Shape-Engineering and Mechanism Investigation of AgCl Microcrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
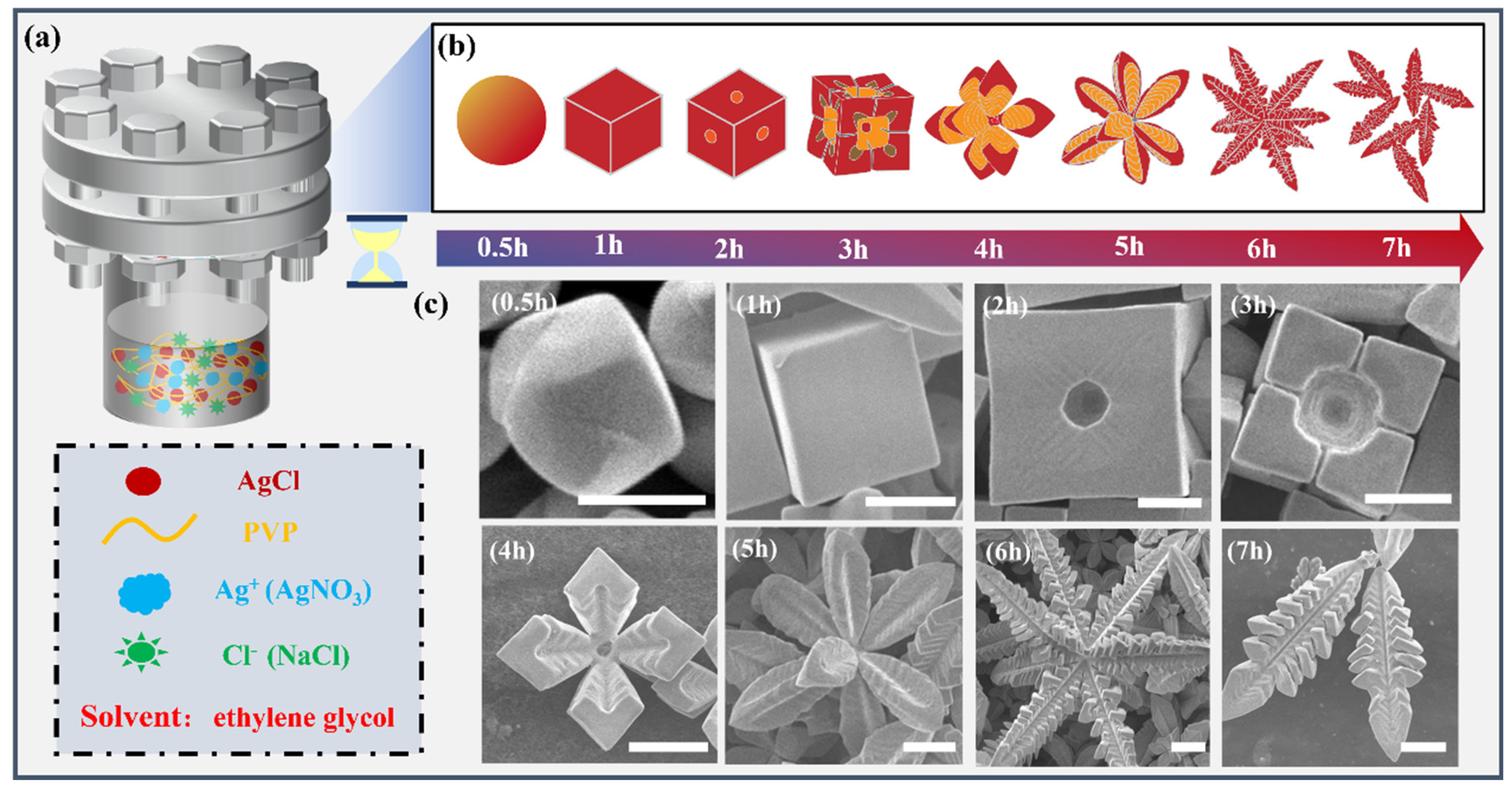
2.3. Preparation of AgCl Microcrystals
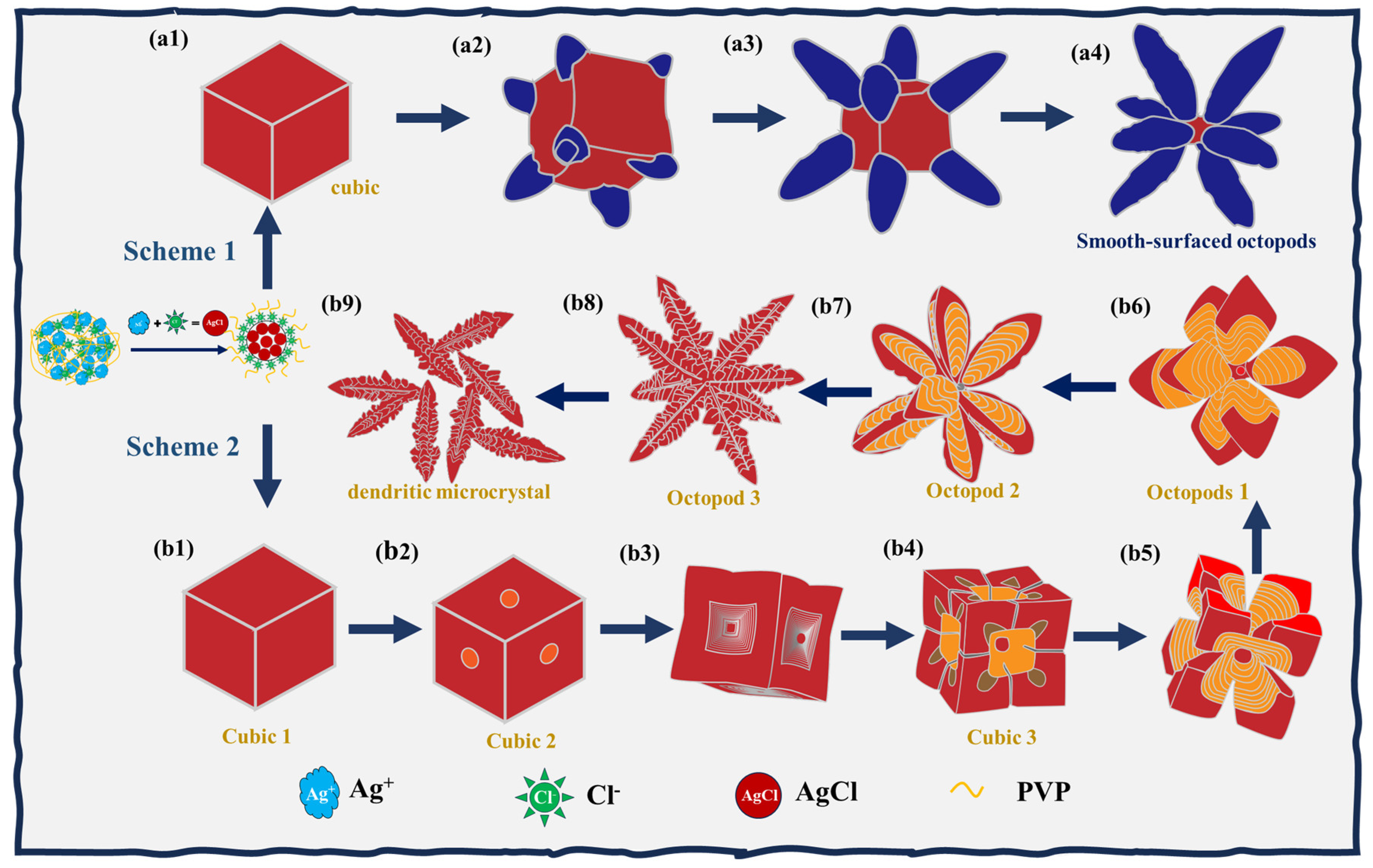
3. Results and Discussion
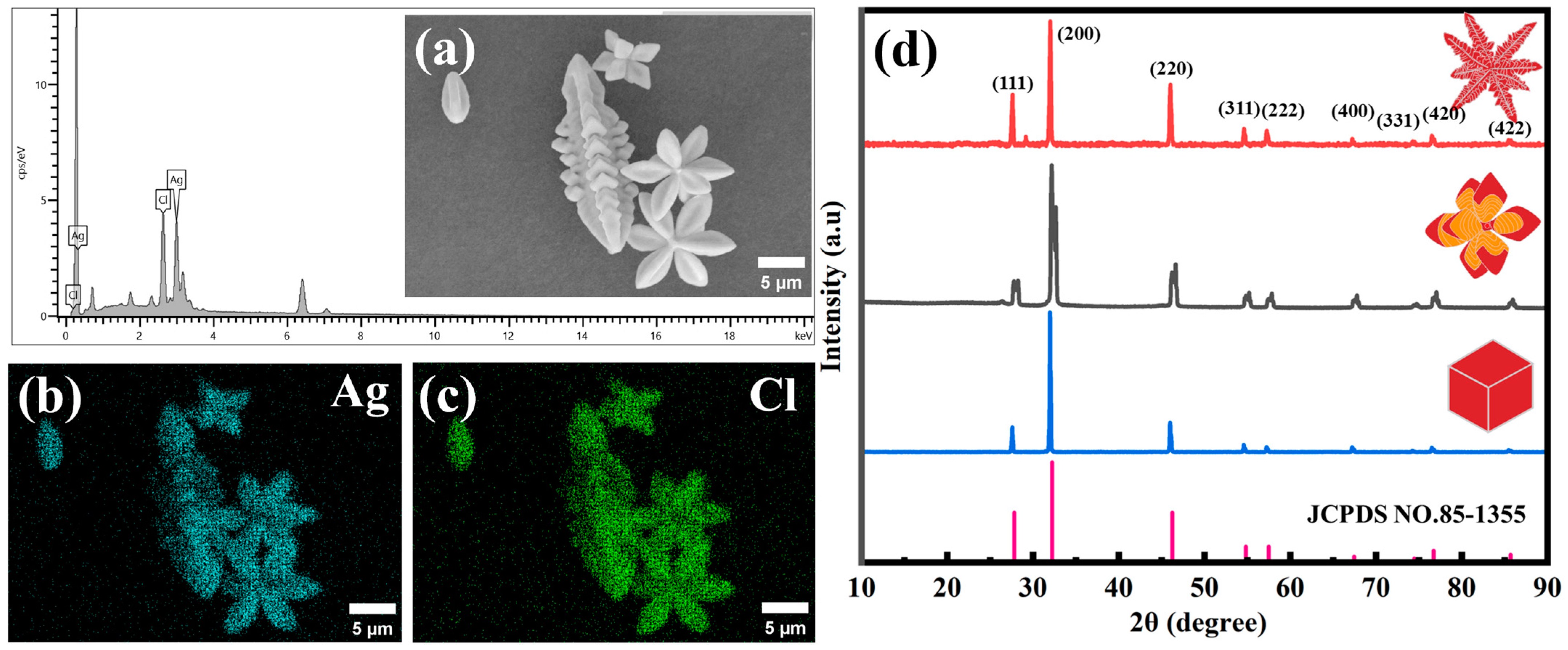
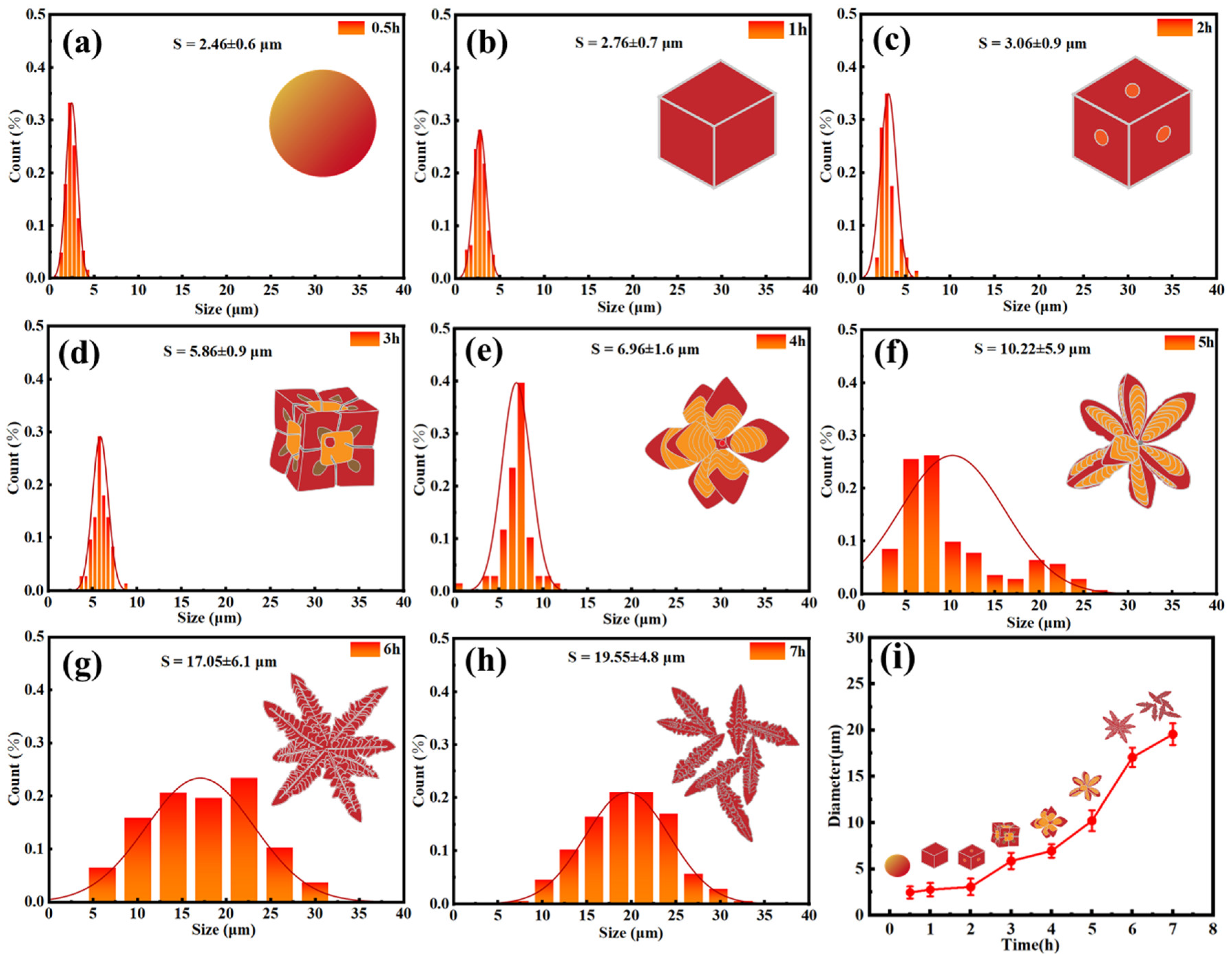
3.1. Effect of Time on the Formation of 3D AgCl Microcrystals
3.2. Effect of NaCl on the Formation of 3D AgCl Microcrystals
3.3. Effect of PVP on the Growth and Etching of 3D AgCl Microcrystals
3.4. Second-Order Etching of 3D AgCl Microcrystals
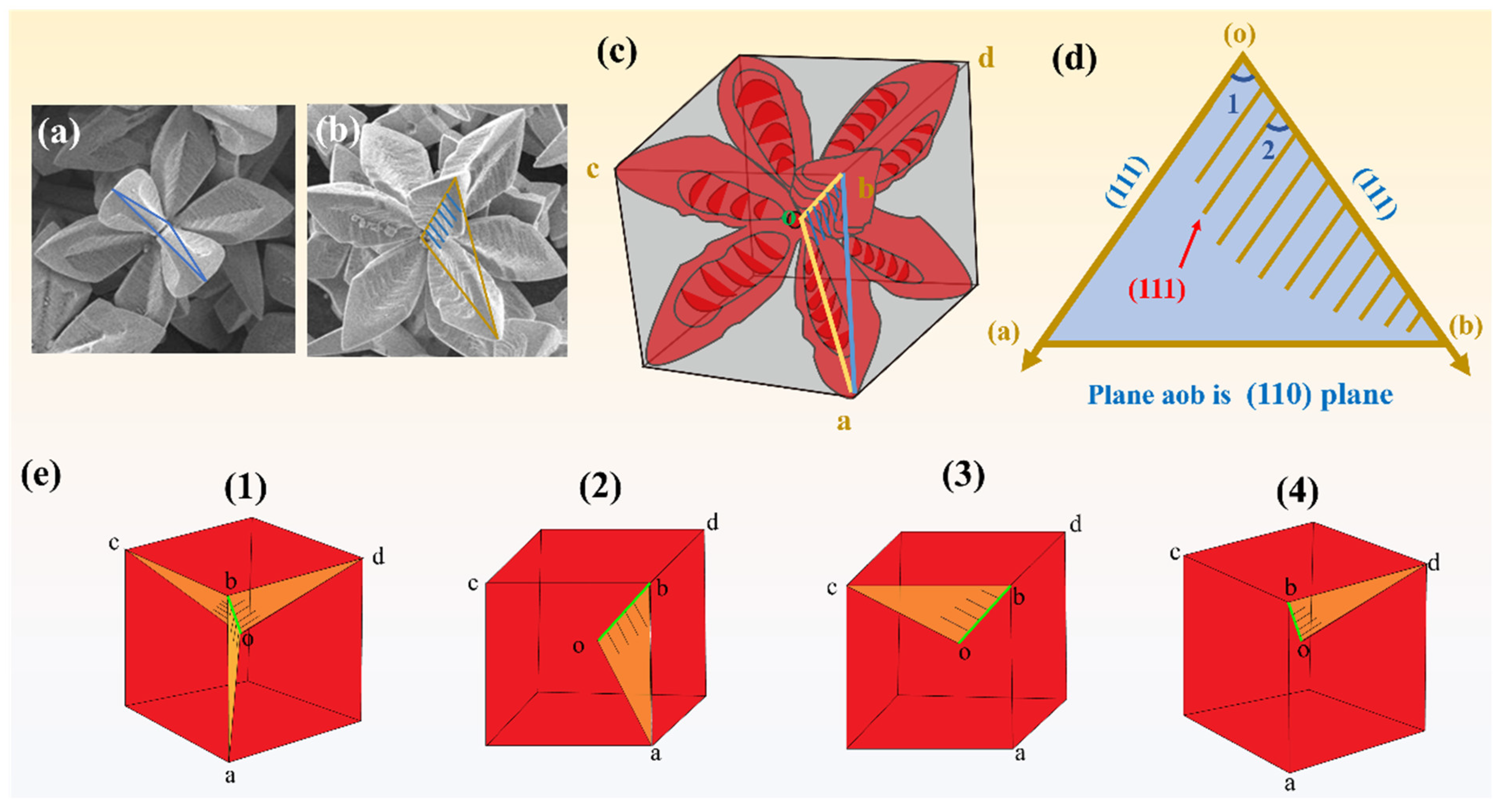
3.5. Growth and Etching Mechanism of 3D AgCl Microcrystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheepborisutikul, S.J.; Ogawa, M. Suppressing the Photocatalytic Activity of Titania by Precisely Controlled Silica Coating. Inorg. Chem. 2021, 60, 6201–6208. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Erfani, A.; Pirouzifard, M.K.; Pirsa, S. Photochromic biodegradable film based on polyvinyl alcohol modified with silver chloride nanoparticles and spirulina; investigation of physicochemical, antimicrobial and optical properties. Food Chem. 2023, 411, 135459. [Google Scholar] [CrossRef]
- Guo, L.; Ji, Y.L.; Xu, H.; Simon, P.; Wu, Z. Regularly Shaped, Single-Crystalline ZnO Nanorods with Wurtzite Structure. J. Am. Chem. Soc. 2002, 124, 14864–14865. [Google Scholar] [CrossRef] [PubMed]
- Lignier, P.; Bellabarba, R.; Tooze, R.P. Scalable strategies for the synthesis of well-defined copper metal and oxide nanocrystals. Chem. Soc. Rev. 2012, 41, 1708–1720. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhou, S.; Xia, Y. Shape Transformation via Etching and Regrowth: A Systematic Study of Pd Nanocrystals with Different Shapes and Twin Structures. Chem. A Eur. J. 2023, 29, e202301465. [Google Scholar] [CrossRef]
- Tao, S.; Yang, M.; Chen, H.; Zhao, S.; Chen, G. Continuous Synthesis of Ag/AgCl/ZnO Composites Using Flow Chemistry and Photocatalytic Application. Ind. Eng. Chem. Res. 2018, 57, 3263–3273. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, X.; Wu, F.; Pang, Y.; Xu, G.; Yuan, Y.; Hsu, H.-Y.; Niu, W. Boosting plasmon-enhanced electrochemistry by in situ surface cleaning of plasmonic nanocatalysts. Nanoscale 2023, 15, 18901–18909. [Google Scholar] [CrossRef]
- Westcott, S.L.; Oldenburg, S.J.; Lee, T.R.; Halas, N.J. Construction of simple gold nanoparticle aggregates with controlled plasmon–plasmon interactions. Chem. Phys. Lett. 1999, 300, 651–655. [Google Scholar] [CrossRef]
- Dong, Q.; Jiao, Z.; Yu, H.; Ye, J.; Bi, Y. Facile synthesis of hollow Ag@AgBr heterostructures with highly efficient visible-light photocatalytic properties. CrystEngComm 2014, 16, 8317–8321. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yang, X.; Lou, Y.; Chen, J. Zn0.5Cd0.5S/MIL-125-NH2(Ti) nanocomposites: Highly efficient and stable photocatalyst for hydrogen production under visible light. Inorg. Chem. Commun. 2020, 112, 107714. [Google Scholar] [CrossRef]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral Silver Nanocrystals with Distinct Scattering Signatures. Angew. Chem. Int. Ed. 2006, 45, 4597–4601. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Zhang, Q.; Zhang, X.; Qin, X.; Dai, Y.; Zhan, J.; Yu, J.; Liu, H.; Lou, Z. Highly Efficient Visible Light Plasmonic Photocatalyst Ag@Ag(Br,I). Chem. A Eur. J. 2010, 16, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ge, J.; Pham, T.; Goebl, J.; Hu, Y.; Lu, Z.; Yin, Y. Reconstruction of Silver Nanoplates by UV Irradiation: Tailored Optical Properties and Enhanced Stability. Angew. Chem. Int. Ed. 2009, 48, 3516–3519. [Google Scholar] [CrossRef]
- An, C.; Peng, S.; Sun, Y. Facile Synthesis of Sunlight-Driven AgCl:Ag Plasmonic Nanophotocatalyst. Adv. Mater. 2010, 22, 2570–2574. [Google Scholar] [CrossRef]
- Guo, X.; Deng, D.; Tian, Q. One pot controllable synthesis of AgCl nanocrystals with different morphology and their photocatalytic activity. Powder Technol. 2017, 308, 206–213. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Zhu, C.; Zhai, Y.; Dong, S. Facile solvothermal synthesis of cube-like Ag@AgCl: A highly efficient visible light photocatalyst. Nanoscale 2011, 3, 2931–2935. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Qin, X.; Zhang, X.; Cheng, H.; Liu, Y.; Wang, S.; Wang, J.; Dai, Y. One-step synthesis of AgCl concave cubes by preferential overgrowth along 〈111〉 and 〈110〉 directions. Chem. Commun. 2012, 48, 3488–3490. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Wang, P.; Wang, Z.; Qin, X.; Zhang, X.; Cheng, H.; Zheng, Z.; Dai, Y. The synthesis of the near-spherical AgCl crystal for visible light photocatalytic applications. Dalton Trans. 2011, 40, 4104–4110. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M. Ag@AgCl: A Highly Efficient and Stable Photocatalyst Active under Visible Light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Xia, J.; Yin, S.; Luo, Z.; Liu, L.; Xu, L. One-Pot Synthesis of Visible-Light-Driven Plasmonic Photocatalyst Ag/AgCl in Ionic Liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, W.; Lu, M.; Guo, Z.; Li, C.; Wang, W. Novel AgCl nanotubes/BiOCl nanosheets composite with improved adsorption capacity and photocatalytic performance. J. Alloys Compd. 2019, 773, 1146–1153. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Q.; Li, H.; Wang, D.; Che, Q.; Wang, J.; Wang, G.; Yang, P. Fabrication and Photocatalytic Activity of Tunable Triangular- and Circular-Like Ag/AgCl Nanoplates. J. Nanosci. Nanotechnol. 2018, 18, 2738–2745. [Google Scholar] [CrossRef]
- Daupor, H.; Wongnawa, S. Flower-like Ag/AgCl microcrystals: Synthesis and photocatalytic activity. Mater. Chem. Phys. 2015, 159, 71–82. [Google Scholar] [CrossRef]
- Lou, S.; Wang, W.; Wang, L.; Zhou, S. In-situ oxidation synthesis of Cu2O/Ag/AgCl microcubes with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2019, 781, 508–514. [Google Scholar] [CrossRef]
- Chandra, S.; Dutta, P.; Biswas, K. Enhancement of the Thermoelectric Performance of 2D SnSe Nanoplates through Incorporation of Magnetic Nanoprecipitates. ACS Appl. Energy Mater. 2020, 3, 9051–9057. [Google Scholar] [CrossRef]
- Kozhina, E.; Kulesh, E.; Bedin, S.; Doludenko, I.; Piryazev, A.; Korolkov, I.; Kozlovskiy, A.; Zdorovets, M.; Rogachev, A.; Shumskaya, A. One-Dimensional Magneto-Optical Nanostructures: Template Synthesis, Structure, Properties, and Application in Spectroscopy Based on Plasmon Resonance. IEEE Magn. Lett. 2022, 13, 1–5. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Jiang, M.; Wang, P.; Whangbo, M. Highly Efficient Photocatalyst: TiO2 Microspheres Produced from TiO2 Nanosheets with a High Percentage of Reactive {001} Facets. Chem. A Eur. J. 2009, 15, 12576–12579. [Google Scholar] [CrossRef]
- Wu, M.; Yan, L.; Li, J.; Wang, L. Synthesis and photocatalytic performance of Ag/AgCl/ZnO tetrapod composites. Res. Chem. Intermed. 2017, 43, 6407–6419. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Liu, H.; Fang, J. One-pot synthesis of high-index faceted AgCl nanocrystals with trapezohedral, concave hexoctahedral structures and their photocatalytic activity. Nanoscale 2015, 7, 11591–11601. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Z.; Axinte, D. Temperature effect on the material removal mechanism of soft-brittle crystals at nano/micron scale. Int. J. Mach. Tools Manuf. 2020, 159, 103620. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Deng, J.; Wang, X.; Guo, G. Microfluidics revealing formation mechanism of intermetallic nanocrystals. Nano Energy 2020, 70, 104565. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, W.; Wang, L.; Zheng, Y.; Su, J.; Liu, N.; Gao, Y. Unveiling the Nucleation Dynamics and Growth Mechanism of Layered MoS2 from Crystalline K2MoS4 by in Situ Transmission Electron Microscopy. Cryst. Growth Des. 2020, 20, 4069–4076. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Fu, C.; Li, T.; Yu, H. Self-templated formation of AgCl/TiO2 hollow octahedra for improved visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 494, 740–748. [Google Scholar] [CrossRef]
- Hanmant Gaikwad, S.; Koratti, A.; Porel Mukherjee, S. Facile tuning of Ag@AgCl cubical hollow nanoframes with efficient sunlight-driven photocatalytic activity. Appl. Surf. Sci. 2019, 465, 413–419. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Mori, K.; Kuwahara, Y.; Yamashita, H. Tailoring the Size and Shape of Colloidal Noble Metal Nanocrystals as a Valuable Tool in Catalysis. Catal. Surv. Asia 2019, 23, 127–148. [Google Scholar] [CrossRef]
- Xia, Y.; Nelli, D.; Ferrando, R.; Yuan, J.; Li, Z.Y. Shape control of size-selected naked platinum nanocrystals. Nat. Commun. 2021, 12, 3019. [Google Scholar] [CrossRef]
- Wang, J.; Qin, Y.; Shi, Q.; Wen, L.; Bi, L. Cl−-Induced selective fabrication of 3D AgCl microcrystals by a one-pot synthesis method. CrystEngComm 2021, 23, 5116–5123. [Google Scholar] [CrossRef]
- Gatemala, H.; Thammacharoen, C.; Ekgasit, S. 3D AgCl microstructures selectively fabricated via Cl−-induced precipitation from [Ag(NH3)2]+. CrystEngComm 2014, 16, 6688–6696. [Google Scholar] [CrossRef]
- Alharbi, N.D.; Salah, N.; Habib, S.S.; Alarfaj, E. Synthesis and characterization of nano- and microcrystalline cubes of pure and Ag-doped LiF. J. Phys. D Appl. Phys. 2013, 46, 035305. [Google Scholar] [CrossRef]
- Dong, H.; Yang, H.; Ning, Y.; Liu, F.; Bradley, R.; Zhao, B.; Wu, W. Large-scale facile green synthesis of porous silver nanocubes on monolithic activated carbon for room-temperature catalytic oxidation of formaldehyde. Appl. Phys. A 2022, 128, 976. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Ma, X.; Zhang, X.; Qin, X.; Wang, Z.; Dai, Y.; Liu, Y. A 3D AgCl Hierarchical Superstructure Synthesized by a Wet Chemical Oxidation Method. Chem. A Eur. J. 2012, 18, 16090–16096. [Google Scholar] [CrossRef]
- Zettsu, N.; McLellan, J.M.; Wiley, B.; Yin, Y.; Li, Z.-Y.; Xia, Y. Synthesis, Stability, and Surface Plasmonic Properties of Rhodium Multipods, and Their Use as Substrates for Surface-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2006, 45, 1288–1292. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.E.; Kim, T.; Suh, K.S. Ionic liquid-assisted synthesis of highly branched Ag:AgCl hybrids and their photocatalytic activity. J. Alloys Compd. 2015, 621, 378–382. [Google Scholar] [CrossRef]
- Gryglewicz, J.; Oleszkiewicz, W.; Ramiączek-Krasowska, M.; Szyszka, A.; Prażmowska, J.; Paszkiewicz, B.; Paszkiewicz, R.; Tłaczała, M. Reactive ion etching of GaN and AlGaN/GaN assisted by Cl2/BCl3. Mater. Sci. Pol. 2011, 29, 260–265. [Google Scholar] [CrossRef]
- Oh, K.H.; Baek, G.B.; Chung, C.W. Etch characteristics of cobalt thin films using high density plasma of halogen gas. Thin Solid Films 2024, 796, 140341. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, X.; Wang, Z.; Zhang, Y.; Tan, G.; Yan, W. New architecture of a variable anode for full-time efficient electrochemical oxidation of organic wastewater with variable Cl− concentration. Appl. Surf. Sci. 2020, 515, 146003. [Google Scholar] [CrossRef]
- Dong, M.; Pan, Y.; Zhu, J.; Jia, H.; Dong, H.; Xu, F. Real-time imaging reveal anisotropic dissolution behaviors of silver nanorods. Nanotechnology 2024, 35, 275703. [Google Scholar] [CrossRef]
- Tian, X.; Anand, U.; Mirsaidov, U.; Zheng, H. Spontaneous Reshaping and Splitting of AgCl Nanocrystals under Electron Beam Illumination. Small 2018, 14, 1803231. [Google Scholar] [CrossRef]
- Tian, X.; Anand, U.; Mirsaidov, U.; Zheng, H. Nanocrystal Dynamics: Spontaneous Reshaping and Splitting of AgCl Nanocrystals under Electron Beam Illumination (Small 48/2018). Small 2018, 14, 1870231. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.-H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.-Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef]
- da Silva, R.R.; Yang, M.; Choi, S.-I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.-Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile Synthesis of Sub-20 nm Silver Nanowires through a Bromide-Mediated Polyol Method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ye, S.; Stewart, I.E.; Alvarez, S.; Wiley, B.J. Synthesis and Purification of Silver Nanowires To Make Conducting Films with a Transmittance of 99%. Nano Lett. 2015, 15, 6722–6726. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Cui, F.; Kuttner, E.; Xie, C.; Chen, H.; Sun, Y.; Dehestani, A.; Schierle-Arndt, K.; Yang, P. Synthesis of Silver Nanowires with Reduced Diameters Using Benzoin-Derived Radicals to Make Transparent Conductors with High Transparency and Low Haze. Nano Lett. 2018, 18, 5329–5334. [Google Scholar] [CrossRef] [PubMed]
- Ruditskiy, A.; Xia, Y. Toward the Synthesis of Sub-15 nm Ag Nanocubes with Sharp Corners and Edges: The Roles of Heterogeneous Nucleation and Surface Capping. J. Am. Chem. Soc. 2016, 138, 3161–3167. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Gilroy, K.D.; Tao, J.; Zhu, C.; Yang, X.; Sun, X.; Xia, Y. Facile Synthesis of Silver Nanocubes with Sharp Corners and Edges in an Aqueous Solution. ACS Nano 2016, 10, 9861–9870. [Google Scholar] [CrossRef]
- Saidi, W.A.; Feng, H.; Fichthorn, K.A. Binding of Polyvinylpyrrolidone to Ag Surfaces: Insight into a Structure-Directing Agent from Dispersion-Corrected Density Functional Theory. J. Phys. Chem. C 2013, 117, 1163–1171. [Google Scholar] [CrossRef]
- Ahn, J.; Wang, D.; Ding, Y.; Zhang, J.; Qin, D. Site-Selective Carving and Co-Deposition: Transformation of Ag Nanocubes into Concave Nanocrystals Encased by Au–Ag Alloy Frames. ACS Nano 2018, 12, 298–307. [Google Scholar] [CrossRef]


| NO. | AgNO3 (mM) | PVP (mM) | NaCl (M) | Time (h) | Results |
|---|---|---|---|---|---|
| a-1 | 0.67 | 240 | 5.5 | 0.5 | Figure S2a |
| a-2 | 0.67 | 240 | 5.5 | 1 | Figure S2b |
| a-3 | 0.67 | 240 | 5.5 | 2 | Figure S2c |
| a-4 | 0.67 | 240 | 5.5 | 3 | Figure S2d |
| a-5 | 0.67 | 240 | 5.5 | 4 | Figure S2e |
| a-6 | 0.67 | 240 | 5.5 | 5 | Figure S2f |
| a-7 | 0.67 | 240 | 5.5 | 6 | Figure S2g |
| a-8 | 0.67 | 240 | 5.5 | 7 | Figure S2h |
| b-1 | 0.67 | 240 | 0 | 6 | Figure 4a |
| b-2 | 0.67 | 240 | 2 | 6 | Figure 4b |
| b-3 | 0.67 | 240 | 3 | 6 | Figure 4c |
| b-4 | 0.67 | 240 | 4 | 6 | Figure 4d |
| c-1 | 0.67 | 0 | 5.5 | 4 | Figure 5a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Wang, Q.; Yin, C.; Li, X.; Yang, R.; Shen, X.; Xin, W. Shape-Engineering and Mechanism Investigation of AgCl Microcrystals. Crystals 2025, 15, 451. https://doi.org/10.3390/cryst15050451
Cai C, Wang Q, Yin C, Li X, Yang R, Shen X, Xin W. Shape-Engineering and Mechanism Investigation of AgCl Microcrystals. Crystals. 2025; 15(5):451. https://doi.org/10.3390/cryst15050451
Chicago/Turabian StyleCai, Chunli, Qian Wang, Changsheng Yin, Xuhuan Li, Rong Yang, Xiaodong Shen, and Wenbo Xin. 2025. "Shape-Engineering and Mechanism Investigation of AgCl Microcrystals" Crystals 15, no. 5: 451. https://doi.org/10.3390/cryst15050451
APA StyleCai, C., Wang, Q., Yin, C., Li, X., Yang, R., Shen, X., & Xin, W. (2025). Shape-Engineering and Mechanism Investigation of AgCl Microcrystals. Crystals, 15(5), 451. https://doi.org/10.3390/cryst15050451





