Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review
Abstract
1. Introduction
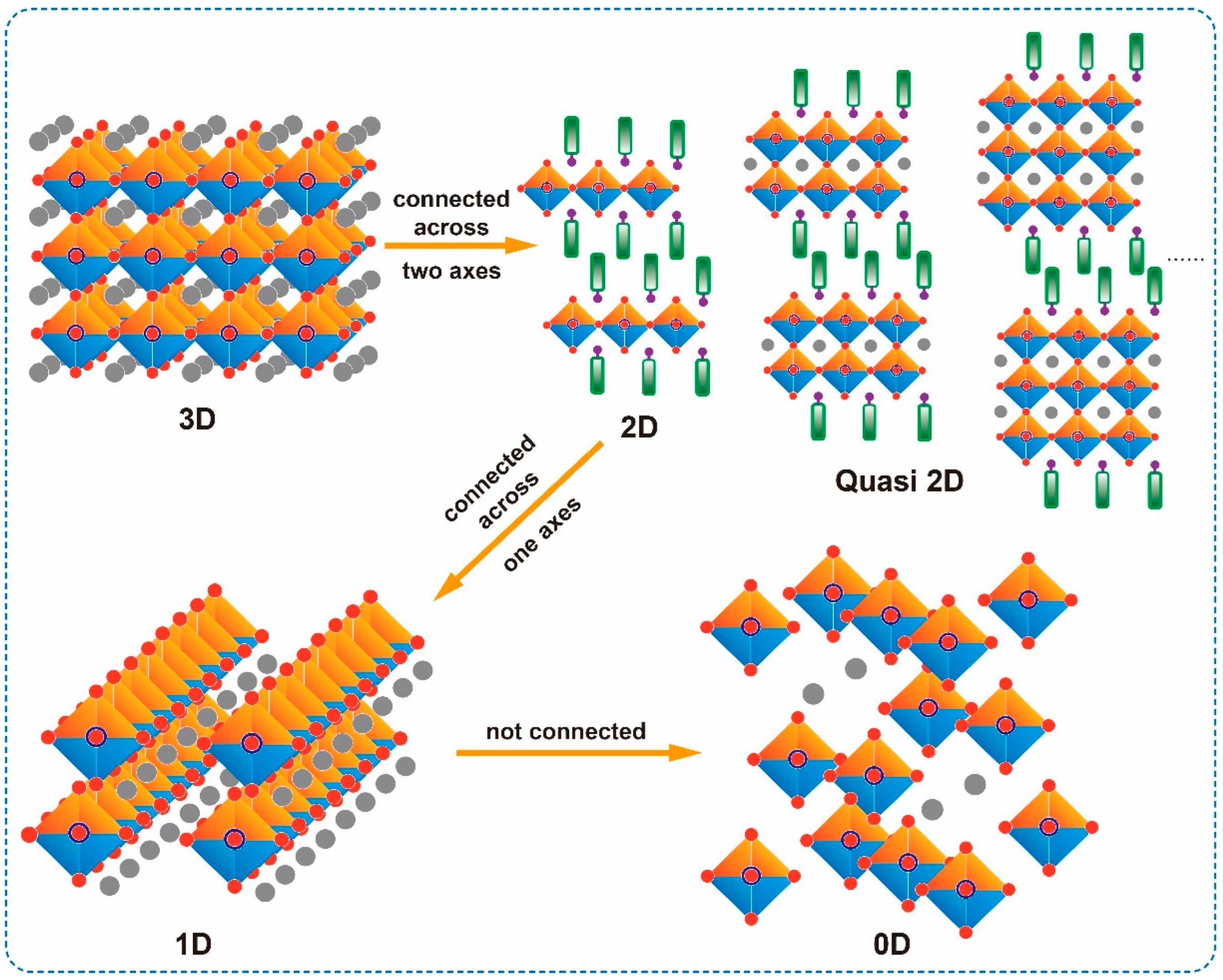
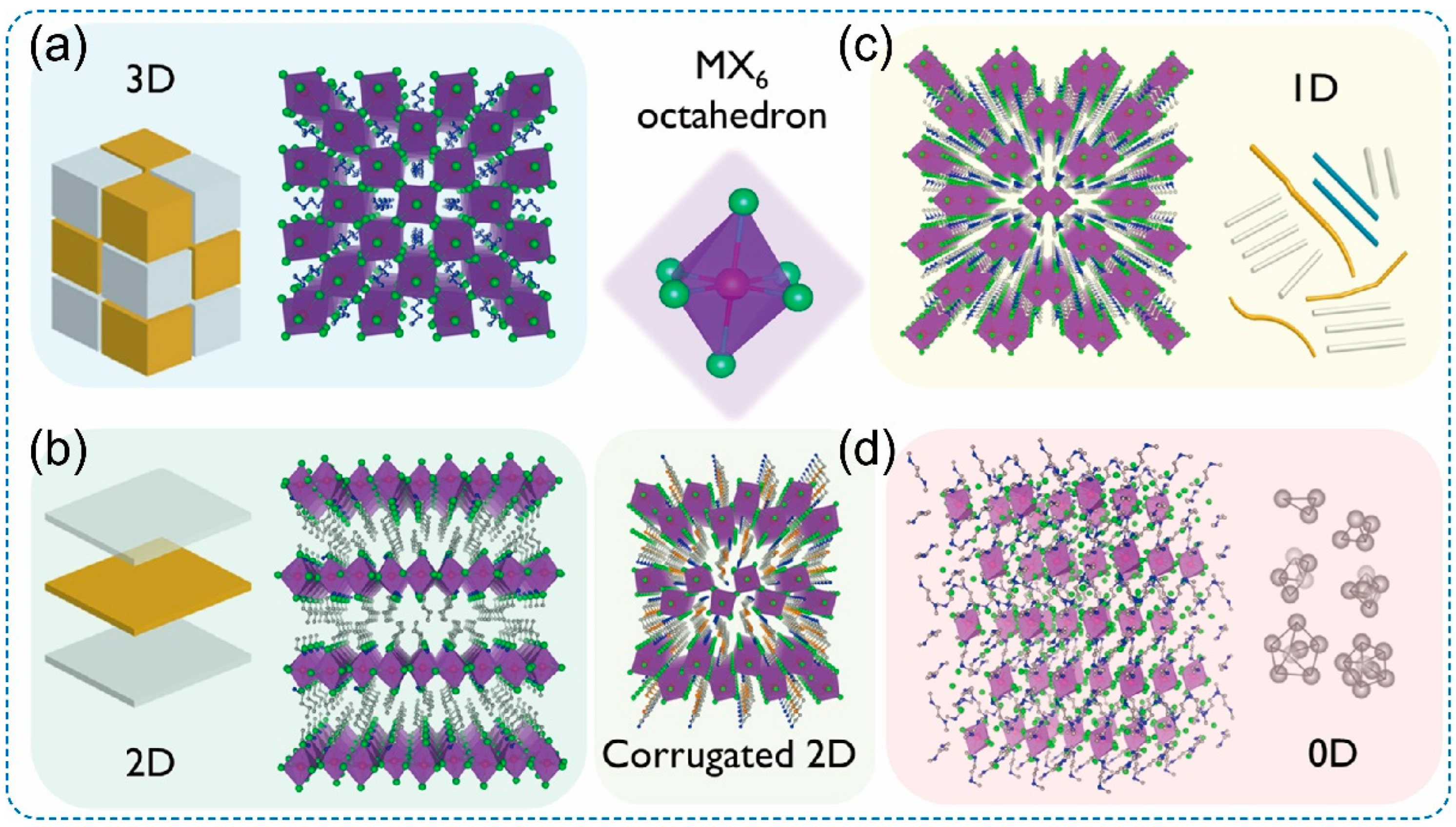
2. Structure of Low-Dimensional OIMHs
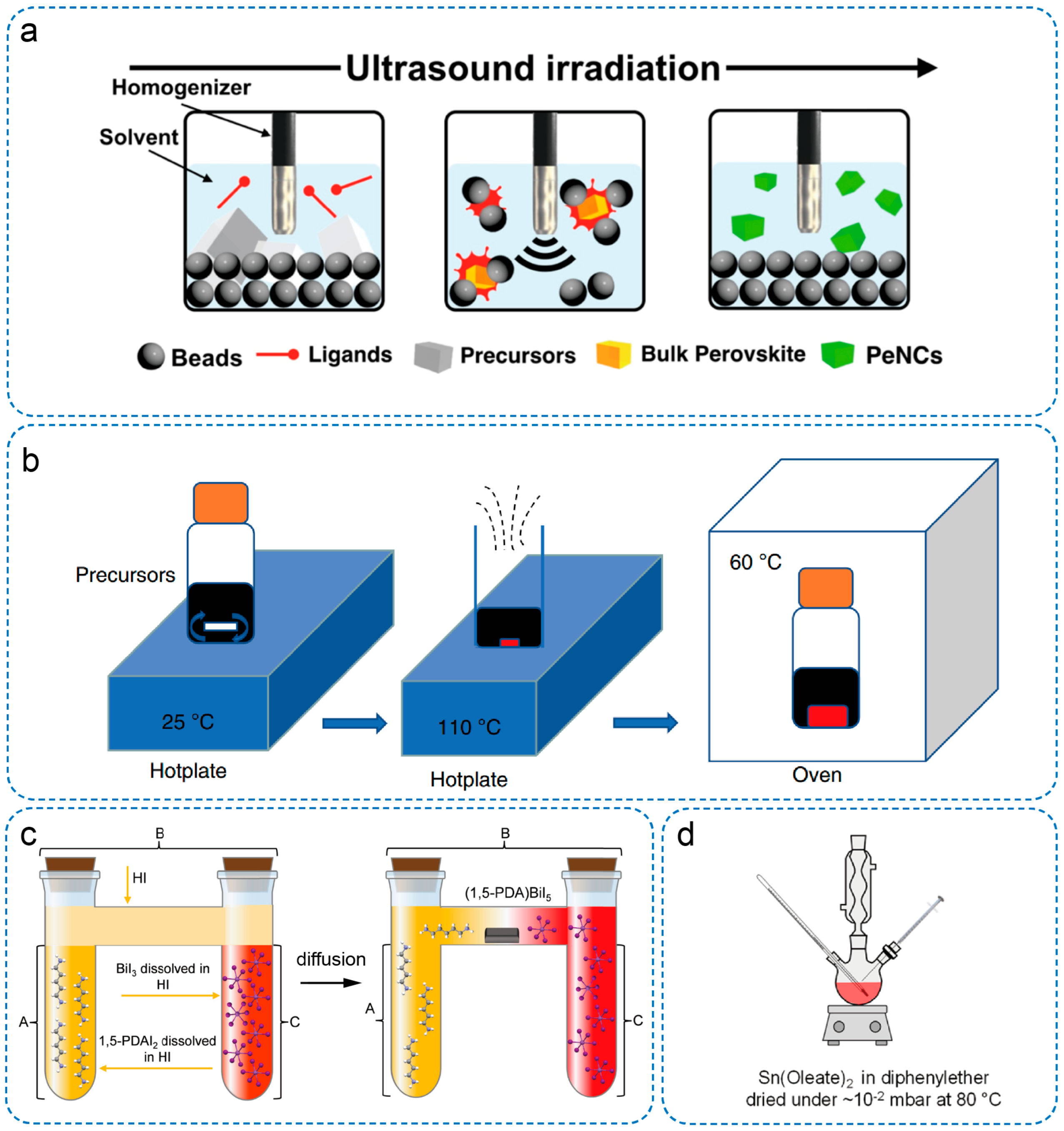
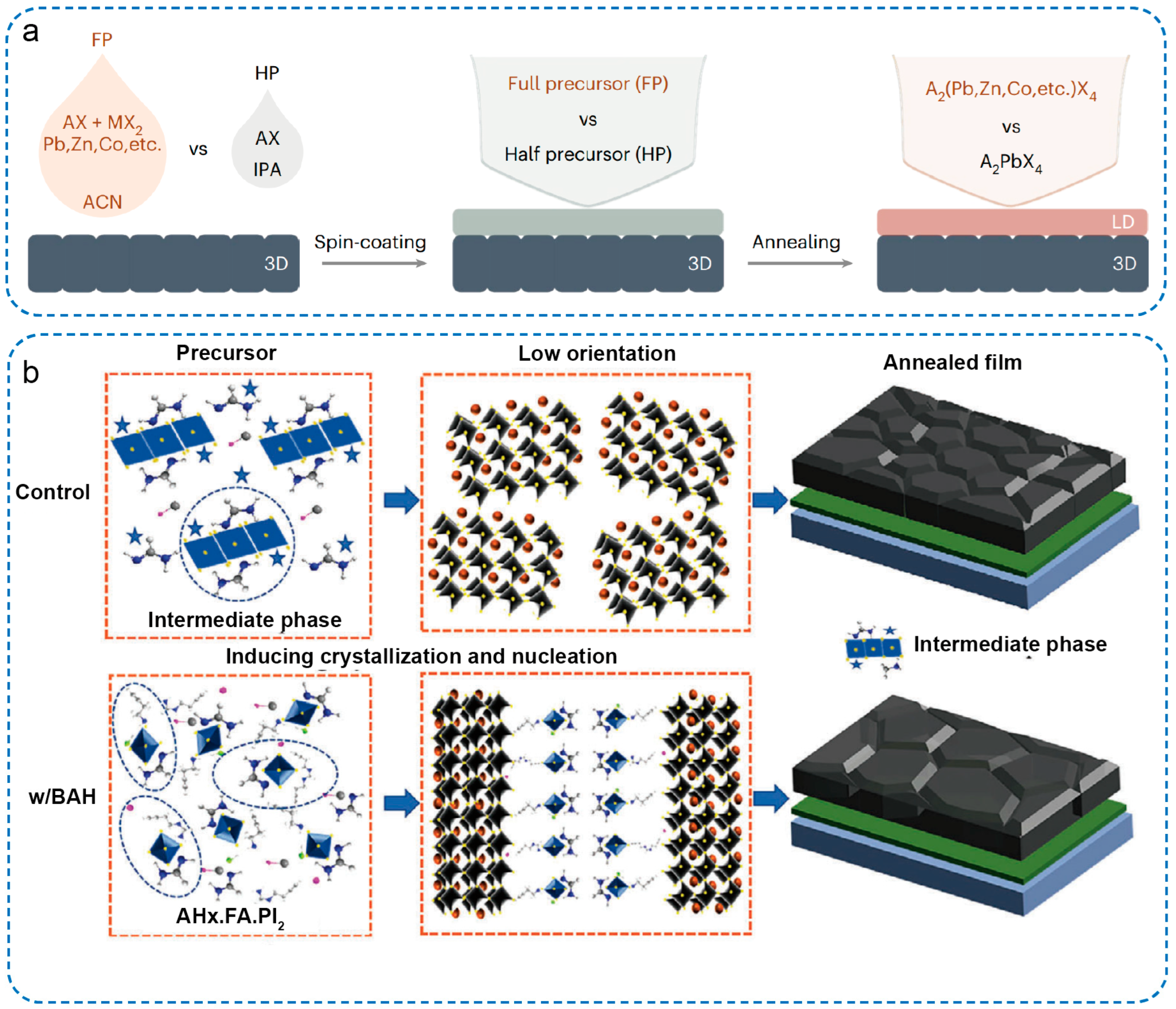
3. Preparation Strategies of Low-Dimensional OIMHs
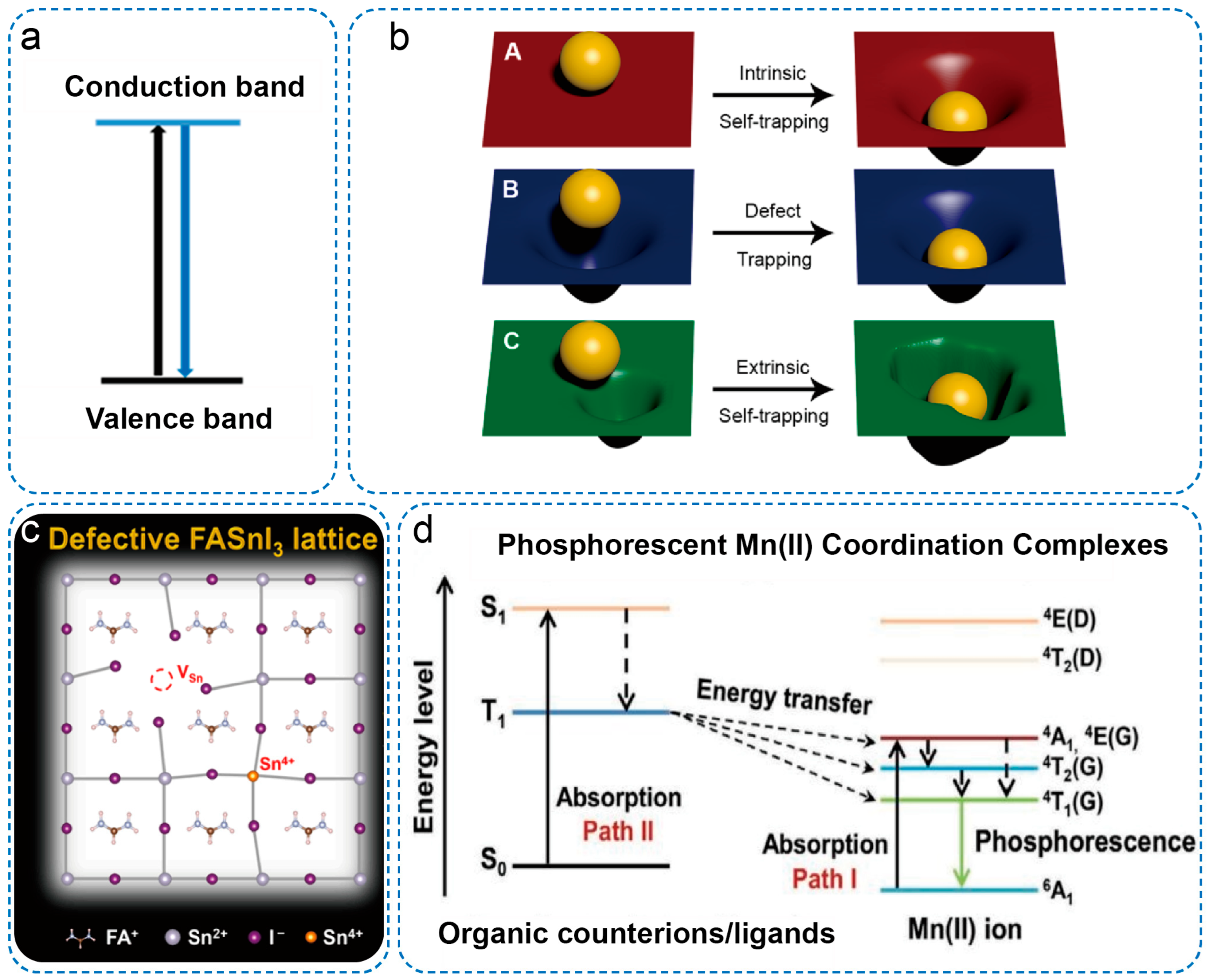
4. Properties of Low-Dimensional OIMHs
5. Optoelectronic Devices Based on Low-Dimensional OIMHs
5.1. White Light-Emitting Diodes
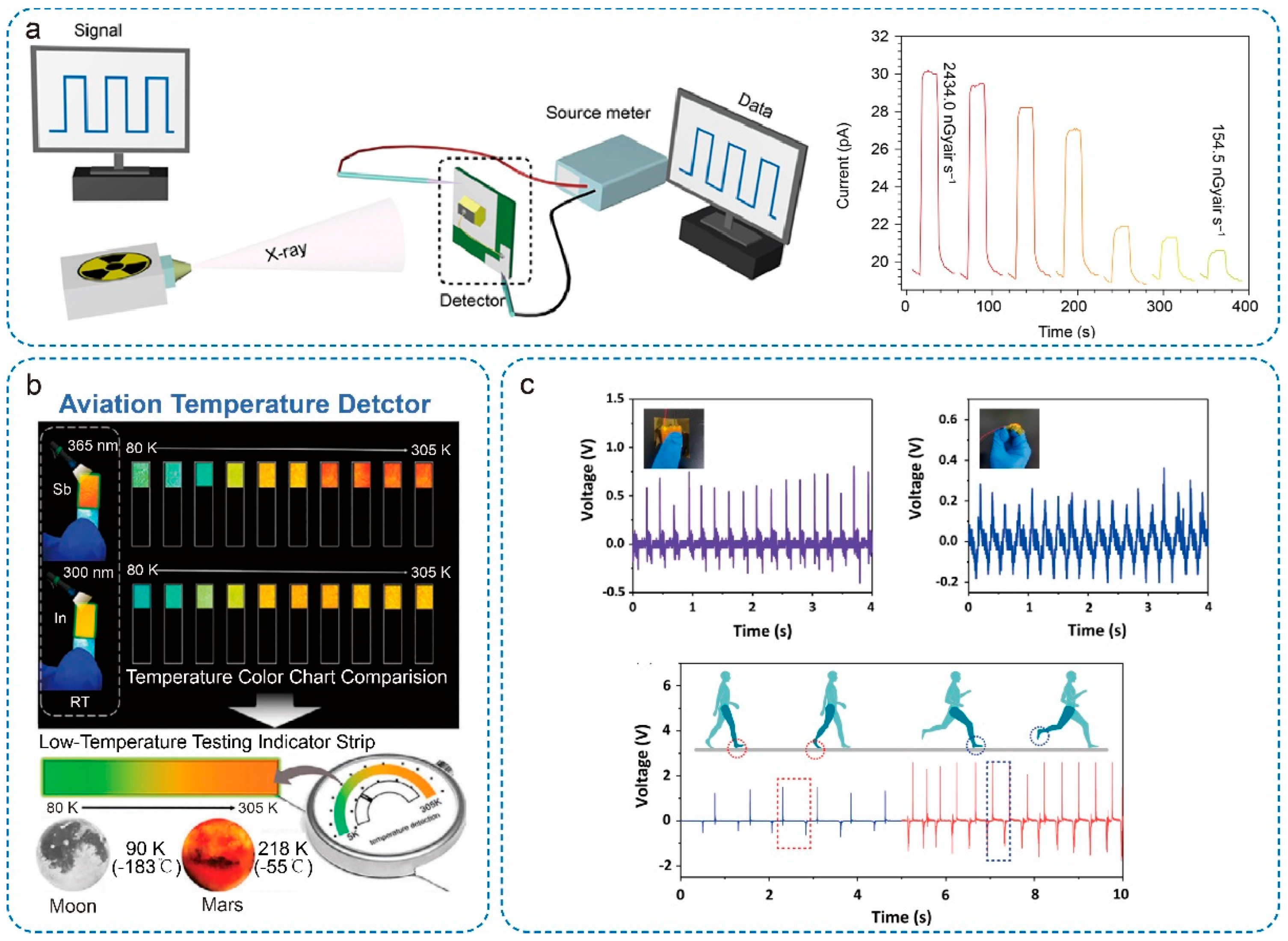
5.2. X-Ray Detectors

5.3. Sensors
5.4. Solar Cells
5.5. Anti-Counterfeiting
5.6. Other Applications
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.-X.; Zheng, S.-T. Three-Dimensional Metal-Halide Open Frameworks. Coord. Chem. Rev. 2021, 430, 213663. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Hu, S.; Xia, B.; Fan, K.-Q.; Gong, L.-K.; Kong, J.-T.; Huang, X.-Y.; Xiao, Z.; Du, K.-Z. Material Design and Optoelectronic Properties of Three-Dimensional Quadruple Perovskite Halides. J. Phys. Chem. Lett. 2019, 10, 5219–5225. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Ma, Z.; Xiao, G.; Wang, K.; Zou, B. Structural Regulation and Optical Behavior of Three-Dimensional Metal Halide Perovskites under Pressure. J. Mater. Chem. C 2020, 8, 12755–12767. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Song, X.-J.; Cheng, H.; Zeng, Y.-L.; Zhang, Y.; Li, P.-F.; Liao, W.-Q.; Xiong, R.-G. A Three-Dimensional Lead Halide Perovskite-Related Ferroelectric. J. Am. Chem. Soc. 2020, 142, 4604–4608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, X.; Chen, Q.; Jiang, T.; Chen, Q.; Liu, X. Metal–Halide Perovskite Nanocrystal Superlattice: Self-Assembly and Optical Fingerprints. Adv. Mater. 2023, 35, 2209279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal Halide Perovskites for Energy Applications. Nat. Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Yang, Y.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the Spotlight: The Organic–Inorganic Hybrid Halide Perovskite for Optoelectronic Applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.S.; Choi, J.; Kim, S.Y.; Jang, H.W. Halide Perovskites for Applications beyond Photovoltaics. Small Methods 2018, 2, 1700310. [Google Scholar] [CrossRef]
- Katz, E.A. Perovskite: Name Puzzle and German-Russian Odyssey of Discovery. Helv. Chim. Acta 2020, 103, e2000061. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Song, Z.; Sun, K.; Meng, Y.; Zhu, Z.; Wang, Y.; Zhang, W.; Bai, Y.; Lu, X.; Tian, R.; Liu, C.; et al. Universal Approach for Managing Iodine Migration in Inverted Single-Junction and Tandem Perovskite Solar Cells. Adv. Mater. 2024, 37, 2410779. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Zhuang, R.; Wu, X.; Tian, C.; Sun, A.; Chen, C.; Guo, Y.; Hua, Y.; Meng, K.; et al. Towards 26% Efficiency in Inverted Perovskite Solar Cells via Interfacial Flipped Band Bending and Suppressed Deep-Level Traps. Energy Environ. Sci. 2024, 17, 1153–1162. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Lozano, G. The Role of Metal Halide Perovskites in Next-Generation Lighting Devices. J. Phys. Chem. Lett. 2018, 9, 3987–3997. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Kshirsagar, A.S.; Wang, Z.; Yin, J.; Wang, Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y. Chemical Stability and Instability of Inorganic Halide Perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. [Google Scholar] [CrossRef]
- Park, B.; Seok, S.I. Intrinsic Instability of Inorganic–Organic Hybrid Halide Perovskite Materials. Adv. Mater. 2019, 31, 1805337. [Google Scholar] [CrossRef]
- Straus, D.B.; Guo, S.; Abeykoon, A.M.; Cava, R.J. Understanding the Instability of the Halide Perovskite CsPbI3 through Temperature-Dependent Structural Analysis. Adv. Mater. 2020, 32, 2001069. [Google Scholar] [CrossRef]
- Slavney, A.H.; Smaha, R.W.; Smith, I.C.; Jaffe, A.; Umeyama, D.; Karunadasa, H.I. Chemical Approaches to Addressing the Instability and Toxicity of Lead–Halide Perovskite Absorbers. Inorg. Chem. 2017, 56, 46–55. [Google Scholar] [CrossRef]
- Ren, M.; Qian, X.; Chen, Y.; Wang, T.; Zhao, Y. Potential Lead Toxicity and Leakage Issues on Lead Halide Perovskite Photovoltaics. J. Hazard. Mater. 2022, 426, 127848. [Google Scholar] [CrossRef] [PubMed]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of Organometal Halide Perovskite Solar Cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Yun, J.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Shi, E.; Gao, Y.; Finkenauer, B.P.; Akriti, A.; Coffey, A.H.; Dou, L. Two-Dimensional Halide Perovskite Nanomaterials and Heterostructures. Chem. Soc. Rev. 2018, 47, 6046–6072. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Hu, Y.; Xu, F.; Yan, Z.; Bai, F.; Jia, G.; Zhang, S. Recent Advances in One-Dimensional Halide Perovskites for Optoelectronic Applications. Nanoscale 2018, 10, 20963–20989. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, H.; Ran, R.; Zhou, W.; Wang, W.; Shao, Z. Beyond Two-Dimension: One- and Zero-Dimensional Halide Perovskites as New-Generation Passivators for High-Performance Perovskite Solar Cells. J. Energy Chem. 2023, 83, 189–208. [Google Scholar] [CrossRef]
- Blancon, J.-C.; Stier, A.V.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Traoré, B.; Pedesseau, L.; Kepenekian, M.; Katsutani, F.; Noe, G.T.; et al. Scaling Law for Excitons in 2D Perovskite Quantum Wells. Nat. Commun. 2018, 9, 2254. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper Hybrid Lead Iodide Perovskite 2D Homologous Semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Novikov, S.A.; Valueva, A.D.; Klepov, V.V. Band Gap Engineering and Photoluminescence Tuning in Halide Double Perovskites. Dalton Trans. 2024, 53, 12442–12449. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Wu, J.; Lin, Z. Regulation and Application of Organic Luminescence from Low-Dimensional Organic–Inorganic Hybrid Metal Halides. J. Mater. Chem. C 2023, 11, 16890–16911. [Google Scholar] [CrossRef]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Yuan, M.; Comin, R.; Voznyy, O.; Beauregard, E.M.; Hoogland, S.; Buin, A.; Kirmani, A.R.; Zhao, K.; Amassian, A.; et al. Ligand-Stabilized Reduced-Dimensionality Perovskites. J. Am. Chem. Soc. 2016, 138, 2649–2655. [Google Scholar] [CrossRef]
- Kundu, J.; Das, D.K. Low Dimensional, Broadband, Luminescent Organic-Inorganic Hybrid Materials for Lighting Applications. Eur. J. Inorg. Chem. 2021, 2021, 4508–4520. [Google Scholar] [CrossRef]
- Tan, J.; Li, D.; Zhu, J.; Han, N.; Gong, Y.; Zhang, Y. Self-Trapped Excitons in Soft Semiconductors. Nanoscale 2022, 14, 16394–16414. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, X.; Chen, Q.; Chen, Q.; Jing, Y.; Zhou, Z.; Zhao, Y.S.; Chen, J.; Liu, X. Highly Stable Lead-Free Perovskite Single Crystals with NIR Emission Beyond 1100 Nm. Adv. Opt. Mater. 2022, 10, 2201254. [Google Scholar] [CrossRef]
- Ghosh, S.; Pradhan, B. Lead-Free Metal Halide Perovskite Nanocrystals: Challenges, Applications, and Future Aspects. ChemNanoMat 2019, 5, 300–312. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Lee, S.; Chaaban, M.; Ma, B. Organic–Inorganic Metal Halide Hybrids beyond Perovskites. Mater. Res. Lett. 2018, 6, 552–569. [Google Scholar] [CrossRef]
- Gonzalez-Carrero, S.; Galian, R.E.; Pérez-Prieto, J. Organic-Inorganic and All-Inorganic Lead Halide Nanoparticles [Invited]. Opt. Express 2016, 24, A285. [Google Scholar] [CrossRef]
- Kirchartz, T.; Yan, G.; Yuan, Y.; Patel, B.K.; Cahen, D.; Nayak, P.K. The State of the Art in Photovoltaic Materials and Device Research. Nat. Rev. Mater. 2025. [Google Scholar] [CrossRef]
- Zhou, K.; Qi, B.; Liu, Z.; Wang, X.; Sun, Y.; Zhang, L. Advanced Organic–Inorganic Hybrid Materials for Optoelectronic Applications. Adv. Funct. Mater. 2024, 34, 2411671. [Google Scholar] [CrossRef]
- Dong, Y.; Han, Y.; Chen, R.; Lin, Y.; Cui, B.-B. Recent Progress of Triplet State Emission in Organic-Inorganic Hybrid Metal Halides. J. Lumin. 2022, 249, 119013. [Google Scholar] [CrossRef]
- Ahmadi, M.; Wu, T.; Hu, B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater. 2017, 29, 1605242. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tang, Y.; Ma, Y.; Hu, B. Spin-Orbital Ordering Effects of Light Emission in Organic–Inorganic Hybrid Metal Halide Perovskites. Adv. Mater. 2024, 2411913. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically Diverse and Multifunctional Hybrid Organic–Inorganic Perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Gu, H.; Xia, J.; Liang, C.; Chen, Y.; Huang, W.; Xing, G. Phase-Pure Two-Dimensional Layered Perovskite Thin Films. Nat. Rev. Mater. 2023, 8, 533–551. [Google Scholar] [CrossRef]
- Bi, C.-Y.; Li, S.-X.; Zhang, H.; Wu, C.-Y.; Dong, Y.-H.; Zhao, F.-H.; Wang, L.; Jing, Z.; He, Y.-C. Three New Low-Dimensional Organic Inorganic Hybrid Metal Halides: Syntheses, Structures and Properties. Solid State Sci. 2024, 151, 107530. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.-H.; Ma, B. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, C.; Tian, Y.; Siegrist, T.; Ma, B. Low-Dimensional Organometal Halide Perovskites. ACS Energy Lett. 2018, 3, 54–62. [Google Scholar] [CrossRef]
- Huo, C.; Cai, B.; Yuan, Z.; Ma, B.; Zeng, H. Two-Dimensional Metal Halide Perovskites: Theory, Synthesis, and Optoelectronics. Small Methods 2017, 1, 1600018. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.; Kuang, D. An Overview for Zero-Dimensional Broadband Emissive Metal-Halide Single Crystals. Adv. Opt. Mater. 2021, 9, 2100544. [Google Scholar] [CrossRef]
- Ge, C.; Xue, Y.Z.B.; Li, L.; Tang, B.; Hu, H. Recent Progress in 2D/3D Multidimensional Metal Halide Perovskites Solar Cells. Front. Mater. 2020, 7, 601179. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Ou, Q.; Ha, S.T.; Qiu, C.; Zhang, H.; Cheng, Y.; Xiong, Q.; Bao, Q. Photonics and Optoelectronics of 2D Metal-Halide Perovskites. Small 2018, 14, 1800682. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper Perovskites in Electrocatalysis. Mater. Horiz. 2020, 7, 2519–2565. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, D.; Li, Y.; Li, X.; Peng, S.; Shao, G.; Xing, G. Ruddlesden–Popper Perovskite for Stable Solar Cells. Energy Environ. Mater. 2018, 1, 221–231. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, S.; Sun, Y.; Liang, Z. Phase Engineering in Quasi-2D Ruddlesden–Popper Perovskites. J. Phys. Chem. Lett. 2018, 9, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, X.; Fu, J.; Wu, Y.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. 2D/Quasi-2D Ruddlesden–Popper Perovskite: A High-Performance Photocatalyst for Hydrogen Evolution. ACS Catal. 2023, 13, 14716–14724. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z.; Xue, Q.; Zheng, Y.; Wu, X.; Huang, Y.; Wang, X.; Qin, C.; Chen, Z.; Chen, C.-C. A Finely Regulated Quantum Well Structure in Quasi-2D Ruddlesden–Popper Perovskite Solar Cells with Efficiency Exceeding 20%. Energy Environ. Sci. 2022, 15, 296–310. [Google Scholar] [CrossRef]
- Dong, R.; Lan, C.; Xu, X.; Liang, X.; Hu, X.; Li, D.; Zhou, Z.; Shu, L.; Yip, S.; Li, C.; et al. Novel Series of Quasi-2D Ruddlesden–Popper Perovskites Based on Short-Chained Spacer Cation for Enhanced Photodetection. ACS Appl. Mater. Interfaces 2018, 10, 19019–19026. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Meng, N.; Wang, W.; Lu, Y.; Dong, J.; Zhao, S.; Qiao, B.; Song, D.; Xu, Z. Modulation Phase Distribution of Ruddlesden–Popper Quasi-2D Perovskites with a Similarly Spaced Dion–Jacobson Phase. ACS Appl. Mater. Interfaces 2023, 15, 42706–42716. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Y.; Gu, H.; Cheng, T.; Xie, Y.; Lin, Y.; Xing, G.; Yin, J.; Cui, B.-B. Efficient Room-Temperature Phosphorescence of 1D Organic–Inorganic Hybrid Metal Halides. Small Struct. 2022, 3, 2200110. [Google Scholar] [CrossRef]
- Rahaman, Z.; Ge, S.; Lin, C.-H.; Cui, Y.; Wu, T. One-Dimensional Molecular Metal Halide Materials: Structures, Properties, and Applications. Small Struct. 2021, 2, 2000062. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, C.; Neu, J.; Zhou, Y.; Han, D.; Chen, S.; Worku, M.; Chaaban, M.; Lee, S.; Berkwits, E.; et al. Bulk Assembly of Corrugated 1D Metal Halides with Broadband Yellow Emission. Adv. Opt. Mater. 2019, 7, 1801474. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, T.; Mondal, D.; Teng, S.; Zhang, Y.; Huang, K.; Wang, D.; Yang, W.; Mahadevan, P.; Zhao, Y.S.; et al. Light-Emitting Metal–Organic Halide 1D and 2D Structures: Near-Unity Quantum Efficiency, Low-Loss Optical Waveguide and Highly Polarized Emission. Angew. Chem. 2021, 133, 13660–13665. [Google Scholar] [CrossRef]
- Duan, D.; Ge, C.; Rahaman, M.Z.; Lin, C.-H.; Shi, Y.; Lin, H.; Hu, H.; Wu, T. Recent Progress with One-Dimensional Metal Halide Perovskites: From Rational Synthesis to Optoelectronic Applications. NPG Asia Mater. 2023, 15, 8. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z. Recent Progress of Zero-Dimensional Luminescent Metal Halides. Chem. Soc. Rev. 2021, 50, 2626–2662. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, B.; Ren, X.; Wang, F. Recent Advances in All-Inorganic Zero-Dimensional Metal Halides. ChemPlusChem 2021, 86, 1577–1585. [Google Scholar] [CrossRef]
- Ghosh, B.; Wu, B.; Mulmudi, H.K.; Guet, C.; Weber, K.; Sum, T.C.; Mhaisalkar, S.; Mathews, N. Limitations of Cs3Bi2I9 as Lead-Free Photovoltaic Absorber Materials. ACS Appl. Mater. Interfaces 2018, 10, 35000–35007. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Ma, L.; Shen, G.; Yang, Q. Air-Stabilized Lead-Free Hexagonal Cs3Bi2I9 Nanocrystals for Ultrahigh-Performance Optical Detection. Adv. Funct. Mater. 2022, 32, 2203072. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Wang, L.; Liu, C.; Wang, K.; Zou, B. Pressure-Induced Emission Enhancement, Band-Gap Narrowing, and Metallization of Halide Perovskite Cs3Bi2I9. Angew. Chem. Int. Ed. 2018, 57, 11213–11217. [Google Scholar] [CrossRef]
- McCall, K.M.; Stoumpos, C.C.; Kontsevoi, O.Y.; Alexander, G.C.B.; Wessels, B.W.; Kanatzidis, M.G. From 0D Cs3Bi2I9 to 2D Cs3Bi2I6Cl3: Dimensional Expansion Induces a Direct Band Gap but Enhances Electron–Phonon Coupling. Chem. Mater. 2019, 31, 2644–2650. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.; Lee, S.; Lin, H.; Ma, B. Recent Advances in Luminescent Zero-Dimensional Organic Metal Halide Hybrids. Adv. Opt. Mater. 2021, 9, 2001766. [Google Scholar] [CrossRef]
- Hong, K.; Le, Q.V.; Kim, S.Y.; Jang, H.W. Low-Dimensional Halide Perovskites: Review and Issues. J. Mater. Chem. C 2018, 6, 2189–2209. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, Z.; Xue, Y.; Ou, Q.; Polavarapu, L.; Zheng, J.; Qi, X.; Bao, Q. Synthesis, Properties, and Optical Applications of Low-Dimensional Perovskites. Chem. Commun. 2016, 52, 13637–13655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Deng, H.; Qiao, K.; Farooq, U.; Ishaq, M.; Yi, F.; Liu, H.; Tang, J.; Song, H. Low-Dimensional Halide Perovskites and Their Advanced Optoelectronic Applications. Nano-Micro Lett. 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, K.; Khan, S.A.; Shabbir, B.; Zhang, Y.; Khan, Q.; Bao, Q. Synthesis and Optical Applications of Low Dimensional Metal-Halide Perovskites. Nanotechnology 2020, 31, 152002. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Bolink, H.J. Making by Grinding: Mechanochemistry Boosts the Development of Halide Perovskites and Other Multinary Metal Halides. Adv. Energy Mater. 2020, 10, 1902499. [Google Scholar] [CrossRef]
- Liang, D.; Sun, Z.; Lu, S.; Zhao, J.; Zhou, Y.; An, K.; Zang, Z. Solvent-Free Grinding Synthesis of Hybrid Copper Halides for White Light Emission. Inorg. Chem. 2023, 62, 7296–7303. [Google Scholar] [CrossRef]
- Ben-Akacha, A.; Zhou, C.; Chaaban, M.; Beery, D.; Lee, S.; Worku, M.; Lin, X.; Westphal, R.; Ma, B. Mechanochemical Synthesis of Zero Dimensional Organic-Inorganic Metal Halide Hybrids. ChemPhotoChem 2021, 5, 326–329. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, W.; Su, P.; Lang, T.; He, H.; Jiang, H.; Jia, B.; Liu, X.; Han, T. Mn-Doped M2CdCl4 (M=CH3NH3+, C2H8N+, and C3H10N+) Layered Hybrid Perovskite and Its Flexible Film Based on Simple Mechanochemical Synthesis. Inorg. Chem. 2024, 63, 2562–2568. [Google Scholar] [CrossRef]
- He, Z.-L.; Wei, J.-H.; Luo, J.-B.; Zhang, Z.-Z.; Kuang, D.-B. Reversible Human-Temperature-Responsive Luminescence Switching in a Mn(II)-Based Metal Halide. J. Mater. Chem. C 2023, 11, 1251–1257. [Google Scholar] [CrossRef]
- Jiang, R.; Peng, G.; Li, Q.; Wang, H.; Ci, Z.; Wang, Q. Manganese (II) Halides for X-Ray Imaging and Moisture Detection. Adv. Mater. Technol. 2024, 9, 2301894. [Google Scholar] [CrossRef]
- Umemoto, K.; Ebe, H.; Sato, R.; Enomoto, J.; Oshita, N.; Kimura, T.; Inose, T.; Nakamura, T.; Chiba, T.; Asakura, S.; et al. Simple Production of Highly Luminescent Organometal Halide Perovskite Nanocrystals Using Ultrasound-Assisted Bead Milling. ACS Sustain. Chem. Eng. 2020, 8, 16469–16476. [Google Scholar] [CrossRef]
- Poli, I.; Petrozza, A. Halide Perovskite Semiconductors Processing: Solvent-Based or Solvent-Free? ACS Energy Lett. 2024, 9, 4596–4597. [Google Scholar] [CrossRef]
- Hoang, M.T.; Ünlü, F.; Martens, W.; Bell, J.; Mathur, S.; Wang, H. Towards the Environmentally Friendly Solution Processing of Metal Halide Perovskite Technology. Green Chem. 2021, 23, 5302–5336. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Dang, Y.; Ju, D.; Wang, L.; Tao, X. Recent Progress in the Synthesis of Hybrid Halide Perovskite Single Crystals. CrystEngComm 2016, 18, 4476–4484. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.; Ye, H.; Zheng, Y.; Wei, Q.; Xia, Y.; Chen, Y.; Zhao, K.; Huang, W.; Liu, S. Centimeter-Sized Single Crystal of Two-Dimensional Halide Perovskites Incorporating Straight-Chain Symmetric Diammonium Ion for X-Ray Detection. Angew. Chem. Int. Ed. 2020, 59, 14896–14902. [Google Scholar] [CrossRef]
- Ng, M.; Halpert, J.E. Single Crystals of Mixed Br/Cl and Sn-Doped Formamidinium Lead Halide Perovskites via Inverse Temperature Crystallization. RSC Adv. 2020, 10, 3832–3836. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Burlakov, V.M.; Saidaminov, M.I.; Alofi, A.; Haque, M.A.; Turedi, B.; Davaasuren, B.; Dursun, I.; Cho, N.; El-Zohry, A.M.; et al. The Role of Surface Tension in the Crystallization of Metal Halide Perovskites. ACS Energy Lett. 2017, 2, 1782–1788. [Google Scholar] [CrossRef]
- Zhuang, R.; Wang, X.; Ma, W.; Wu, Y.; Chen, X.; Tang, L.; Zhu, H.; Liu, J.; Wu, L.; Zhou, W.; et al. Highly Sensitive X-Ray Detector Made of Layered Perovskite-like (NH4)3Bi2I9 Single Crystal with Anisotropic Response. Nat. Photonics 2019, 13, 602–608. [Google Scholar] [CrossRef]
- Gao, L.; Luo, X.; Sun, J.; Li, Q.; Yan, Q. Room-Temperature Solvent Evaporation Induced Crystallization: A General Strategy for Growth of Halide Perovskite Single Crystals by Applying the Le Chatelier’s Principle. Small 2023, 19, 2303687. [Google Scholar] [CrossRef]
- Ling, C.; Xia, Y.; Xiao, X.; Chen, X.; Zheng, Z.; Xia, M.; Hu, Y.; Mei, A.; Rong, Y.; Han, H. Modeling and Balancing the Solvent Evaporation of Thermal Annealing Process for Metal Halide Perovskites and Solar Cells. Small Methods 2022, 6, 2200161. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, Z.; Ye, H.; Li, Q.; Hu, M.; Yang, Z.; Liu, S. Two-Dimensional (PEA)2 PbBr4 Perovskite Single Crystals for a High Performance UV-Detector. J. Mater. Chem. C 2019, 7, 1584–1591. [Google Scholar] [CrossRef]
- Liu, W.; Xie, H.; Cai, W.; Zhang, R.; Xu, B.; Yang, C. Rapid Synthesis of Two Photoluminescent Pyridinium Manganese-Based Halides by an Anti-Solvent Method. J. Alloys Compd. 2023, 968, 172173. [Google Scholar] [CrossRef]
- Guan, J.; Zheng, Y.; Cheng, P.; Han, W.; Han, X.; Wang, P.; Xin, M.; Shi, R.; Xu, J.; Bu, X.-H. Free Halogen Substitution of Chiral Hybrid Metal Halides for Activating the Linear and Nonlinear Chiroptical Properties. J. Am. Chem. Soc. 2023, 145, 26833–26842. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Fang, C.-L.; Feng, Y.; Liu, S.-Y.; Zhang, Y.; Ye, H.-Y.; Miao, L.-P.; Liu, L. One-Dimensional Lead-Free Perovskite Single Crystals with High X-Ray Response Grown by Liquid Phase Diffusion. J. Mater. Chem. C 2023, 11, 134–140. [Google Scholar] [CrossRef]
- Ghimire, S.; Oldenburg, K.; Bartling, S.; Lesyuk, R.; Klinke, C. Structural Reconstruction in Lead-Free Two-Dimensional Tin Iodide Perovskites Leading to High Quantum Yield Emission. ACS Energy Lett. 2022, 7, 975–983. [Google Scholar] [CrossRef]
- Long, Z.; Yang, S.; Pi, J.; Zhou, D.; Wang, Q.; Yang, Y.; Wu, H.; Qiu, J. All-Inorganic Halide Perovskite (CsPbX3, X=Cl, Br, I) Quantum Dots Synthesized via Fast Anion Hot Injection by Using Trimethylhalosilanes. Ceram. Int. 2022, 48, 35474–35479. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Liu, C.; Wang, X.; Zhang, Q.; Liu, J.; Wang, M.; Liu, Y. Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties. Materials 2024, 17, 2173. [Google Scholar] [CrossRef]
- Wang, X.; Bai, T.; Meng, X.; Ji, S.; Zhang, R.; Zheng, D.; Yang, B.; Jiang, J.; Han, K.; Liu, F. Filling Chlorine Vacancy with Bromine: A Two-Step Hot-Injection Approach Achieving Defect-Free Hybrid Halogen Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2022, 14, 46857–46865. [Google Scholar] [CrossRef] [PubMed]
- Fattal, H.; Creason, T.D.; Delzer, C.J.; Yangui, A.; Hayward, J.P.; Ross, B.J.; Du, M.-H.; Glatzhofer, D.T.; Saparov, B. Zero-Dimensional Hybrid Organic–Inorganic Indium Bromide with Blue Emission. Inorg. Chem. 2021, 60, 1045–1054. [Google Scholar] [CrossRef]
- Han, K.; Jin, J.; Su, B.; Xia, Z. Molecular Dimensionality and Photoluminescence of Hybrid Metal Halides. Trends Chem. 2022, 4, 1034–1044. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Sun, C.; Li, D.-Y.; Dong, Y.-H.; Wang, C.-L.; Zhao, H.-F.; Jiang, H.; Jing, Z.-H.; Lei, X.-W. Organic–Inorganic Hybrid Heterometallic Halides with Low-Dimensional Structures and Red Photoluminescence Emissions. Inorg. Chem. 2019, 58, 10304–10312. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.; Marohn, J.; McLendon, G. Dynamics of Electron-Hole Pair Recombination in Semiconductor Clusters. J. Phys. Chem. 1990, 94, 4356–4363. [Google Scholar] [CrossRef]
- Peng, H.; Yao, S.; Guo, Y.; Zhi, R.; Wang, X.; Ge, F.; Tian, Y.; Wang, J.; Zou, B. Highly Efficient Self-Trapped Exciton Emission of a (MA)4Cu2Br6 Single Crystal. J. Phys. Chem. Lett. 2020, 11, 4703–4710. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Karunadasa, H.I. White-Light Emission from Layered Halide Perovskites. Acc. Chem. Res. 2018, 51, 619–627. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect Emissions in ZnO Nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Chen, J.-K.; Zhang, B.-B.; Shirahata, N.; Sun, H.-T. Defect-Induced Bandgap Widening and Abnormal Photoluminescence in Formamidinium Tin Iodide Perovskite Microcrystals. ACS Mater. Lett. 2024, 6, 3218–3225. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J. Defect-Related Luminescent Materials: Synthesis, Emission Properties and Applications. Chem. Soc. Rev. 2012, 41, 7938. [Google Scholar] [CrossRef]
- Wang, H.-R.; Yang, X.-G.; Qin, J.-H.; Ma, L.-F. Long-Lived Room Temperature Phosphorescence of Organic–Inorganic Hybrid Systems. Inorg. Chem. Front. 2021, 8, 1942–1950. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef]
- Hong, X.; Ishihara, T.; Nurmikko, A.V. Dielectric Confinement Effect on Excitons in PbI4-Based Layered Semiconductors. Phys. Rev. B 1992, 45, 6961–6964. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Ding, J.; Nurmikko, A.V. Dielectric Confinement Effect for Exciton and Biexciton States in Pbla-Based Two-Dimensional Semiconductor Structures. Surf. Sci. 1992, 267, 323–326. [Google Scholar] [CrossRef]
- Katan, C.; Mercier, N.; Even, J. Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Perovskite Semiconductors. Chem. Rev. 2019, 119, 3140–3192. [Google Scholar] [CrossRef]
- Yin, J.; Maity, P.; Naphade, R.; Cheng, B.; He, J.-H.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Tuning Hot Carrier Cooling Dynamics by Dielectric Confinement in Two-Dimensional Hybrid Perovskite Crystals. ACS Nano 2019, 13, 12621–12629. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Ma, J.; Shen, H.; Li, L.; Duan, X.; Li, D. Self-Trapped State Enabled Filterless Narrowband Photodetections in 2D Layered Perovskite Single Crystals. Nat. Commun. 2019, 10, 806. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhang, T.; Gong, X.; Sun, Q.; Pan, H.; Shen, Y.; Ahmad, S.; Wang, M. Layered Ruddlesden–Popper Efficient Perovskite Solar Cells with Controlled Quantum and Dielectric Confinement Introduced via Doping. Adv. Funct. Mater. 2019, 29, 1903293. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Yi, L. Defect Emission in Cs3Cu2I5 and CsCu2I3 Halide Films. J. Lumin. 2023, 254, 119516. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, W.K.; Chen, H.Y.; Gwo, S. Green, Yellow, and Orange Defect Emission from ZnO Nanostructures: Influence of Excitation Wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]
- Collins, R.J. Mechanism and Defect Responsible for Edge Emission in CdS. J. Appl. Phys. 1959, 30, 1135–1140. [Google Scholar] [CrossRef]
- Binsma, J.J.M.; Giling, L.J.; Bloem, J. LUMINESCENCE OF CuInS2.
- Shi, T.; Su, Z.; Li, J.; Liu, C.; Yang, J.; He, X.; Yun, D.; Peng, Q.; Lu, C. Distinct Point Defect Behaviours in Body-Centered Cubic Medium-Entropy Alloy NbZrTi Induced by Severe Lattice Distortion. Acta Mater. 2022, 229, 117806. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, D.; Morris, O.P.; Zhitomirsky, D.; Grossman, J.C. Origins of the Stokes Shift in PbS Quantum Dots: Impact of Polydispersity, Ligands, and Defects. ACS Nano 2018, 12, 2838–2845. [Google Scholar] [CrossRef]
- Zhao, H.; Kalt, H. Energy-Dependent Huang-Rhys Factor of Free Excitons. Phys. Rev. B 2003, 68, 125309. [Google Scholar] [CrossRef]
- Lin, C. Fluorescence Line-Narrowing Difference Spectra: Dependence of Huang–Rhys Factor on Excitation Wavelength.
- Zhang, D.; Cao, X.; Liu, C.; Chen, M.; Ye, W.; Zhou, J.; Fan, X.; You, G.; Zheng, C.; Ning, J.; et al. Abnormal Temperature Dependence of Huang–Rhys Factor and Exciton Recombination Kinetics in CsPbBr3 Perovskite Quantum Dots. J. Phys. Chem. Lett. 2024, 15, 11015–11021. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Song, B.; Luo, J.; Tang, J. Light Emission of Self-Trapped Excitons in Inorganic Metal Halides for Optoelectronic Applications. Adv. Mater. 2022, 34, 2201008. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.; Huang, Z.; Wei, J.; Wang, X.; Chen, H.; Kuang, D. Intrinsic Self-Trapped Emission in 0D Lead-Free (C4H14N2)2In2Br10 Single Crystal. Angew. Chem. 2019, 131, 15581–15586. [Google Scholar] [CrossRef]
- Li, Q.; Xu, B.; Chen, Z.; Han, J.; Tan, L.; Luo, Z.; Shen, P.; Quan, Z. Excitation-Dependent Emission Color Tuning of 0D Cs2InBr5·H2O at High Pressure. Adv. Funct. Mater. 2021, 31, 2104923. [Google Scholar] [CrossRef]
- Das, D.K.; Bakthavatsalam, R.; Hathwar, V.R.; Pallepogu, R.; Kundu, J. Intrinsic vs. Extrinsic STE Emission Enhancement in Ns2 Ion Doped Metal (Cd, In) Halide Hybrids. J. Mater. Chem. C 2023, 11, 3855–3864. [Google Scholar] [CrossRef]
- Zorenko, Y.; Voloshinovskii, A.; Savchyn, V.; Voznyak, T.; Nikl, M.; Nejezchleb, K.; Mikhailin, V.; Kolobanov, V.; Spassky, D. Exciton and Antisite Defect-related Luminescence in Lu3Al5O12 and Y3Al5O12 Garnets. Phys. Status Solidi B 2007, 244, 2180–2189. [Google Scholar] [CrossRef]
- Muñoz Ramo, D.; Shluger, A.L.; Gavartin, J.L.; Bersuker, G. Theoretical Prediction of Intrinsic Self-Trapping of Electrons and Holes in Monoclinic HfO2. Phys. Rev. Lett. 2007, 99, 155504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, H.; Dong, Z.; Jin, T.; Pang, J.; Liu, Y.; Zheng, Z.; Niu, G.; Xu, L.; Tang, J. Direct X-Ray Detectors Made of Zero-Dimensional Hybrid Metal Halide Perovskite Single Crystals. J. Mater. Chem. C 2024, 12, 6288–6296. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, T.; Zhang, Y.; Yao, S.; Han, L.; Mu, D.; Song, H.; He, J.; Zhang, F.; Xu, X. A Zero-Dimensional Perovskite with High-Efficiency Luminescence and Transient Response for Advanced Information Encryption. Chem. Eng. J. 2024, 496, 153939. [Google Scholar] [CrossRef]
- Zhou, B.; Fang, F.; Liu, Z.; Zhong, H.; Zhou, K.; Hu, H.; Min, J.; Zheng, F.; Fang, S.; Nie, J.; et al. Self-Trapped Exciton Emission in Highly Polar 0D Hybrid Ammonium/Hydronium-Based Perovskites Triggered by Antimony Doping. J. Am. Chem. Soc. 2024, 146, 15198–15208. [Google Scholar] [CrossRef]
- Wang, S.; Yao, Y.; Kong, J.; Zhao, S.; Sun, Z.; Wu, Z.; Li, L.; Luo, J. Highly Efficient White-Light Emission in a Polar Two-Dimensional Hybrid Perovskite. Chem. Commun. 2018, 54, 4053–4056. [Google Scholar] [CrossRef]
- Mao, L.; Wu, Y.; Stoumpos, C.C.; Wasielewski, M.R.; Kanatzidis, M.G. White-Light Emission and Structural Distortion in New Corrugated Two-Dimensional Lead Bromide Perovskites. J. Am. Chem. Soc. 2017, 139, 5210–5215. [Google Scholar] [CrossRef]
- Febriansyah, B.; Borzda, T.; Cortecchia, D.; Neutzner, S.; Folpini, G.; Koh, T.M.; Li, Y.; Mathews, N.; Petrozza, A.; England, J. Metal Coordination Sphere Deformation Induced Highly Stokes-Shifted, Ultra Broadband Emission in 2D Hybrid Lead-Bromide Perovskites and Investigation of Its Origin. Angew. Chem. 2020, 132, 10883–10888. [Google Scholar] [CrossRef]
- Levine, I.; Menzel, D.; Musiienko, A.; MacQueen, R.; Romano, N.; Vasquez-Montoya, M.; Unger, E.; Mora Perez, C.; Forde, A.; Neukirch, A.J.; et al. Revisiting Sub-Band Gap Emission Mechanism in 2D Halide Perovskites: The Role of Defect States. J. Am. Chem. Soc. 2024, 146, 23437–23448. [Google Scholar] [CrossRef]
- Park, D.Y.; An, S.-J.; Lee, C.; Nguyen, D.A.; Lee, K.-N.; Jeong, M.S. Investigation of Chemical Origin of White-Light Emission in Two-Dimensional (C4H9NH3)2PbBr4 via Infrared Nanoscopy. J. Phys. Chem. Lett. 2019, 10, 7942–7948. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Q.; Chen, Y.; Ali, N.; Ren, Z.; Bi, G.; Wu, H. Self-Trapped Exciton Emission in an Sn(II)-Doped All-Inorganic Zero-Dimensional Zinc Halide Perovskite Variant. Nanoscale 2021, 13, 15285–15291. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, Y.; Zhu, J.; Gong, Y.; Li, C. Mn-Doped 2D Sn-Based Perovskites with Energy Transfer from Self-Trapped Excitons to Dopants for Warm White Light-Emitting Diodes. J. Mater. Chem. C 2020, 8, 8502–8506. [Google Scholar] [CrossRef]
- Xing, Z.; Zhou, Z.; Zhong, G.; Chan, C.C.S.; Li, Y.; Zou, X.; Halpert, J.E.; Su, H.; Wong, K.S. Barrierless Exciton Self-Trapping and Emission Mechanism in Low-Dimensional Copper Halides. Adv. Funct. Mater. 2022, 32, 2207638. [Google Scholar] [CrossRef]
- Du, M.-H. Emission Trend of Multiple Self-Trapped Excitons in Luminescent 1D Copper Halides. ACS Energy Lett. 2020, 5, 464–469. [Google Scholar] [CrossRef]
- Long, J.; Wei, Q.; Shen, X.; Yang, C.; Zhang, G.; Ke, B.; Liang, W.; Zhong, X.; Zou, B. Energy Transfer and Self-Trapping Exciton Luminescence in Sb3+ -Doped Two-Dimensional Layered Dion–Jacobson Phase Cadmium-Based Perovskites. J. Phys. Chem. C 2024, 128, 304–314. [Google Scholar] [CrossRef]
- Luo, B.; Liang, D.; Sun, S.; Xiao, Y.; Lian, X.; Li, X.; Li, M.-D.; Huang, X.-C.; Zhang, J.Z. Breaking Forbidden Transitions for Emission of Self-Trapped Excitons in Two Dimensional (F2CHCH2NH3)2CdBr4 Perovskite through Pb Alloying. J. Phys. Chem. Lett. 2020, 11, 199–205. [Google Scholar] [CrossRef]
- Wei, Q.; Meng, X.; Lin, W.; Ge, S.; Han, X.; Chen, L.; Zeng, R.; Zou, B. Green Triplet Self-Trapped Exciton Emission in Layered Rb3Cd2Cl7: Sb3+ Perovskite: Comparison with RbCdCl3: Sb3+. J. Phys. Chem. Lett. 2022, 13, 8436–8446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ke, W.; Traoré, B.; Guo, P.; Hadar, I.; Kepenekian, M.; Even, J.; Katan, C.; Stoumpos, C.C.; Schaller, R.D.; et al. Two-Dimensional Dion–Jacobson Hybrid Lead Iodide Perovskites with Aromatic Diammonium Cations. J. Am. Chem. Soc. 2019, 141, 12880–12890. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Zhou, C.; Li, P.; Zhu, L.; Sun, S.; Feng, X.; Sun, Y.; Zhao, G. Mechanism for Tunable Broadband White Photoluminescence of One-Dimensional (C4N2H14)2Pb1-xMnxBr4 Perovskite Microcrystals. J. Lumin. 2020, 221, 117045. [Google Scholar] [CrossRef]
- Hou, A.; Fan, L.; Xiong, Y.; Lin, J.; Liu, K.; Chen, M.; Guo, Z.; Zhao, J.; Liu, Q. Zero-Dimensional Halides with ns2 Electron (Sb3+) Activation to Generate Broad Photoluminescence. Inorg. Chem. 2023, 62, 12501–12509. [Google Scholar] [CrossRef]
- Morad, V.; Yakunin, S.; Benin, B.M.; Shynkarenko, Y.; Grotevent, M.J.; Shorubalko, I.; Boehme, S.C.; Kovalenko, M.V. Hybrid 0D Antimony Halides as Air-Stable Luminophores for High-Spatial-Resolution Remote Thermography. Adv. Mater. 2021, 33, 2007355. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Yang, S.; Hong, F.; Sun, L.; Han, P.; Pullerits, T.; Deng, W.; Han, K. Lead-Free Silver-Bismuth Halide Double Perovskite Nanocrystals. Angew. Chem. 2018, 130, 5457–5461. [Google Scholar] [CrossRef]
- Shen, Y.; Yin, J.; Cai, B.; Wang, Z.; Dong, Y.; Xu, X.; Zeng, H. Lead-Free, Stable, High-Efficiency (52%) Blue Luminescent FA3Bi2Br9 Perovskite Quantum Dots. Nanoscale Horiz. 2020, 5, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Han, K. Ultrafast Dynamics of Self-Trapped Excitons in Lead-Free Perovskite Nanocrystals. J. Phys. Chem. Lett. 2021, 12, 8256–8262. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-Free, Air-Stable All-Inorganic Cesium Bismuth Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 12471–12475. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, X.; Mao, X.; Yang, H.; Chen, Z.; Xu, L. Ultrahigh X-Ray Imaging Spatial Resolution Enabled by an 0D Mn(II) Hybrid Scintillator. Adv. Funct. Mater. 2024, 34, 2404003. [Google Scholar] [CrossRef]
- Xu, L.; Sun, C.; Xiao, H.; Wu, Y.; Chen, Z. Green-Light-Emitting Diodes Based on Tetrabromide Manganese(II) Complex through Solution Process. Adv. Mater. 2017, 29, 1605739. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, W.; Zhang, H.; Tian, Y.; Lin, G.; Xiao, W.; Yu, X.; Qiu, J.; Xu, X.; Yang, Y.; et al. Highly Efficient and Tunable Emission of Lead-Free Manganese Halides toward White Light-Emitting Diode and X-Ray Scintillation Applications. Adv. Funct. Mater. 2021, 31, 2009973. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, T.; Ji, S.; Zhao, H.; Meng, X.; Zhang, R.; Jiang, J.; Liu, F. Ultraviolet Emission from Cerium-Based Organic-Inorganic Hybrid Halides and Their Abnormal Anti-Thermal Quenching Behavior. Adv. Funct. Mater. 2023, 33, 2303399. [Google Scholar] [CrossRef]
- Bai, T.; Wang, Q.; Bai, Y.; Meng, Q.; Zhao, H.; Wen, Z.; Sun, H.; Huang, L.; Jiang, J.; Huang, D.; et al. From Dopant to Host: Solution Synthesis and Light-Emitting Applications of Organic-Inorganic Lanthanide-Based Metal Halides. Small Struct. 2024, 5, 2400096. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Han, K.; Xia, Z. Rigid Phase Formation and Sb3+ Doping of Tin (IV) Halide Hybrids toward Photoluminescence Enhancement and Tuning for Anti-Counterfeiting and Information Encryption. Angew. Chem. 2024, 136, e202408653. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Ge, L.; Liu, S.; Lv, W.; Yu, Y.; Tang, Y.; Han, C.; Li, M.; Tao, Y.; et al. Ultralong Red Room-Temperature Phosphorescence of 2D Organic–Inorganic Metal Halide Perovskites for Afterglow Red LEDs and X-Ray Scintillation Applications. Inorg. Chem. 2023, 62, 16538–16546. [Google Scholar] [CrossRef] [PubMed]
- Ema, K.; Umeda, K.; Toda, M.; Yajima, C.; Arai, Y.; Kunugita, H.; Wolverson, D.; Davies, J.J. Huge Exchange Energy and Fine Structure of Excitons in an Organic-Inorganic Quantum Well Material. Phys. Rev. B 2006, 73, 241310. [Google Scholar] [CrossRef]
- Ema, K.; Inomata, M.; Kato, Y.; Kunugita, H.; Era, M. Nearly Perfect Triplet-Triplet Energy Transfer from Wannier Excitons to Naphthalene in Organic-Inorganic Hybrid Quantum-Well Materials. Phys. Rev. Lett. 2008, 100, 257401. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Meier, F.; Zhao, D.; Abe, Y.; Gao, Y.; Chen, B.; Salim, T.; Chia, E.E.M.; Qiao, X.; Deibel, C.; et al. Efficient Room-Temperature Phosphorescence from Organic–Inorganic Hybrid Perovskites by Molecular Engineering. Adv. Mater. 2018, 30, 1707621. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, D. Simultaneous Long-Persistent Blue Luminescence and High Quantum Yield within 2D Organic–Metal Halide Perovskite Micro/Nanosheets. Angew. Chem. 2019, 131, 15272–15279. [Google Scholar] [CrossRef]
- Chen, X.; Ge, L.; Tang, Y.; Han, C.; Yu, Y.; Liu, S.; Li, M.; Zhang, P.; Xu, L.; Yin, J.; et al. Achieving Ultralong Room-Temperature Phosphorescence in Two-Dimensional Metal Halide Perovskites by Alkyl Chain Engineering. J. Phys. Chem. Lett. 2023, 14, 8638–8647. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Molokeev, M.S.; Sun, J.; Xu, D.; Xia, Z. Tunable Photoluminescence in Sb3+ -Doped Zero-Dimensional Hybrid Metal Halides with Intrinsic and Extrinsic Self-Trapped Excitons. J. Mater. Chem. C 2020, 8, 5058–5063. [Google Scholar] [CrossRef]
- Zhou, C.; Lee, S.; Lin, H.; Neu, J.; Chaaban, M.; Xu, L.-J.; Arcidiacono, A.; He, Q.; Worku, M.; Ledbetter, L.; et al. Bulk Assembly of Multicomponent Zero-Dimensional Metal Halides with Dual Emission. ACS Mater. Lett. 2020, 2, 376–380. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Zhou, G.; Molokeev, M.S.; Zhao, J.; Morad, V.; Kovalenko, M.V.; Xia, Z. Hybrid Metal Halides with Multiple Photoluminescence Centers. Angew. Chem. Int. Ed. 2019, 58, 18670–18675. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Liu, Y.; Li, C.; Li, Y.; Li, Q.; Wei, Y.; Zhang, L.; Xu, B.; Chang, X.; et al. Integrated Afterglow and Self-Trapped Exciton Emissions in Hybrid Metal Halides for Anti-Counterfeiting Applications. Adv. Mater. 2022, 34, 2200607. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Yan, T.; Wu, Y.; Gong, Z.; Chen, Z.; Yue, C.; Yan, D.; Lei, X. Synchronously Improved Multiple Afterglow and Phosphorescence Efficiencies in 0D Hybrid Zinc Halides with Ultrahigh Anti-Water Stabilities. Angew. Chem. Int. Ed. 2024, 63, e202412350. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yan, C.; Chun, F.; Li, W.; Fu, X.; Yang, W. A Review of Low-Dimensional Metal Halide Perovskites for Blue Light Emitting Diodes. J. Alloys Compd. 2021, 883, 160727. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Lyu, M.; Yun, J.; Hao, M.; Wang, L. Progress and Perspective in Low-Dimensional Metal Halide Perovskites for Optoelectronic Applications. Sol. RRL 2018, 2, 1700186. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Liu, X.; Shi, Z.; Fang, X.; He, J. Low-Dimensional Metal Halide Perovskite Photodetectors. Adv. Mater. 2021, 33, 2003309. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lei, Z.; Yu, X.; Lin, C.; Huang, J.; Huang, C.; Hu, L.; Li, F.; Vinu, A.; Yi, J.; et al. Low-Dimensional Metal-Halide Perovskites as High-Performance Materials for Memory Applications. Small 2022, 18, 2203311. [Google Scholar] [CrossRef]
- Hu, H.; Morris, S.A.; Qiao, X.; Zhao, D.; Salim, T.; Chen, B.; Chia, E.E.M.; Lam, Y.M. Molecular Engineering of Two-Dimensional Hybrid Perovskites with Broadband Emission for White Light-Emitting Diodes. J. Mater. Chem. C 2018, 6, 10301–10307. [Google Scholar] [CrossRef]
- Li, S.; Xu, J.; Li, Z.; Zeng, Z.; Li, W.; Cui, M.; Qin, C.; Du, Y. One-Dimensional Lead-Free Halide with Near-Unity Greenish-Yellow Light Emission. Chem. Mater. 2020, 32, 6525–6531. [Google Scholar] [CrossRef]
- Huang, J.; Su, B.; Song, E.; Molokeev, M.S.; Xia, Z. Ultra-Broad-Band-Excitable Cu(I)-Based Organometallic Halide with Near-Unity Emission for Light-Emitting Diode Applications. Chem. Mater. 2021, 33, 4382–4389. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Yuan, Z.; Lin, H.; Chen, B.; Clark, R.; Dilbeck, T.; Zhou, Y.; Hurley, J.; Neu, J.; et al. Highly Efficient Broadband Yellow Phosphor Based on Zero-Dimensional Tin Mixed-Halide Perovskite. ACS Appl. Mater. Interfaces 2017, 9, 44579–44583. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Kou, T.; Zhou, Y.; Liang, Y.; Wu, Z.; Huang, J.; Chang, T.; Peng, C.; Wei, Q.; et al. Lead-Free MnII -Based Red-Emitting Hybrid Halide (CH6N3)2MnCl4 toward High Performance Warm WLEDs. J. Mater. Chem. C 2021, 9, 4895–4902. [Google Scholar] [CrossRef]
- Zhou, G.; Ding, J.; Jiang, X.; Zhang, J.; Molokeev, M.S.; Ren, Q.; Zhou, J.; Li, S.; Zhang, X.-M. Coordination Units of Mn2+ Modulation toward Tunable Emission in Zero-Dimensional Bromides for White Light-Emitting Diodes. J. Mater. Chem. C 2022, 10, 2095–2102. [Google Scholar] [CrossRef]
- Wei, J.-H.; Liao, J.-F.; Zhou, L.; Luo, J.-B.; Wang, X.-D.; Kuang, D.-B. Indium-Antimony-Halide Single Crystals for High-Efficiency White-Light Emission and Anti-Counterfeiting. Sci. Adv. 2021, 7, eabg3989. [Google Scholar] [CrossRef] [PubMed]
- Spahn, M. X-Ray Detectors in Medical Imaging. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2013, 731, 57–63. [Google Scholar] [CrossRef]
- Rowlands, J.A. Material Change for X-Ray Detectors. Nature 2017, 550, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.J.; Rowlands, J.A. X-Ray Detectors for Digital Radiography. Phys. Med. Biol. 1997, 42, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Koch, A.; Nikl, M. Scintillator Materials for X-Ray Detectors and Beam Monitors. MRS Bull. 2017, 42, 451–457. [Google Scholar] [CrossRef]
- Shonde, T.B.; Chaaban, M.; Liu, H.; Olasupo, O.J.; Ben-Akacha, A.; Gonzalez, F.G.; Julevich, K.; Lin, X.; Winfred, J.S.R.V.; Stand, L.M.; et al. Molecular Sensitization Enabled High Performance Organic Metal Halide Hybrid Scintillator. Adv. Mater. 2023, 35, 2301612. [Google Scholar] [CrossRef]
- Lin, N.; Wang, R.; Zhang, S.; Lin, Z.; Chen, X.; Li, Z.; Lei, X.; Wang, Y.; Yue, C. 0D Hybrid Cuprous Halide as an Efficient Light Emitter and X-Ray Scintillator. Laser Photonics Rev. 2023, 17, 2300427. [Google Scholar] [CrossRef]
- Molokeev, M.; Golovnev, N.; Zolotov, A.; Zhang, S.; Xia, Z. Screening High-Performance Hybrid Halides Scintillators: A Comprehensive Analysis and Prediction Model. Chem. Mater. 2025, 37, 1255–1263. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Feng, Y.; Liu, X.-T.; Cao, L.-Y.; Xu, Q.-Y.; Qu, H.; Zhao, T.; Li, Y.; Lin, G. Organic–Inorganic Hybrid Halide X-Ray Scintillator with High Antiwater Stability. Inorg. Chem. 2024, 63, 16224–16232. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Zhang, F.; Niu, S.; Zhu, M.; Shi, Z.; Shen, G. Stable Organic-Inorganic Hybrid Sb(III) Halide Scintillator for Nonplanar Ultra-Flexible X-Ray Imaging. Adv. Funct. Mater. 2025, 35, 2412597. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Xing, G.; Lin, S.; Yan, Y.; Zhou, Q.; Chen, J.; Zhu, W.; Chen, B.; Liu, S.; et al. Direct Ink Writing of Single-Crystal-Assembled Perovskite Thick Films for High-Performance X-ray Flat-Panel Detectors. Adv. Funct. Mater. 2025, 2423403. [Google Scholar] [CrossRef]
- Lu, J.; Qian, R.; Lu, S.; Wang, S.; Zheng, F.; Guo, G. High-Resolution X-Ray Circular Polarization Imaging Enabled by Luminescent Photopolymerized Chiral Metal-Organic Polymers. Adv. Funct. Mater. 2024, 34, 2410219. [Google Scholar] [CrossRef]
- Qi, J.; Guo, Y.; Wu, J.; Huang, Q.; Xu, J.; Yan, S.; Liu, W.; Guo, S. Near Ultraviolet-Excitable Cyan-Emissive Hybrid Copper(I) Halides Nonlinear Optical Crystals with Near-Unity Photoluminescence Quantum Yield and High-Efficiency X-ray Scintillation. Angew. Chem. Int. Ed. 2024, 63, e202407074. [Google Scholar] [CrossRef]
- Su, B.; Jin, J.; Han, K.; Xia, Z. Ceramic Wafer Scintillation Screen by Utilizing Near-Unity Blue-Emitting Lead-Free Metal Halide (C8H20N)2Cu2Br4. Adv. Funct. Mater. 2023, 33, 2210735. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Zhang, J.; Zhao, W.; Tang, Y.; Han, C.; Chen, X.; Xu, L.; Chen, R.; Li, M.; et al. Mn(II)-Based Metal Halide with Near-Unity Quantum Yield for White LEDs and High-Resolution X-Ray Imaging. Inorg. Chem. 2024, 63, 10296–10303. [Google Scholar] [CrossRef]
- Shibuya, K.; Koshimizu, M.; Murakami, H.; Muroya, Y.; Katsumura, Y.; Asai, K. Development of Ultra-Fast Semiconducting Scintillators Using Quantum Confinement Effect. Jpn. J. Appl. Phys. 2004, 43, L1333–L1336. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Z.; Luo, J.; Yuan, J.-H.; Wang, H.; Xie, Z.; Pan, W.; Wu, H.; Xue, K.-H.; Liu, L.; et al. Self-Wavelength Shifting in Two-Dimensional Perovskite for Sensitive and Fast Gamma-Ray Detection. Nat. Commun. 2023, 14, 2808. [Google Scholar] [CrossRef]
- Jin, J.; Han, K.; Hu, Y.; Xia, Z. Zn2+ Doping in Organic Manganese(II) Bromide Hybrid Scintillators toward Enhanced Light Yield for X-Ray Imaging. Adv. Opt. Mater. 2023, 11, 2300330. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Peng, G.; Qiu, F.; Li, Z.; Lei, Y.; Deng, Y.; Wang, H.; Liu, Z.; Jin, Z. Organic Cation Design of Manganese Halide Hybrids Glass toward Low-Temperature Integrated Efficient, Scaling, and Reproducible X-Ray Detector. Adv. Opt. Mater. 2023, 11, 2300216. [Google Scholar] [CrossRef]
- Zhang, W.; Sui, P.; Zheng, W.; Li, L.; Wang, S.; Huang, P.; Zhang, W.; Zhang, Q.; Yu, Y.; Chen, X. Pseudo-2D Layered Organic-Inorganic Manganese Bromide with a Near-Unity Photoluminescence Quantum Yield for White Light-Emitting Diode and X-Ray Scintillator. Angew. Chem. Int. Ed. 2023, 62, e202309230. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Wang, X.; Zhang, P.; Zhu, J.; Zhang, X.; Gao, J.; Wang, S.; Liang, G.; Zhang, D.; Gao, L.; et al. Highly Luminescent Zero-Dimensional Organic Copper Halides for X-Ray Scintillation. J. Phys. Chem. Lett. 2021, 12, 6919–6926. [Google Scholar] [CrossRef]
- He, Q.; Zhou, C.; Xu, L.; Lee, S.; Lin, X.; Neu, J.; Worku, M.; Chaaban, M.; Ma, B. Highly Stable Organic Antimony Halide Crystals for X-Ray Scintillation. ACS Mater. Lett. 2020, 2, 633–638. [Google Scholar] [CrossRef]
- Liu, L.; Hu, H.; Pan, W.; Gao, H.; Song, J.; Feng, X.; Qu, W.; Wei, W.; Yang, B.; Wei, H. Robust Organogel Scintillator for Self-healing and Ultra-flexible X-ray Imaging. Adv. Mater. 2024, 36, 2311206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Zhu, J. Low-dimensional Metal Halide Perovskites and Related Optoelectronic Applications. InfoMat 2020, 2, 341–378. [Google Scholar] [CrossRef]
- Lu, X.; Lin, R.; Ding, Y.; Xia, M.; Zheng, W.; Huang, F. Mixed Low-dimensional Metal Halide Perovskite Single Crystal for Low-detection-limit X-ray Detection via Oriented Ion Migration. InfoMat 2024, 6, e12604. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Mao, Y.; Guo, C.; Zhang, J.; Molokeev, M.S.; Xia, Z.; Zhang, X. Temperature/Component-Dependent Luminescence in Lead-Free Hybrid Metal Halides for Temperature Sensor and Anti-Counterfeiting. Adv. Funct. Mater. 2024, 34, 2401860. [Google Scholar] [CrossRef]
- An, L.-C.; Zhao, C.; Zhao, Y.; Zhang, Y.; Li, K.; Stroppa, A.; Li, W.; Bu, X.-H. Chiral 1D Hybrid Metal Halides with Piezoelectric Energy Harvesting and Sensing Properties. Small Struct. 2023, 4, 2300135. [Google Scholar] [CrossRef]
- Gao, W.; Leng, M.; Hu, Z.; Li, J.; Li, D.; Liu, H.; Gao, L.; Niu, G.; Tang, J. Reversible Luminescent Humidity Chromism of Organic–Inorganic Hybrid PEA2 MnBr4 Single Crystals. Dalton Trans. 2020, 49, 5662–5668. [Google Scholar] [CrossRef]
- Chao, L.; Wang, Z.; Xia, Y.; Chen, Y.; Huang, W. Recent Progress on Low Dimensional Perovskite Solar Cells. J. Energy Chem. 2018, 27, 1091–1100. [Google Scholar] [CrossRef]
- Yusoff, A.R.B.M.; Nazeeruddin, M.K. Low-Dimensional Perovskites: From Synthesis to Stability in Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1702073. [Google Scholar] [CrossRef]
- Ye, S.; Rao, H.; Feng, M.; Xi, L.; Yen, Z.; Seng, D.H.L.; Xu, Q.; Boothroyd, C.; Chen, B.; Guo, Y.; et al. Expanding the Low-Dimensional Interface Engineering Toolbox for Efficient Perovskite Solar Cells. Nat. Energy 2023, 8, 284–293. [Google Scholar] [CrossRef]
- Li, Y.; Duan, Y.; Liu, Z.; Yang, L.; Li, H.; Fan, Q.; Zhou, H.; Sun, Y.; Wu, M.; Ren, X.; et al. In Situ Synthesized Low-Dimensional Perovskite for >25% Efficiency Stable MA-Free Perovskite Solar Cells. Adv. Mater. 2024, 36, 2310711. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.; Kim, G.Y.; Jo, W. Autonomous Control of Ion Migration at α-FAPbI3 Heterointerfaces via Interfacial-Self-Assembled 2D Perovskite. Adv. Energy Mater. 2024, 14, 2402117. [Google Scholar] [CrossRef]
- Febriansyah, B.; Koh, T.M.; Lekina, Y.; Jamaludin, N.F.; Bruno, A.; Ganguly, R.; Shen, Z.X.; Mhaisalkar, S.G.; England, J. Improved Photovoltaic Efficiency and Amplified Photocurrent Generation in Mesoporous n = 1 Two-Dimensional Lead–Iodide Perovskite Solar Cells. Chem. Mater. 2019, 31, 890–898. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Chen, J.; Qin, H.; Wu, J.; Zhang, Q.; Zheng, J.; Li, X.; Sun, Y.; He, Y.; et al. Zero-Dimensional Organic–Inorganic Hybrid Zinc Halides for Multiple Applications in Anti-Counterfeiting, X-Ray Imaging and White LEDs. Adv. Opt. Mater. 2024, 12, 2301864. [Google Scholar] [CrossRef]
- Ren, Q.; Zhou, G.; Mao, Y.; Zhang, N.; Zhang, J.; Zhang, X.-M. Optical Activity Levels of Metal Centers Controlling Multi-Mode Emissions in Low-Dimensional Hybrid Metal Halides for Anti-Counterfeiting and Information Encryption. Chem. Sci. 2024, 15, 16536–16545. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Shi, H.-S.; Zeng, L.-R.; Ge, H.; Hou, Y.-H.; Wu, X.-M.; Yue, C.-Y.; Lei, X.-W. Highly Emissive Zero-Dimensional Antimony Halide for Anti-Counterfeiting and Confidential Information Encryption-Decryption. Chem. Eng. J. 2022, 431, 134336. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, Y.; Chen, Y.; Zuo, M.; Hu, F.; Deng, M.; Wang, B.; Sun, H.; Huang, W.; Wu, D. Halide Modulated Room-Temperature Phosphorescence from One-Dimensional Metal–organic Halides for Time-Resolved Anti-Counterfeiting. J. Lumin. 2025, 277, 120907. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Xiao, Y.; Rao, L.; Zeng, R.; Xu, K.; Huang, X.-C.; Zhang, J.Z.; Luo, B. Efficient Triplet Energy Transfer in a 0D Metal Halide Hybrid with Long Persistence Room Temperature Phosphorescence for Time-Resolved Anti-Counterfeiting. Inorg. Chem. Front. 2023, 10, 7167–7175. [Google Scholar] [CrossRef]
- Xu, T.; Cai, P.; Ai, Q.; He, Q.; Si, J.; Yao, X.; Liu, Z. Regulated Room Temperature Phosphorescence from Zero-Dimensional Organometallic Halide Hybrids for Anti-Counterfeiting and Encryption. J. Lumin. 2022, 248, 118979. [Google Scholar] [CrossRef]
- Ma, W.; Qian, Q.; Qaid, S.M.H.; Zhao, S.; Liang, D.; Cai, W.; Zang, Z. Water-Molecule-Induced Reversible Fluorescence in a One-Dimensional Mn-Based Hybrid Halide for Anticounterfeiting and Digital Encryption–Decryption. Nano Lett. 2023, 23, 8932–8939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Q.; Wen, Z.; Sun, H.; Ji, S.; Meng, X.; Zhang, R.; Jiang, J.; Tang, Z.; Liu, F. Excitation Wavelength-Dependent Fluorescence of a Lanthanide Organic Metal Halide Cluster for Anti-Counterfeiting Applications. Angew. Chem. Int. Ed. 2023, 62, e202316336. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ou, W.; Luo, J.; Kuang, D. Zero-Dimensional Zn-Based Halides with Ultra-Long Room-Temperature Phosphorescence for Time-Resolved Anti-Counterfeiting. Angew. Chem. Int. Ed. 2022, 61, e202207985. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Sun, M.; Zhao, P.; Fang, X.; Zhang, J.; Guo, Y.; Zhao, G. Room Temperature Phosphorescence in Chiral 0D Zn(II) Metal Halide Crystals for Multiple Anti-Counterfeiting. Adv. Opt. Mater. 2024, 12, 2301843. [Google Scholar] [CrossRef]
- Li, W.; Mao, X.; Yin, H.; Wang, Y.; Wang, Y.; Chen, J.; Han, K.; Zhang, R. Guest-Dependent Stimuli-Responsive Photoluminescence in 0D Antimony Chlorides for Anticounterfeiting and Encryption Applications. Adv. Funct. Mater. 2025, 35, 2413049. [Google Scholar] [CrossRef]
- Li, K.-J.; Zhao, Y.-Y.; Sun, M.-E.; Chen, G.-S.; Zhang, C.; Liu, H.-L.; Li, H.-Y.; Zang, S.-Q.; Mak, T.C.W. Zero-Dimensional Zinc Halide Organic Hybrids with Excellent Optical Waveguide Properties. Cryst. Growth Des. 2022, 22, 3295–3302. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Hu, B.; Lei, X.-W.; Li, R.-Q.; Mi, F.-Q.; Gao, H.; Li, Y.; Wu, F.; Wang, C.-L.; Lin, N. Novel Three-Dimensional Semiconducting Materials Based on Hybrid d10 Transition Metal Halogenides as Visible Light-Driven Photocatalysts. Inorg. Chem. 2017, 56, 10962–10970. [Google Scholar] [CrossRef]
- Lei, X.-W.; Yue, C.-Y.; Zhao, J.-Q.; Han, Y.-F.; Yang, J.-T.; Meng, R.-R.; Gao, C.-S.; Ding, H.; Wang, C.-Y.; Chen, W.-D. Low-Dimensional Hybrid Cuprous Halides Directed by Transition Metal Complex: Syntheses, Crystal Structures, and Photocatalytic Properties. Cryst. Growth Des. 2015, 15, 5416–5426. [Google Scholar] [CrossRef]
- Seo, J.; McGillicuddy, R.D.; Slavney, A.H.; Zhang, S.; Ukani, R.; Yakovenko, A.A.; Zheng, S.-L.; Mason, J.A. Colossal Barocaloric Effects with Ultralow Hysteresis in Two-Dimensional Metal–Halide Perovskites. Nat. Commun. 2022, 13, 2536. [Google Scholar] [CrossRef]


| Aspect | Solvent-Based Methods | Solvent-Free Methods |
|---|---|---|
| Scalability | High, but solvent management can be challenging | Moderate, often requiring specialized equipment |
| Morphology control | Excellent (via solubility, temperature, surfactants) | Limited, mainly controlled by reaction parameters |
| Low-dimensional control | Effective for nanostructure synthesis | Less precise, but advancing in mechanochemistry |
| Environmental impact | Solvent waste, energy-intensive drying steps | Green chemistry-friendly, minimal waste |
| Efficiency | High reaction rates, better dispersion | May require high energy input (e.g., milling) |
| Process Simplicity | Complex (multi-step synthesis) | Simple, direct solid-state reactions |
| Materials | Crystal System | Dim | Photo Yield (Photons/MeV × 103) | Lifetime | Peak Position (nm) | Resolution (lp/mm) | Detection Limit (nGy/s) | PLQY (%) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| (C6H13NH3)2PbI4 | Monoclinic orthorhombic | 2D | - | 3.8 ns | 558 | - | - | - | [199] |
| PEA2PbBr4 | Triclinic | 2D | 38.8 | 11.9 ns | 430 | - | - | - | [200] |
| C4H12NMnCl3 | Hexagonal | 1D | 50.5 | 758.95 μs | 635 | - | 24.2 | 91.8 | [159] |
| TPPen2MnBr4 | Monoclinic | 0D | 43 | 298.04 μs | 515 | 4.6 | 696.9 | 97.3 | [201] |
| TPPen2Mn0.9Zn0.1Br4 | Monoclinic | 0D | 68 | 296.34 μs | 515 | 11.2 | 204.1 | 97.7 | [201] |
| HTP2MnBr4 | Monoclinic | 0D | 38 | 318.11 μs | 520 | 17.3 | 130 | 98.66 | [202] |
| MTP2MnBr4 | Trigonal | 0D | 67 | 331 ms | 516 | 6.2 | 82.4 | 99.5 | [203] |
| (4BTP)2MnBr4 | Monoclinic | 0D | 98 | 292.31 μs | 524 | 21.3 | 37.4 | 96.26% | [198] |
| (TBA)CuCl2 | Monoclinic | 0D | 23.4 | 28.7 μs | 510 | - | 92.8 | [204] | |
| (BzTPP)2Cu2I4 | Monoclinic | 0D | 27.7 | 1.93 μs | 558 | 4.9 | 352 | 44.2 | [190] |
| (C8H20N)2Cu2Br4 | Monoclinic | 0D | 91.3 | 33.4 μs | 468 | 9.6 | 52.1 | 99.7 | [197] |
| (PPN)2SbCl5 | Monoclinic | 0D | 49 | 4.1 μs | 635 | - | 191.4 | - | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhu, H.; Sheng, M.; Shao, B.; He, Y.; Liu, Z.; Zhou, G. Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review. Crystals 2025, 15, 364. https://doi.org/10.3390/cryst15040364
Wang S, Zhu H, Sheng M, Shao B, He Y, Liu Z, Zhou G. Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review. Crystals. 2025; 15(4):364. https://doi.org/10.3390/cryst15040364
Chicago/Turabian StyleWang, Suqin, Hui Zhu, Ming Sheng, Bo Shao, Yu He, Zhuang Liu, and Guangtao Zhou. 2025. "Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review" Crystals 15, no. 4: 364. https://doi.org/10.3390/cryst15040364
APA StyleWang, S., Zhu, H., Sheng, M., Shao, B., He, Y., Liu, Z., & Zhou, G. (2025). Advances of Low-Dimensional Organic-Inorganic Hybrid Metal Halide Luminescent Materials: A Review. Crystals, 15(4), 364. https://doi.org/10.3390/cryst15040364






