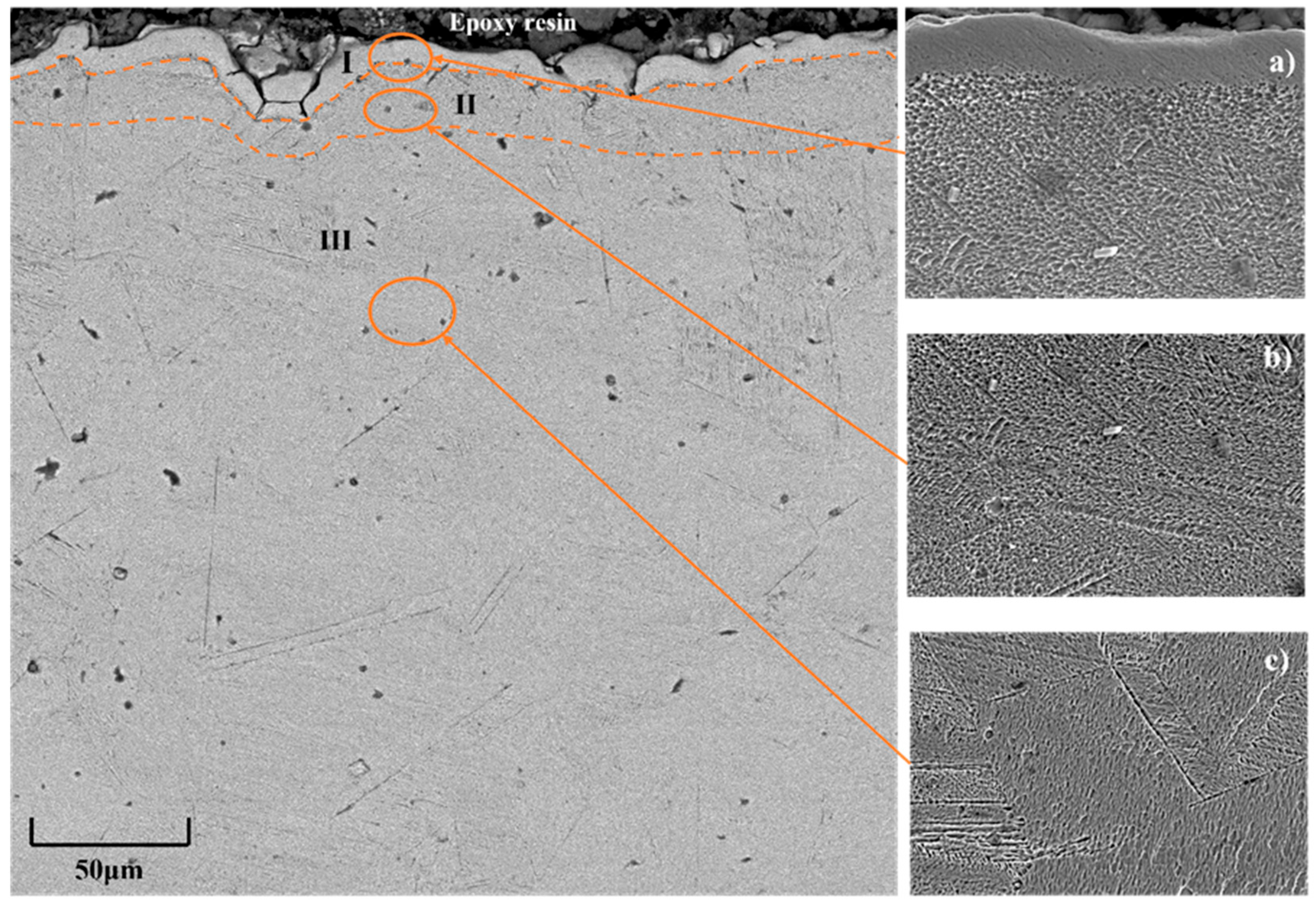
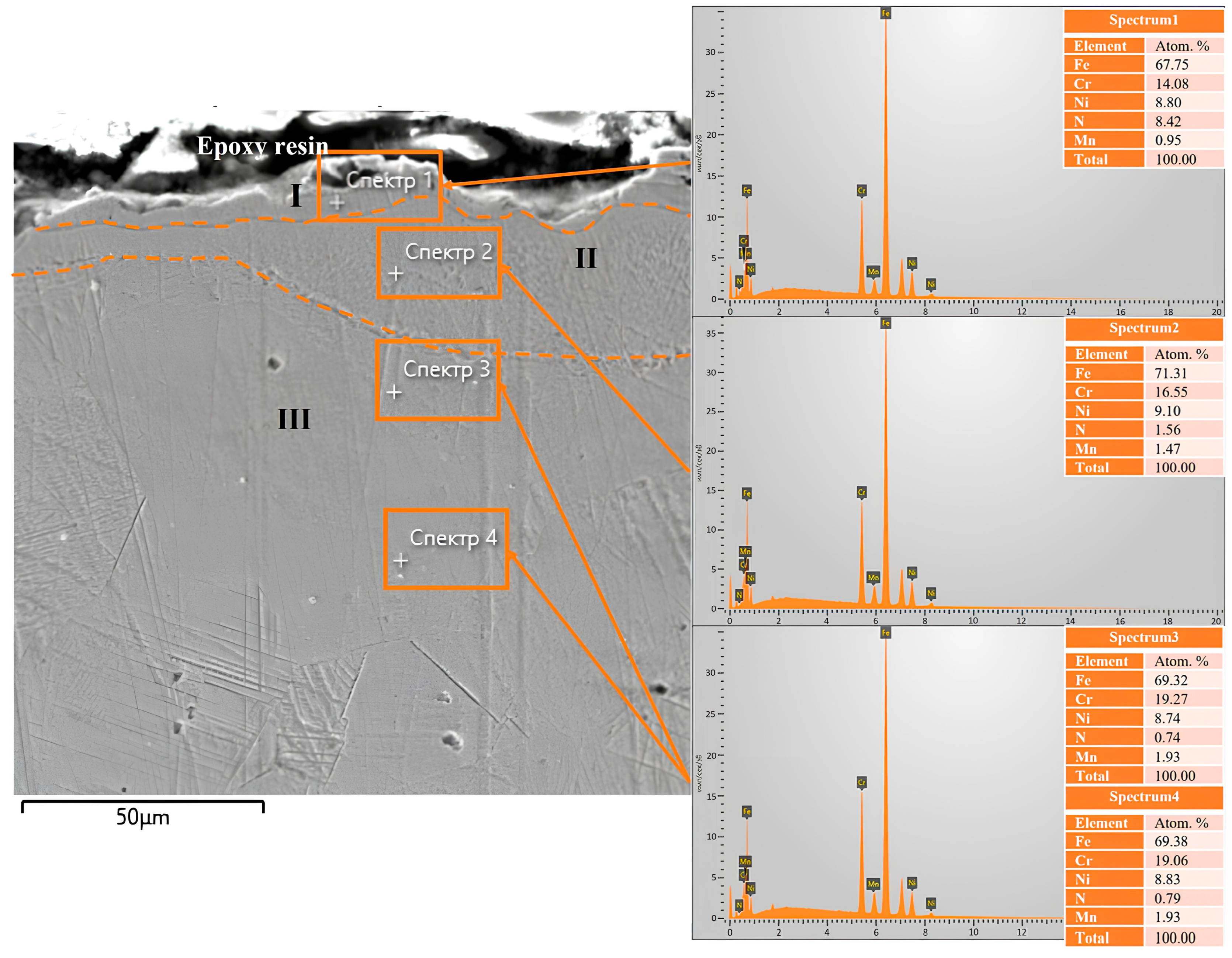
The scanning electron microscope image of the cross-section of 12Kh18N10T steel after electrolytic plasma nitriding (
Figure 5) clearly shows a distinct three-layer structure of the modified surface layer. Zone I, located directly beneath the epoxy layer, appears as a bright, non-etching surface layer approximately 7–8 μm thick, enriched with nitrogen. According to X-ray phase analysis, nitride phases CrN and Fe
3N are formed in this zone, as confirmed by the presence of diffraction reflections from the (110), (222), and (202) planes. The formation of the nitride zone is attributed to the high concentration of active nitrogen species in the plasma, as confirmed by emission spectroscopy, which detected intense lines of N
2+ (λ = 427.7 nm) and N I (λ = 819.8 nm). Energy-dispersive X-ray analysis (spectrum 1) revealed the highest nitrogen content in this zone, amounting to 8.42 at.% (
Figure 5a). Zone II is a transitional sublayer approximately 8–12 μm thick, characterized by a gradual decrease in nitrogen content (down to 1.56 at.% according to spectrum 2) and the formation of a solid solution of nitrogen in the austenitic lattice (FeN
0.0499). This structural state is confirmed by the shift of γ-phase diffraction maxima, indicating distortion of the crystal lattice. Morphologically, the zone exhibits a more ductile structure with reduced contrast, as seen in the magnified fragment (
Figure 5b). Zone III corresponds to the unmodified part of the material, retaining the original austenitic structure, as confirmed by characteristic γ-Fe peaks from the (111), (200), (311), and (222) planes, as well as a consistently low nitrogen content (less than 0.1 at.% according to spectrum 3). The structure of this zone is homogeneous and typical for stainless steels prior to nitriding (
Figure 5c) [
29].
Energy-dispersive X-ray spectroscopy (EDS) was performed on the cross-section of 12X18H10T steel after EPN to assess the elemental distribution in the surface layer. In the scanning electron microscopy image (
Figure 6), four analysis zones were selected along the depth from the surface into the material. In spectrum 1, corresponding to the uppermost part of the layer (the nitride zone), the highest nitrogen content—8.42 at.%—was observed, indicating the formation of a saturated nitride layer containing CrN and Fe
3N phases previously identified by X-ray diffraction. A decrease in chromium content (14.08 at.%) was also observed in this region compared to deeper areas, which may be attributed to its involvement in nitride formation. In spectrum 2 (at the boundary between the nitride and transition zones), the nitrogen content significantly decreases to 1.56 at.%, while the chromium content increases to 16.55 at.%, which may indicate the initial stage of nitride phase decomposition and inward diffusion of nitrogen. In spectra 3 and 4, corresponding to the transition zone and the bulk of the original material, the nitrogen concentrations were 0.74 at.% and 0.83 at.%, respectively, reflecting the gradual saturation of the nitrogen solid solution in γ-Fe (FeN
0.0499 phase), as also confirmed by the X-ray phase analysis results. Thus, EDS analysis reveals a distinct nitrogen distribution gradient with depth, corresponding to the three-zone structure observed in microstructural studies: the nitride zone (I), the transition zone (II), and the unsaturated matrix (III). These findings are in good agreement with the SEM, X-ray diffraction, and emission spectroscopy results, confirming the successful formation of a nitrided surface layer during EPN.
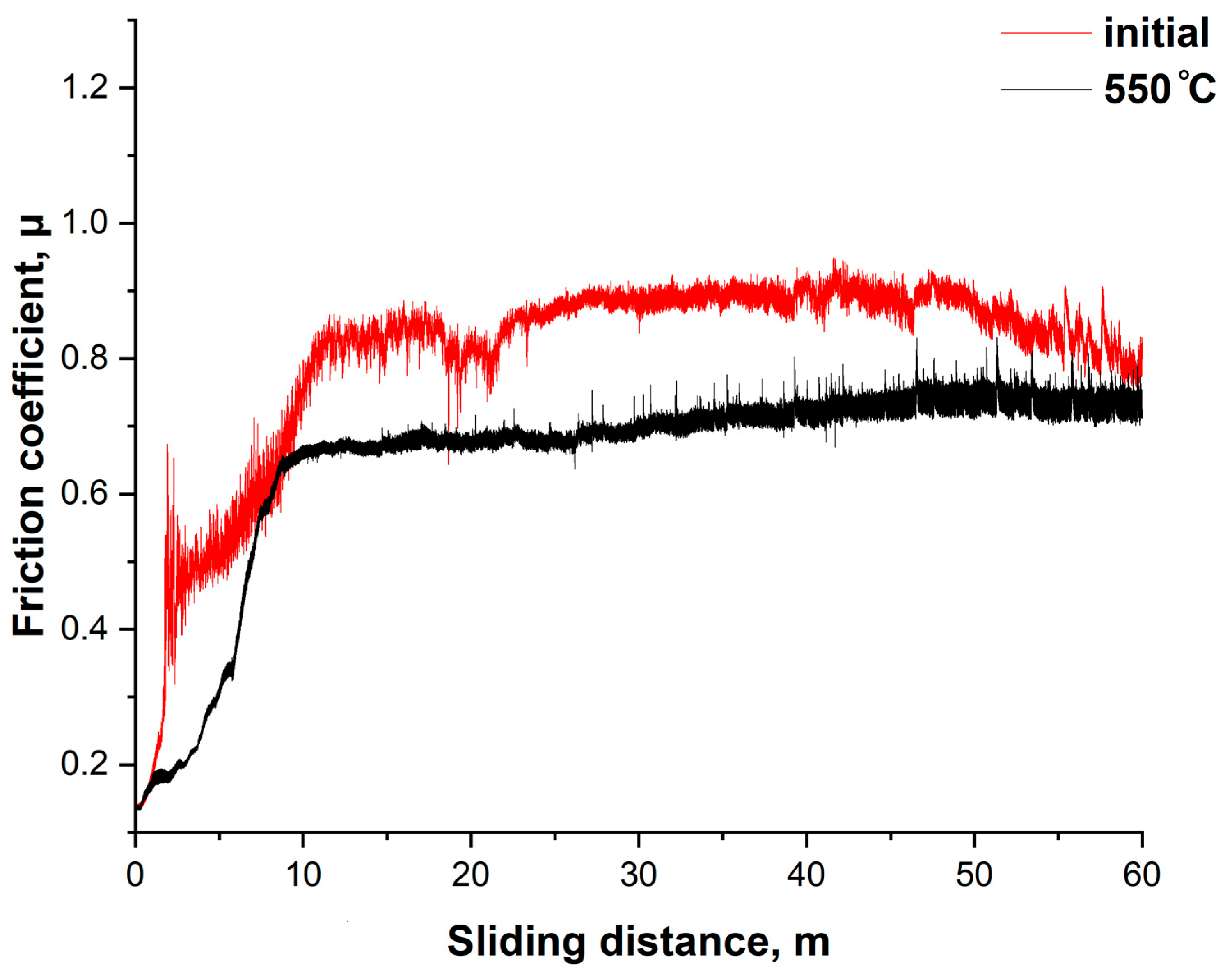
Figure 7 shows the dependence of the friction coefficient (μ) on the sliding distance for the initial and nitrided (at 550 °C) samples of 12Kh18N10T stainless steel. The tests were conducted under dry sliding conditions using the ball-on-disk configuration. The initial sample exhibited a gradual increase in the friction coefficient to approximately 0.8, with pronounced instability throughout the test. Significant fluctuations indicate unstable friction, likely accompanied by micro-scuffing and adhesive interactions. This corresponds to the typical behavior of unmodified austenitic steel under dry contact conditions. After cathodic electrolytic plasma nitriding at 550 °C, a significant improvement in tribological performance was observed. The friction coefficient reaches a stable value of approximately 0.6 already in the initial stages of sliding (~10 m) and remains nearly constant throughout the entire test. The reduction in friction and its stability can be explained by the formation of nitrided phases (Fe
3N, CrN), which possess enhanced hardness and wear resistance, reducing the intensity of adhesive contacts and preventing local seizure. The nitrided layer thickness of 7–8 μm at 550 °C contributes to a reduction in the friction coefficient by more than 20% and ensures stable friction throughout the entire cycle, confirming the effectiveness of the treatment in terms of improving the operational properties of the steel under dry friction conditions [
30].
4. Conclusions
The conducted studies showed that cathodic electrolytic plasma nitriding (EPN) of austenitic stainless steel 12Kh18N10T at 550 °C for 10 min in an aqueous electrolyte containing Na2CO3, CO(NH2)2, and NH4Cl results in the formation of a complex multi-layered structure of the nitrided layer. Emission spectral analysis confirmed the presence of active nitrogen species in the plasma (N2+, N I), as well as hydrogen and oxygen, which ensures efficient surface saturation. The calculation of the electron density based on the Hα line broadening showed a value of approximately 8.5 × 1018 cm−3, indicating a high degree of ionization in the processing zone. X-ray phase analysis revealed the formation of the nitride phases CrN and Fe3N in the surface zone, as well as a solid solution of nitrogen FeN0.0499 in the austenitic lattice. These structural changes are accompanied by a gradient phase distribution with depth, as confirmed by microstructural observations and the results of energy-dispersive X-ray analysis (EDS), which revealed the maximum nitrogen concentration near the surface and its gradual decrease with depth into the material. The high effectiveness of EPN in forming a nitrided layer on 12Kh18N10T steel with a pronounced zonal structure has been established, including a surface nitride zone, a transition region, and the retained austenitic matrix. The microhardness distribution showed that after EPN at 550 °C, the maximum hardness is approximately 480 HV near the surface, with a sharp drop to around 180–220 HV at depths greater than 15 μm. This indicates the presence of a hardened strengthening zone with a sharp transition to the matrix, confirming the phase gradient of the layer. Tribological tests using the ball-on-disk configuration showed a reduction in the friction coefficient by more than 20% compared to the initial state. The friction coefficient stabilizes at around 0.6 for nitrided samples, compared to ~0.8 for the initial samples, a phenomenon that is associated with the formation of hard nitride phases and a reduction in adhesive interaction.
Future work will focus on investigating the effect of preliminary mechanical activation of the surface on the structure, phase composition, and properties of the nitrided layer, with the aim of enhancing its uniformity, density, and operational characteristics.