Preparation of CuO-Bi2O3-MgO/SiO2 Spherical Catalyst and Its Formaldehyde Acetylenation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Catalysts Preparation
2.3. Catalysts Evaluation
3. Results and Discussion
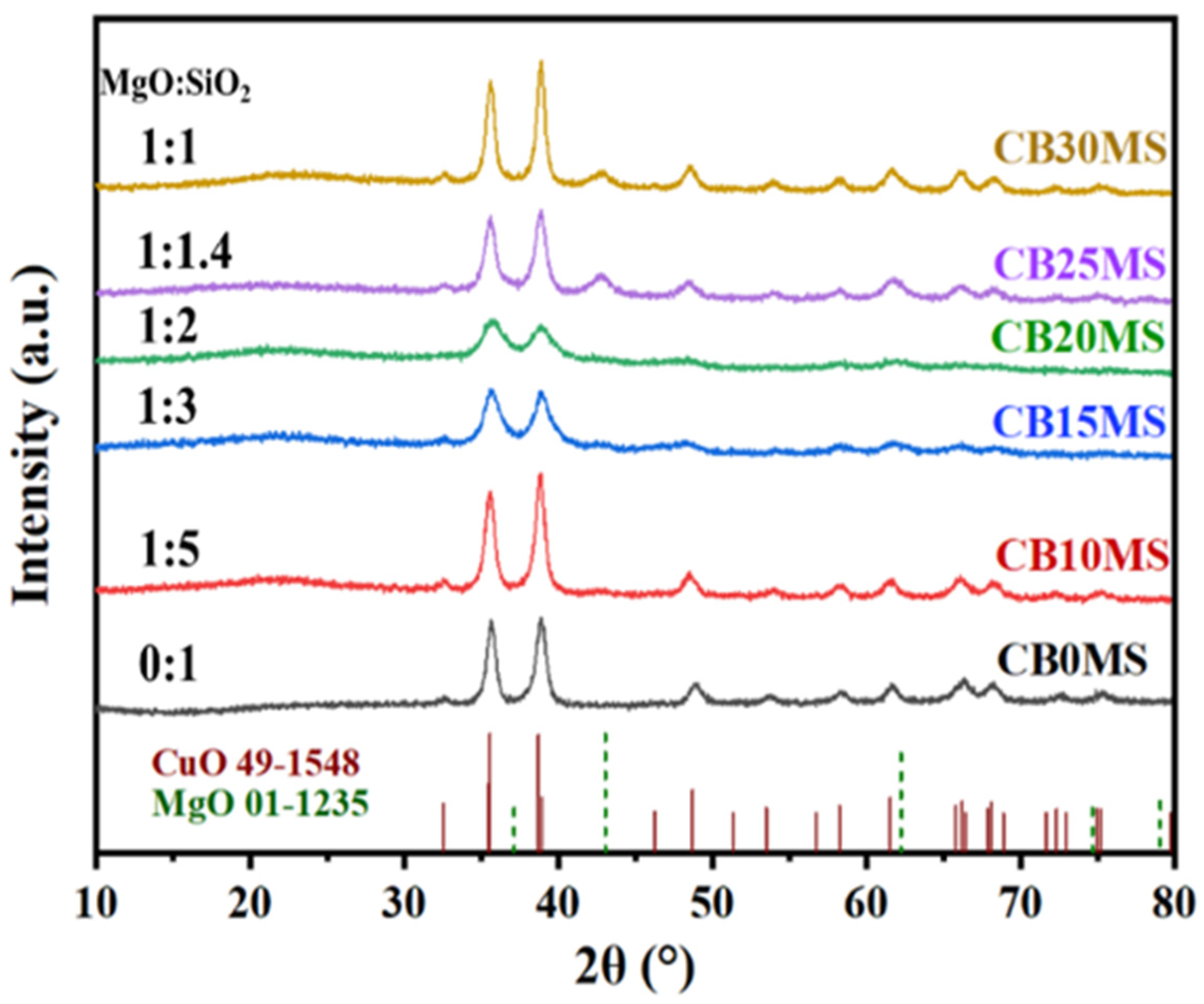
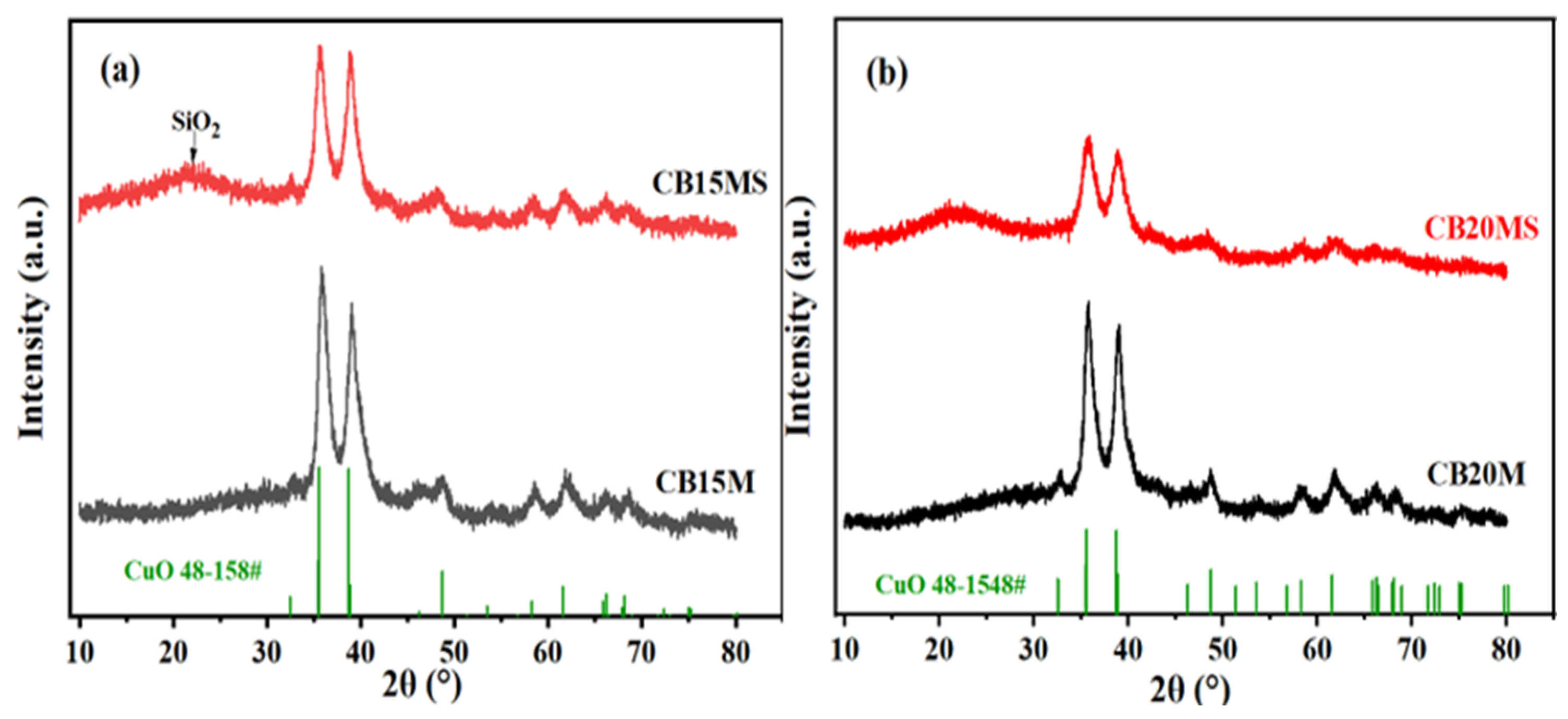
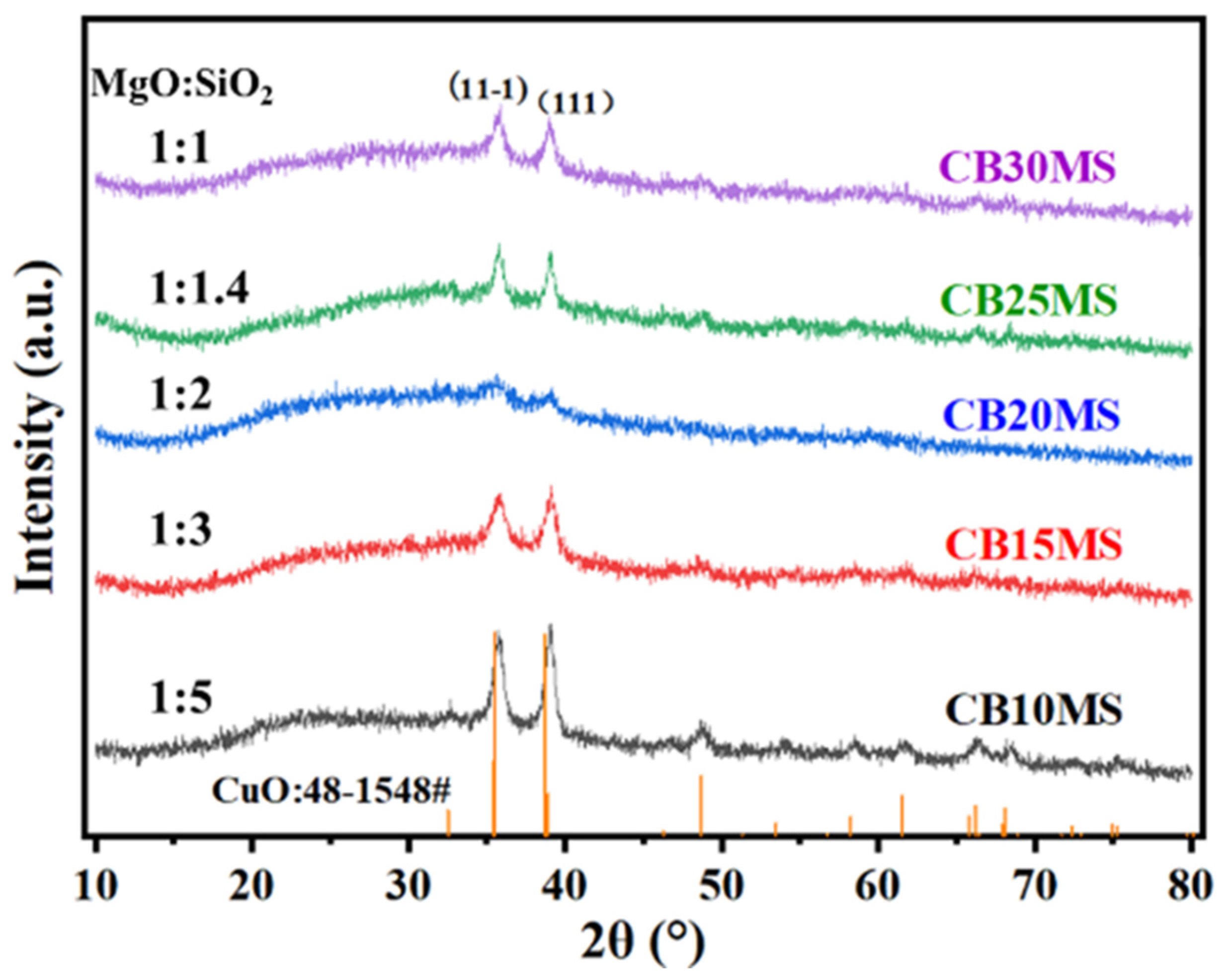
3.1. X-Ray Diffraction Characterization of Catalyst
3.2. N2 Physical Adsorption Characterization of Catalyst
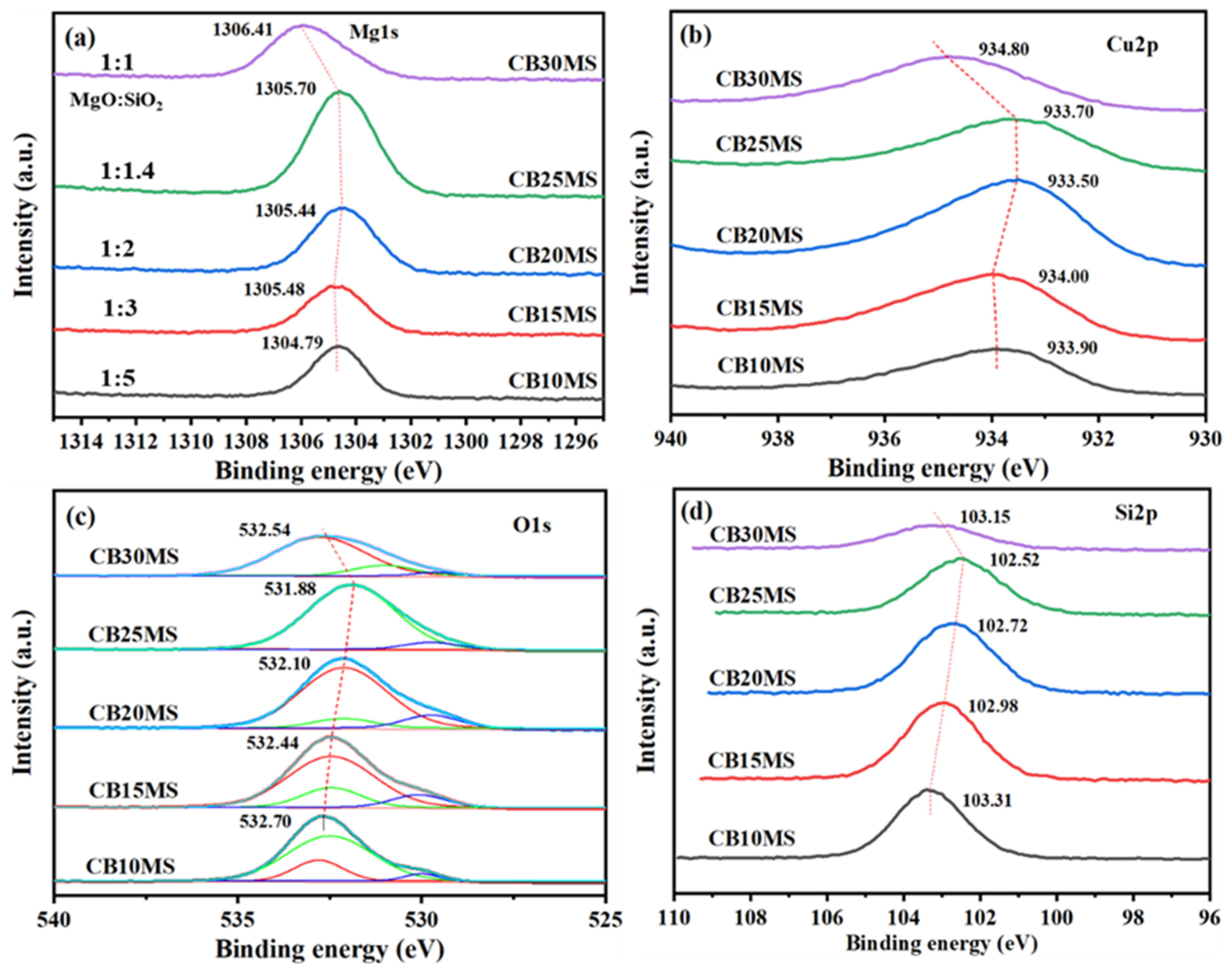
3.3. XPS Characterization of Catalyst
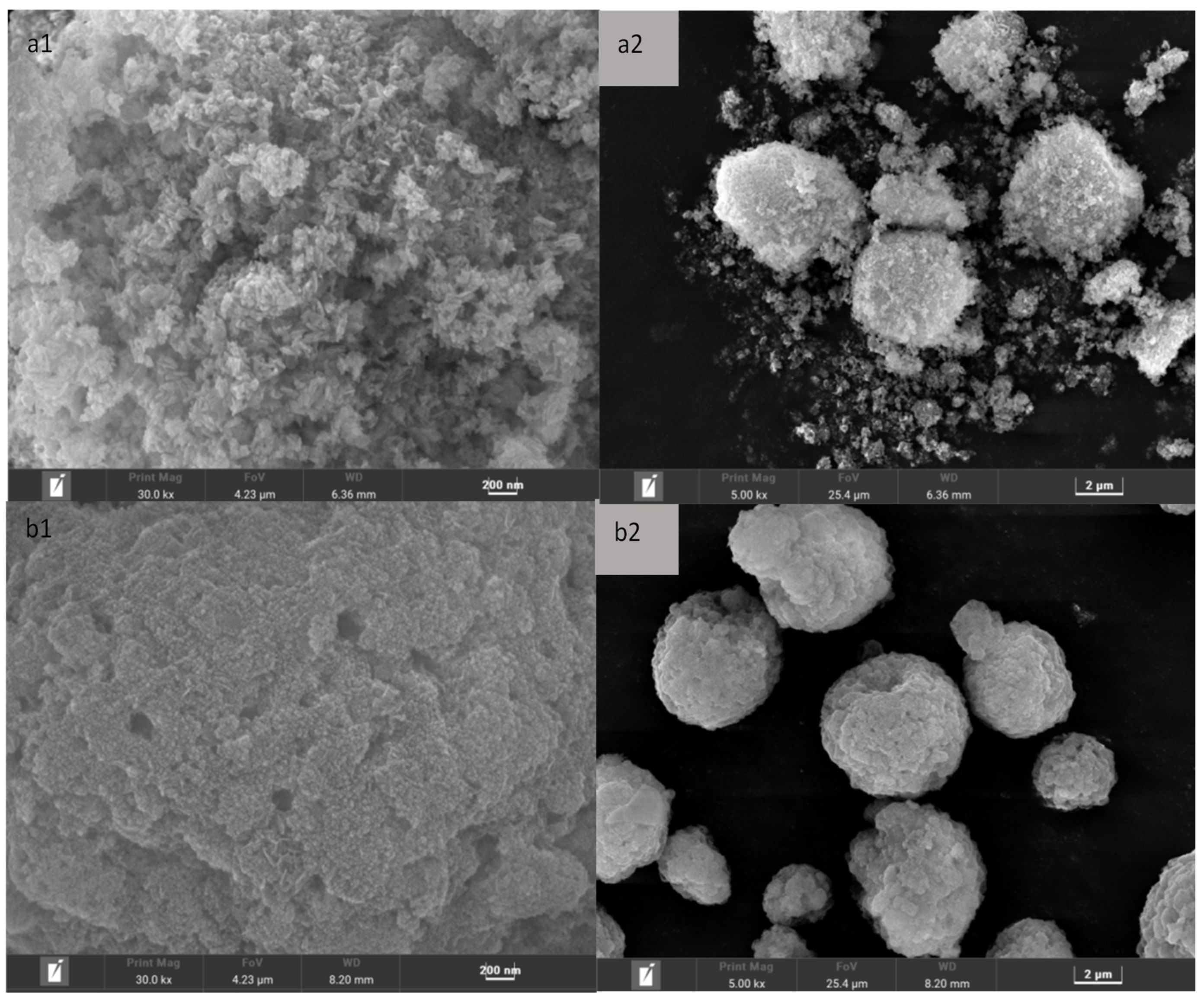
3.4. SEM Characterization of Catalyst
3.5. HR-TEM Characterization of Catalyst
3.6. The Influence of Different Mg/SiO2 Ratios on the Activity and Structure of Catalysts
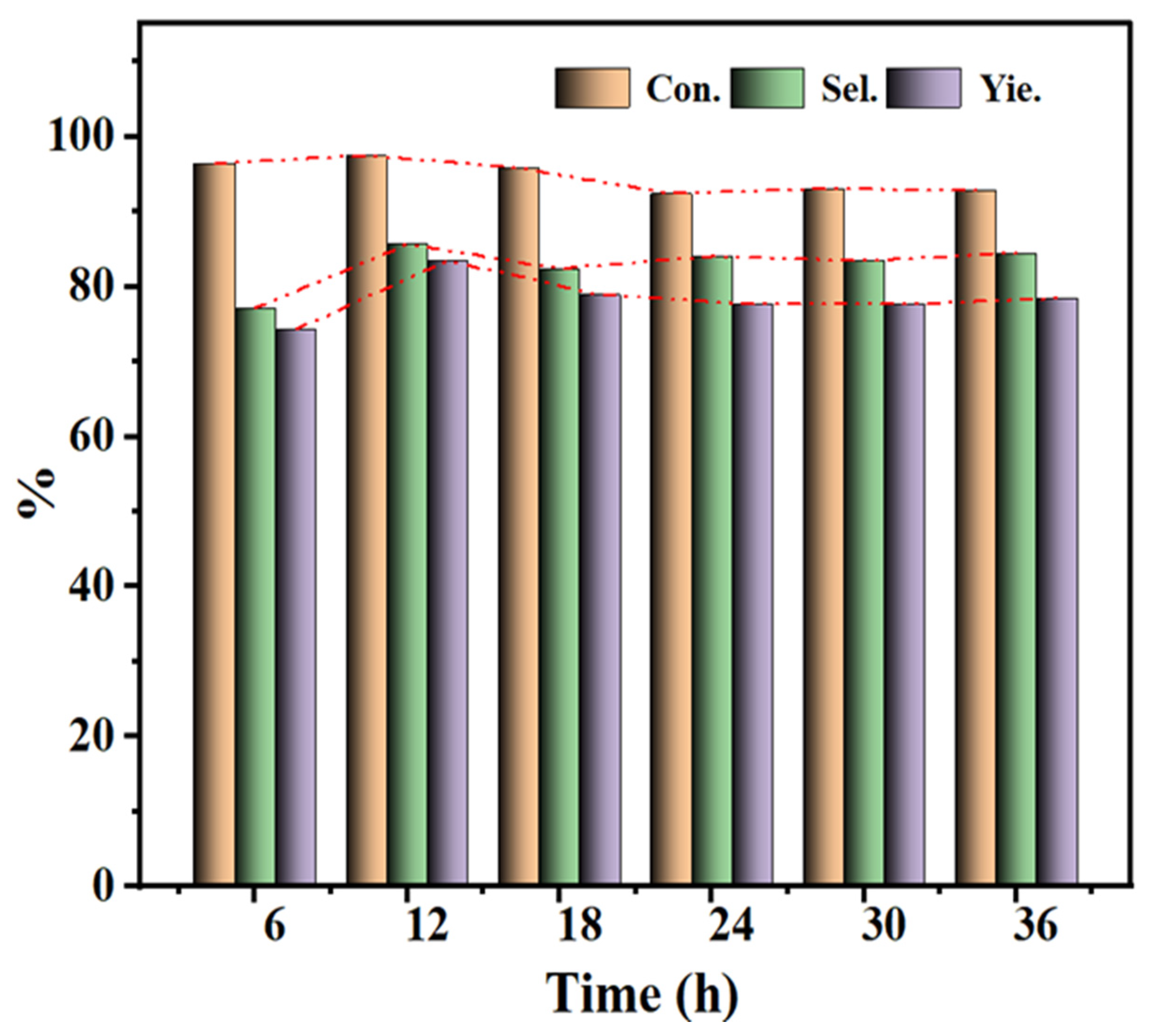
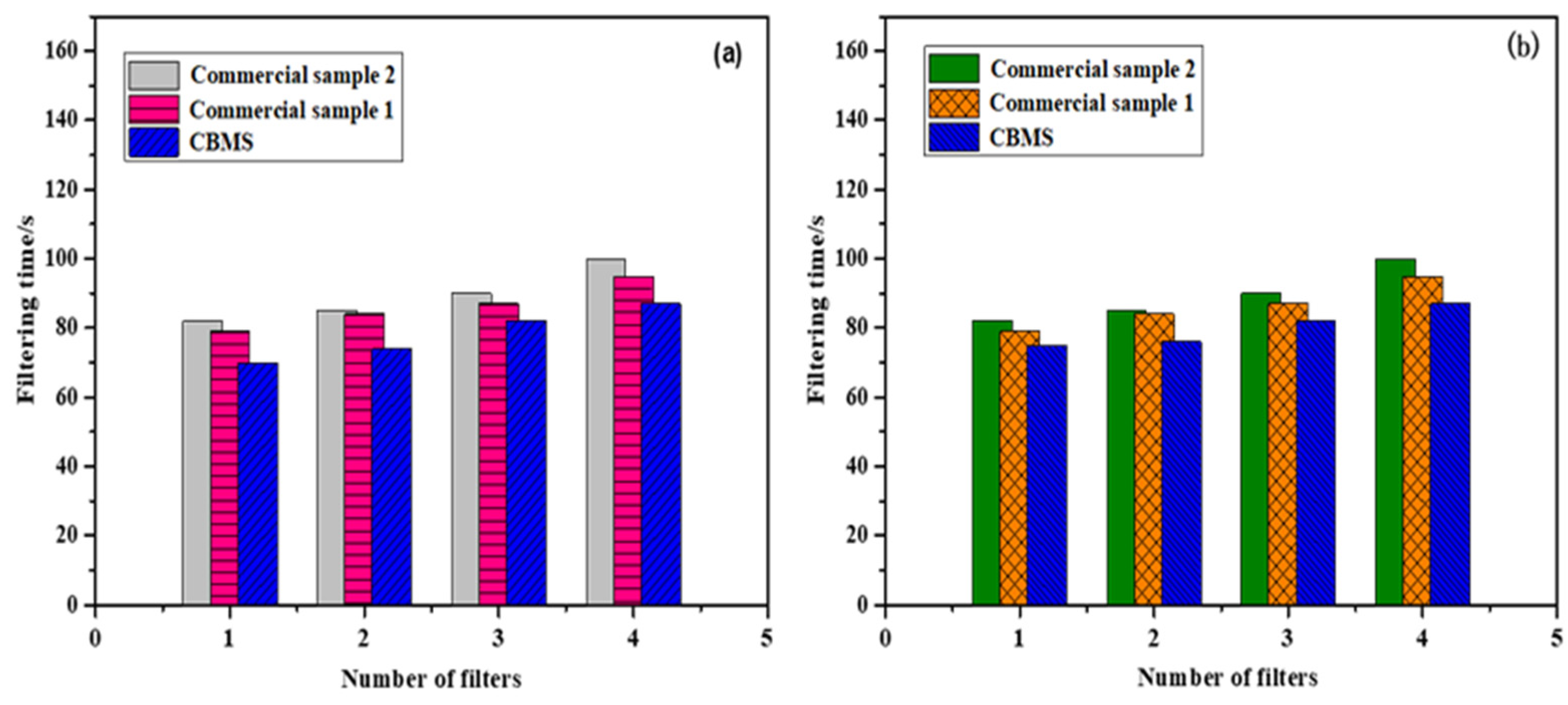
3.7. CBMS Catalyst Activity and Filtration Performance
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, G.; Ma, F.Y.; Luo, P.; Zhang, X.L.; Li, X.D.; Li, G.X. Preparation of CuO-Bi2O3/SiO2@TiO2 core-shell structured catalysts and its catalytic performance for synthesis of 1,4-butynediol by ethynylation of formaldehyde. Catal. Commun. 2024, 187, 106889. [Google Scholar] [CrossRef]
- Tanielyan, S.; Schmidt, S.; Marin, N.; Alvez, G.; Augustine, R. Selective hydrogenation of 2-butyne-1,4-diol to 1,4-butanediol over particulate Raney® nickel catalysts. Top. Catal. 2010, 53, 1145–1149. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.M.; Di, X.; Yin, D.D.; Li, W.Z.; Liang, C.H. One-step synthesis of Pt@ZIF-8 catalyst for the selective hydrogenation of 1,4-butynediol to 1,4-butenediol. Chin. J. Catal. 2016, 37, 1555–1561. [Google Scholar] [CrossRef]
- Nadgeri, J.M.; Telkar, M.M.; Rode, C.V. Hydrogenation activity and selectivity behavior of supported palladium nanoparticles. Catal. Commun. 2008, 9, 441–446. [Google Scholar] [CrossRef]
- Xu, B.C.; Ge, X.D. Studies on the low-pressure synthesis of 1,4-butynediol from butadiene slurry: I. Performance of different support-typeetylating catalysts. J. ECUST 1989, 15, 272. [Google Scholar]
- Huang, Q.F. Preparation of Complex Oxide Catalyst of ZSM-5 Zeolite and Catalytic Properties for Synthesis of Butynediol. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2004. [Google Scholar]
- Ma, Z.Q.; Zhang, H.X.; Li, H.T. Preparation of core-shell CuO-Bi2O3@meso-SiO2 catalyst and its catalytic performance for formaldehyde ethynylation. Ind. Catal. 2015, 23, 344–348. [Google Scholar]
- Wang, J.J.; Li, H.T.; Ma, Z.Q.; Wang, Z.P.; Guo, J.Y.; Zhao, Y.X. Preparation of magnetic CuO-Bi2O3/Fe3O4-SiO2-MgO catalyst and its catalytic performance for formaldehyde ethynylation. J. Chem. Ind. Eng. 2015, 66, 2098–2104. [Google Scholar]
- Guan, G.; Ma, F.Y.; Luo, P.; Zhang, X.L.; Li, X.D.; Li, G.X. Facile one-pot synthesis of CuO-Bi2O3/MgAl2O4 catalyst and its performance in the ethynylation of formaldehyde. Heliyon 2024, 10, e38721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of Formaldehyde over Binary Cu-Based Catalysts: Study on Synergistic Effect between Cu+ Species and Acid/Base Sites. Nanomaterials 2019, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species. Nanomaterials 2019, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Trotuş, I.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, Z.J.; Wang, Y.Z.; Li, H.T.; Wang, S.A.; Luo, M.; Zhao, J.L.; Zhao, Y.X. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance. J. Mol. Catal. 2012, 26, 233–238. [Google Scholar]
- Jing, Q.S.; Fei, J.H.; Mo, L.Y.; Zheng, X.M. Effective reforming of methane with CO2 and O2 to low H2/CO ratio syngas over Ni/MgO-SiO2 using fluidized bed reactor. Energy Conv. Manag. 2004, 20, 3127–3137. [Google Scholar] [CrossRef]
- Taifan, W.E.; Baltrusaitis, J. In-Situ Spectroscopic Insights on the Molecular Structure of the MgO/SiO2 Catalytic Active Site During Ethanol Conversion to 1,3-Butadiene. J. Phys. Chem. C 2018, 122, 20894–20906. [Google Scholar] [CrossRef]
- Li, S.X.; Men, Y.; Wang, J.G.; Liu, S.; Wang, X.F.; Ji, F.; Chai, S.S.; Song, Q.L. Morphological Control of Inverted MgO-SiO2 Composite Catalysts for Effificient Conversion of Ethanol to 1,3-Butadiene. Appl. Catal. A-Gen. 2019, 577, 1–9. [Google Scholar]
- Kviele, S.; Aguero, A.; Sneeden, R. Transformation of Ethanol into 1,3-Butadiene over Magnesium Oxide/Silica Catalysts. Appl. Catal. 1998, 43, 117–131. [Google Scholar]
- Kyriienko, P.I.; Larina, O.V.; Balakin, D.Y.; Stetsuk, A.O.; Nychiporuk, Y.M.; Soloviev, S.O.; Orlyk, S.M. 1,3-Butadiene production from aqueous ethanol over ZnO/MgO-SiO2 catalysts: Insight into H2O effect on catalytic performance. Appl. Catal. A-Gen. 2021, 616, 118081. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Malmusi, A.; Recch, C.; Cavani, F. Understanding the role of Gallium as a new promoter of MgO-SiO2 catalysts for the conversion of Ethanol into Butadiene. Chemcatchem 2017, 9, 2128–2135. [Google Scholar] [CrossRef]
- Liu, H.W.; Hu, Q.; Fan, G.L.; Yang, L.; Li, F. Surface synergistic effect in well-dispersed Cu/MgO catalysts for highly efficient vapor-phase hydrogenation of carbonyl compounds. Catal. Sci. Technol. 2015, 5, 3960–3969. [Google Scholar] [CrossRef]
- Ueda, W.; Yokoyama, T.; Moro-oka, Y. Enhancment of surface base property of magnesium oxide by the combination of metal ion. Chem. Lett. 1985, 14, 1059–1062. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Li, H.; Liu, Z.R.; Xia, P.F.; Xie, Y.H.; Xiong, D.H. Synthesis of 0D/3D CuO/ZnO Heterojunction with Enhanced Photocatalytic Activity. J. Phys. Chem. C 2018, 122, 9531–9539. [Google Scholar] [CrossRef]






| Catalyst | CuO Grain Size, nm |
|---|---|
| CB15M | 8.4 |
| CB15MS | 7.7 |
| CB20M | 8.0 |
| CB20MS | 6.4 |
| Samples | MgO/SiO2 Ratio | Specific Surface Area a | Pore Volume c | dpore a | dCuO b |
|---|---|---|---|---|---|
| m2·g−1 | cm3·g−1 | nm | nm | ||
| CB10MS | 1:5 | 60 | 0.318 | 21.26 | 11.8 |
| CB15MS | 1:3 | 96 | 0.518 | 21.65 | 7.7 |
| CB20MS | 1:2 | 83 | 0.312 | 14.99 | 6.4 |
| CB25MS | 1:1.4 | 80 | 0.369 | 18.45 | 11.9 |
| CB30MS | 1:1 | 62 | 0.293 | 18.97 | 14.4 |
| Sample | Conv.% | Sel.% | Yel.% |
|---|---|---|---|
| CBMS | 94.0 | 96.3 | 90.5 |
| Sample1 | 90.5 | 95.6 | 86.5 |
| Sample 2 | 92.9 | 95.6 | 88.8 |
| Sample | Particle Size Distribution (D50, μm) | ||
|---|---|---|---|
| Before Reaction | After Reaction | Change Value | |
| CBMS | 13.8 | 12.0 | 1.8 |
| Sample 1 | 11.3 | 10.6 | 0.7 |
| Sample 2 | 14.0 | 10.5 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Guan, G.; Tao, H.; Su, J.-J.; Luo, P.; Ma, F.-Y.; Li, X.-D. Preparation of CuO-Bi2O3-MgO/SiO2 Spherical Catalyst and Its Formaldehyde Acetylenation Performance. Crystals 2025, 15, 454. https://doi.org/10.3390/cryst15050454
Wang X-Y, Guan G, Tao H, Su J-J, Luo P, Ma F-Y, Li X-D. Preparation of CuO-Bi2O3-MgO/SiO2 Spherical Catalyst and Its Formaldehyde Acetylenation Performance. Crystals. 2025; 15(5):454. https://doi.org/10.3390/cryst15050454
Chicago/Turabian StyleWang, Xiang-Yu, Gang Guan, Hao Tao, Jun-Jian Su, Ping Luo, Feng-Yun Ma, and Xiao-Ding Li. 2025. "Preparation of CuO-Bi2O3-MgO/SiO2 Spherical Catalyst and Its Formaldehyde Acetylenation Performance" Crystals 15, no. 5: 454. https://doi.org/10.3390/cryst15050454
APA StyleWang, X.-Y., Guan, G., Tao, H., Su, J.-J., Luo, P., Ma, F.-Y., & Li, X.-D. (2025). Preparation of CuO-Bi2O3-MgO/SiO2 Spherical Catalyst and Its Formaldehyde Acetylenation Performance. Crystals, 15(5), 454. https://doi.org/10.3390/cryst15050454






