Ammonia Content in Natural Taranakite: An Experimental Study of Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Scanning Electron Microscopy (SEM)
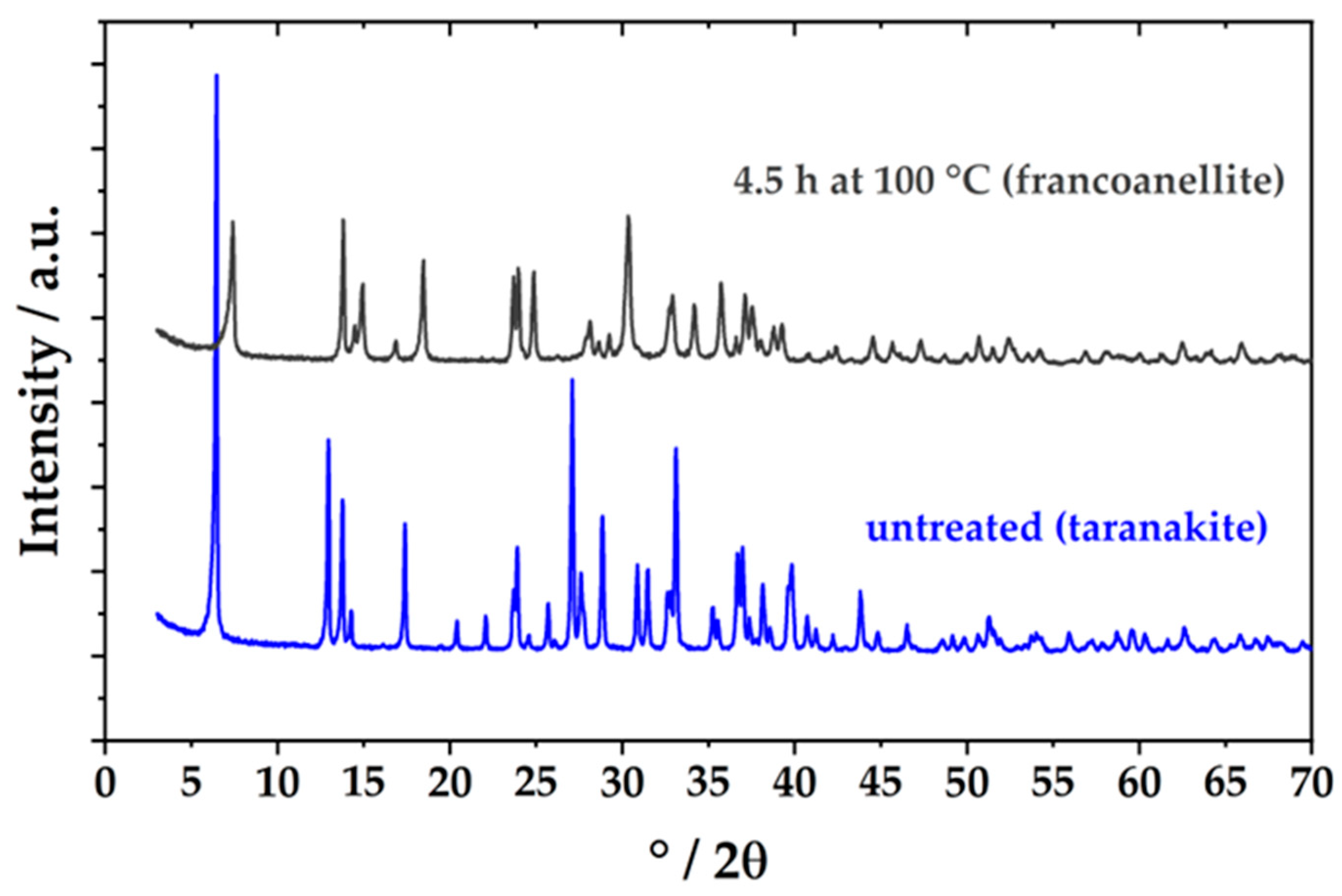
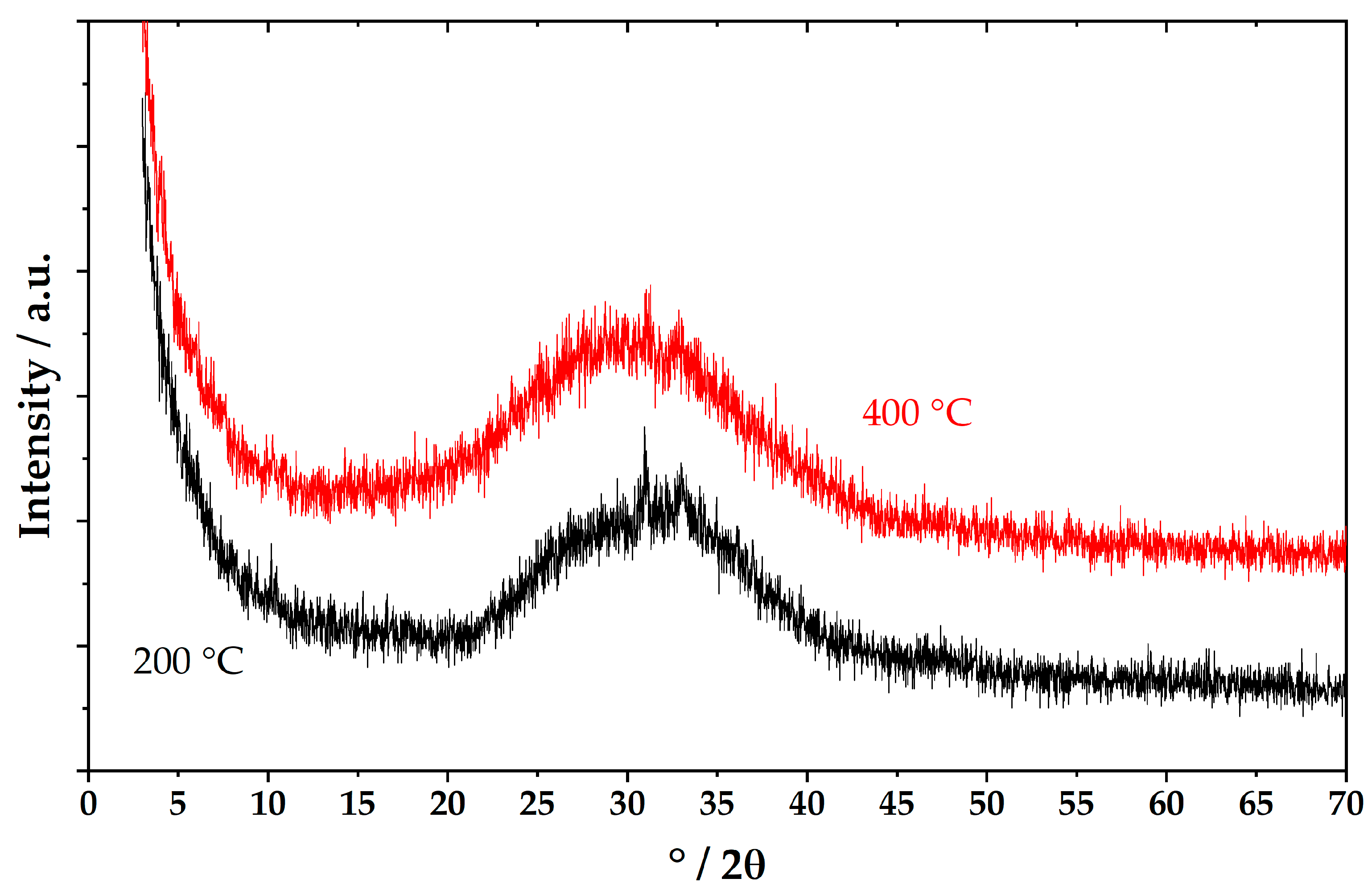
2.3. Powder X-Ray Diffraction (PXRD)
2.4. Thermogravimetric Analysis and Coupled Infra-Red Spectra on Exhaust Gases
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscopy |
| PXRD | Powder X-ray diffractometry |
| TG-DTA | Thermogravimetry differential thermal analysis |
| FT-IR | Fourier transform infrared spectroscopy |
References
- First Taranakite Report. In NZ Exhibition, 1865 Reports and Awards of the Jurors with Hector and Skey. 1865, pp. 423–425. Available online: https://books.google.it/books?id=icIRAAAAYAAJ&ie=ISO-8859-1&redir_esc=y (accessed on 1 July 2024).
- Dick, S.; Gobner, U.; Weib, A.; Robl, C.; Grobmann, G.; Ohms, G.; Zeiske, T. Taranakite-the Mineral with the Longest Crystallographic Axis. Inorganica Chim. Acta 1998, 269, 47–57. [Google Scholar] [CrossRef]
- Giurgiu, A.; Onac, B.P.; Tămaş, T.; Fornós, J.J.; Giurgiu, A.M. Evolution of Guano under Different Environmental Conditions: A Mineralogical Approach. In Proceedings of the 16th International Congress of Speleology, Brno, Czech Republic, 21–28 July 2013; Volume 3, pp. 483–485. [Google Scholar]
- Prabhudesai, S.S.; Kadrekar, S.B. Reaction Products from Fertilizer Phosphorus in Lateritic Soils of Konkan Region. J. Indian. Soc. Soil Sci. 1984, 32, 52–56. [Google Scholar]
- Audra, P.; De Waele, J.; Bentaleb, I.; Chroňáková, A.; Krištůfek, V.; D’Angeli, I.M.; Carbone, C.; Madonia, G.; Vattano, M.; Scopelliti, G.; et al. Guano-Related Phosphate-Rich Minerals in European Caves. Int. J. Speleol. 2019, 48, 75–105. [Google Scholar] [CrossRef]
- Dumitraş, D.G.; Marincea, Ş. Leucophosphite and Associated Minerals in the Fossil Bat Guano Deposit in Gaura cu Muscă Cave, Locvei Mountains, Romania. Minerals 2025, 15, 180. [Google Scholar] [CrossRef]
- Meyer, G.; Bell, M.J.; Lombi, E.; Doolette, C.L.; Brunetti, G.; Novotny, E.H.; Klysubun, W.; Zhang, Y.; Kopittke, P.M. Phosphorus Speciation in the Fertosphere of Highly Concentrated Fertilizer Bands. Geoderma 2021, 403, 115208. [Google Scholar] [CrossRef]
- Kittrick, J.A.; Jackson, M.L. Electron-microscope observations of the reaction of phosphate with minerals, leading to a unified theory of phosphate fixation in soils. J. Soil Sci. 1956, 7, 81–88. [Google Scholar] [CrossRef]
- Tamimi, Y.N. Reaction of Ammonium Phosphate with Gibbsite and Clays. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 1964. [Google Scholar]
- Liu, C.; Huang, P.M.; Zhou, J.M. Residence Time Effect on Iron Perturbation of Taranakite Formation. Soil Sci. Soc. Am. J. 2002, 66, 109–116. [Google Scholar] [CrossRef]
- Zhou, J.M.; Liu, C.; Huang, P.M. Perturbation of Taranakite Formation by Ferrous and Ferric Iron under Acidic Conditions. Soil Sci. Soc. Am. J. 2000, 64, 885–892. [Google Scholar] [CrossRef]
- Schwieger, W.; Meyer Zu Altenschildesche, H.; Kokotailo, G.T.; Fyfe, C.A. Low Temperature Syntheses of Highly-Ordered Framework Materials: The Aluminium Phosphate Ammonium Taranakite. Z. Für Anorg. Und Allg. Chem. 1998, 624, 1712–1717. [Google Scholar] [CrossRef]
- Meyer, G.; Bell, M.J.; Doolette, C.L.; Brunetti, G.; Zhang, Y.; Lombi, E.; Kopittke, P.M. Plant-Available Phosphorus in Highly Concentrated Fertilizer Bands: Effects of Soil Type, Phosphorus Form, and Coapplied Potassium. J. Agric. Food Chem. 2020, 68, 7571–7580. [Google Scholar] [CrossRef]
- Murrell, T.S.; Mikkelsen, R.L.; Sulewski, G.; Norton, R.; Thompson Editors, M.L. Improving Potassium Recommendations for Agricultural Crops; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Rousselet, A.; Xu, B.; Mercier, C.A.; Gauffinet, S. Investigation of aluminum nitrate as a set retarder of magnesium potassium phosphate cement: Mechanisms involved in diluted suspension. Cem. Concr. Res. 2021, 150, 106608. [Google Scholar] [CrossRef]
- Chaparadza, A.; Majoni, S. The Effects of Aluminum and Silicon Phosphates on Thermal Stability and Flammability of Polystyrene. J. Chem. Sci. 2022, 134, 11–21. [Google Scholar] [CrossRef]
- Arlidge, E.Z.; Farmer, V.C.; Mitchell, B.D.; Mitchell, W.A. Infra-red, X-ray and thermal analysis of some aluminium and ferric phosphates. J. Appl. Chem. 1963, 13, 17–27. [Google Scholar] [CrossRef]
- Dick, S.; Zeiskeb, T. Francoanellite K3Al5(HPO4)6(PO4)2·12H2O: Structure and Synthesis by Topochemical Dehydration of Taranakite. Z. Naturforsch 1998, 53, 711–719. [Google Scholar] [CrossRef]
- Balenzano, F.; Dell’anna, L.; Di Pierro, M.; Italy, B. Francoanellite, H6K3Al5(PO4)8·13H2O, a new mineral from the caves of Castellana, Puglia, southern Italy. Neues Jahrb. Mineral. Monatshefte 1976, 2, 49–57. Available online: https://zh.mindat.org/reference.php?id=16150032 (accessed on 1 July 2024).
- Dal Poggetto, G.; Fortunato, M.; Cardinale, A.M.; Leonelli, C. Thermal, Chemical and Mechanical Characterization of Recycled Corundum Powder in Metakaolin-Based Geopolymer Binder. Appl. Clay Sci. 2023, 237, 106875. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Vecchio Ciprioti, S.; Fortunato, M.; Catauro, M. Thermal Behavior and Antibacterial Studies of a Carbonate Mg–Al-Based Layered Double Hydroxide (LDH) for in Vivo Uses. J. Therm. Anal. Calorim. 2023, 148, 1523–1532. [Google Scholar] [CrossRef]
- Król, M.; Dechnik, J.; Szymczak, P.; Handke, B.; Szumera, M.; Stoch, P. Thermal Behavior of Clinoptilolite. Crystals 2024, 14, 646. [Google Scholar] [CrossRef]
- Frazier, A.W.; Taylor, A.W. Characterization of Taranakites and Ammonium Aluminum Phosphates. Soil Sci. Soc. Am. J. 1965, 29, 545–547. [Google Scholar] [CrossRef]
- Frye, W.W.; Hutcheson, T.B. Release of NH4+ in Soils by Oven Drying. Soil. Sci. Soc. Am. J. 1981, 41, 889–892. [Google Scholar] [CrossRef]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific Inc. Driver Version: 9.13.1229, Nicolet IS20, Firmware Version: 1.02, Pat. Available online: www.Thermoscientific.Com/Pm_molspec (accessed on 1 January 2025).
- Song, X.; Zhao, J.; Li, Y.; Sun, Z.; Yu, J. Thermal Decomposition Mechanism of Ammonium Sulfate Catalyzed by Ferric Oxide. Front. Chem. Sci. Eng. 2013, 7, 210–217. [Google Scholar] [CrossRef]
- Mooney, R.C.E. The Crystal Structure of Aluminum Phosphate and Gallium Phosphate, Low-Cristobalite Type. Acta Cryst. 1956, 9, 728–734. [Google Scholar] [CrossRef]
- Graetsch, H.E. Thermal expansion and thermally induced variations of the crystal structure of AlPO4 low cristobalite. Neues Jahrb. Mineral. Monatshefte 2003, 7, 289–301. [Google Scholar] [CrossRef]
- Graetsch, H.E. Two forms of aluminium phosphate tridymite from X-ray powder data. Acta Cryst. 2000, C56, 401–403. [Google Scholar] [CrossRef]

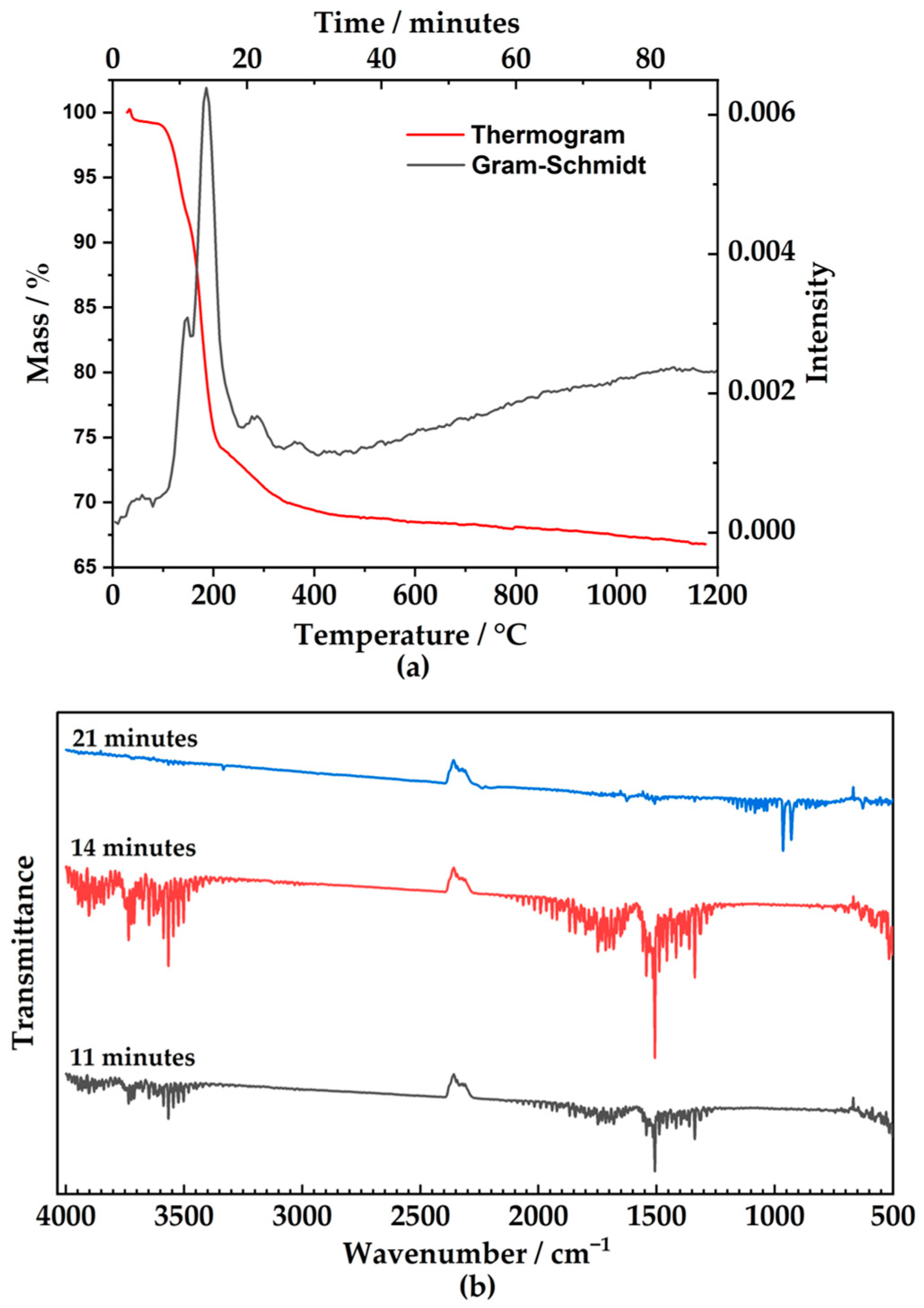

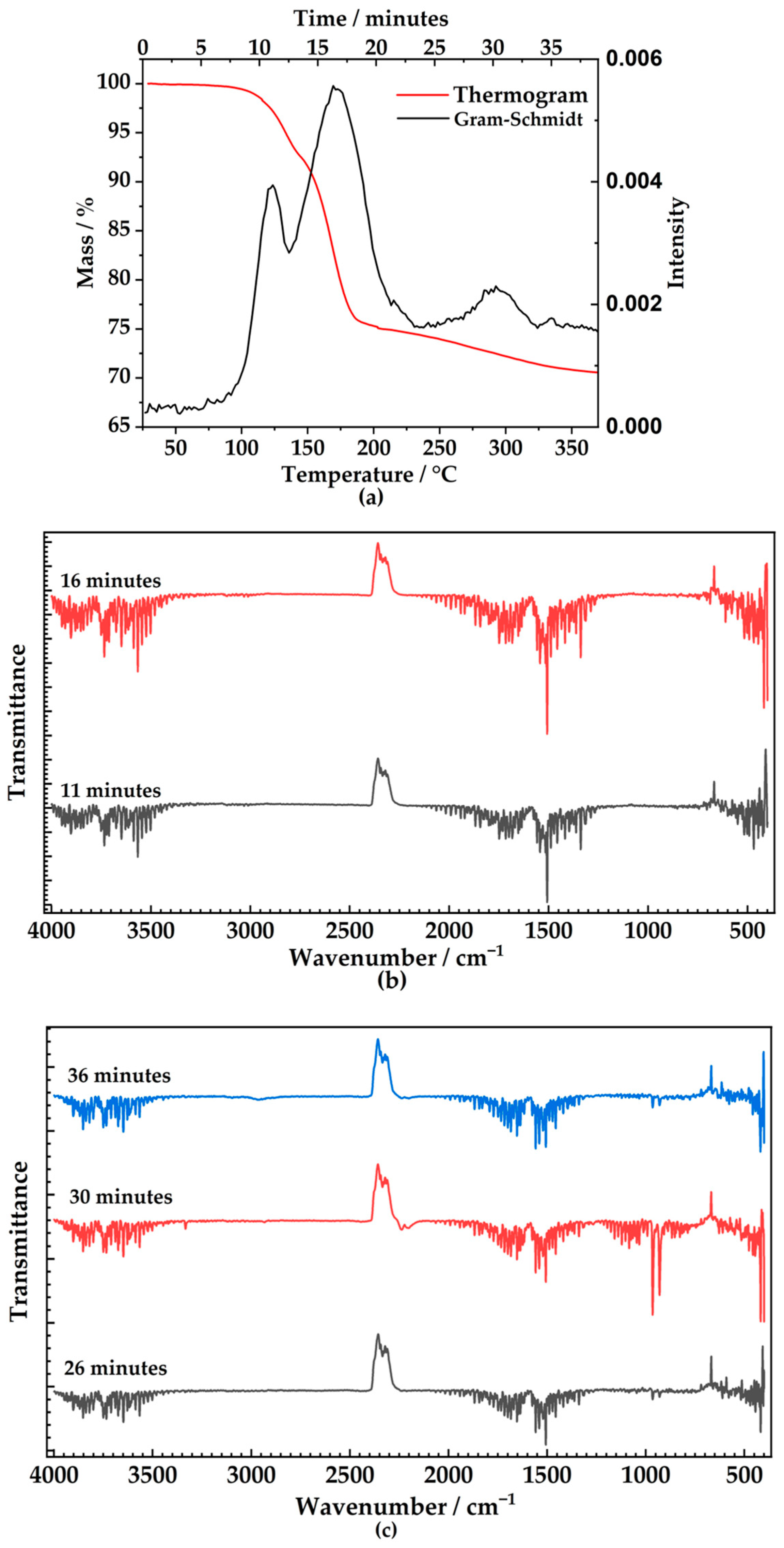
| Temperature Range | Time (Gram–Schmidt) | Main Peaks/Clusters in the Spectrum | Gas Composition | Mass Loss (mg) | Molar Loss (mmol) | Ratio Gas mols/Taranakite mols |
|---|---|---|---|---|---|---|
| 80–140 °C | 11 min | 3400–3700 cm−1 1200–2000 cm−1 | Water | 1.53 | 0.05 | ~6 |
| 141–215 °C | 16 min | 3400–3700 cm−1 1200–2000 cm−1 | Water | 3.12 | 0.14 | ~12 |
| 240–325 °C | 30 min | 3400–3700 cm−1 1200–2000 cm−1 | Water | 0.57 | 0.03 | ~2 |
| Two peaks 929–964 cm−1 | Ammonia | 0.32 | 0.02 | ~1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casale, M.; Galliano, Y.; Carbone, C.; Cardinale, A.M. Ammonia Content in Natural Taranakite: An Experimental Study of Thermal Stability. Crystals 2025, 15, 378. https://doi.org/10.3390/cryst15040378
Casale M, Galliano Y, Carbone C, Cardinale AM. Ammonia Content in Natural Taranakite: An Experimental Study of Thermal Stability. Crystals. 2025; 15(4):378. https://doi.org/10.3390/cryst15040378
Chicago/Turabian StyleCasale, Michael, Yuri Galliano, Cristina Carbone, and Anna Maria Cardinale. 2025. "Ammonia Content in Natural Taranakite: An Experimental Study of Thermal Stability" Crystals 15, no. 4: 378. https://doi.org/10.3390/cryst15040378
APA StyleCasale, M., Galliano, Y., Carbone, C., & Cardinale, A. M. (2025). Ammonia Content in Natural Taranakite: An Experimental Study of Thermal Stability. Crystals, 15(4), 378. https://doi.org/10.3390/cryst15040378






