Synthesis of MXene Composites Using Thiourea as a Nitrogen–Sulfur Precursor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
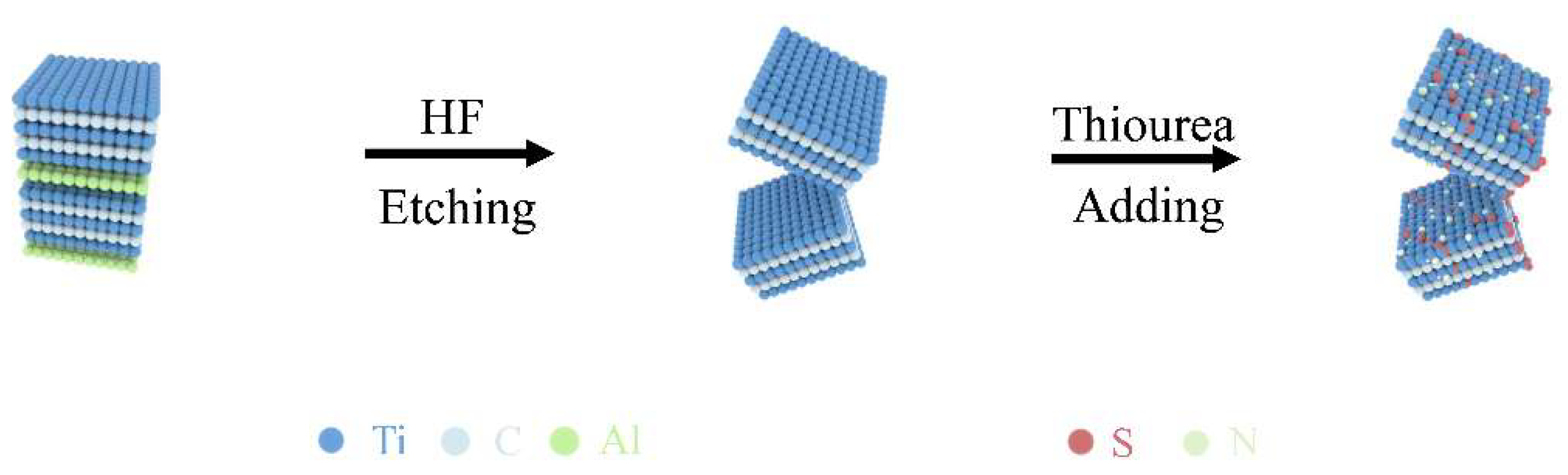
2.2. Preparation of MXene
2.3. Preparation of Thiourea-Ti3C2
2.4. Characterization
2.5. Electrode Preparation
2.6. Electrochemical Measurements
3. Results
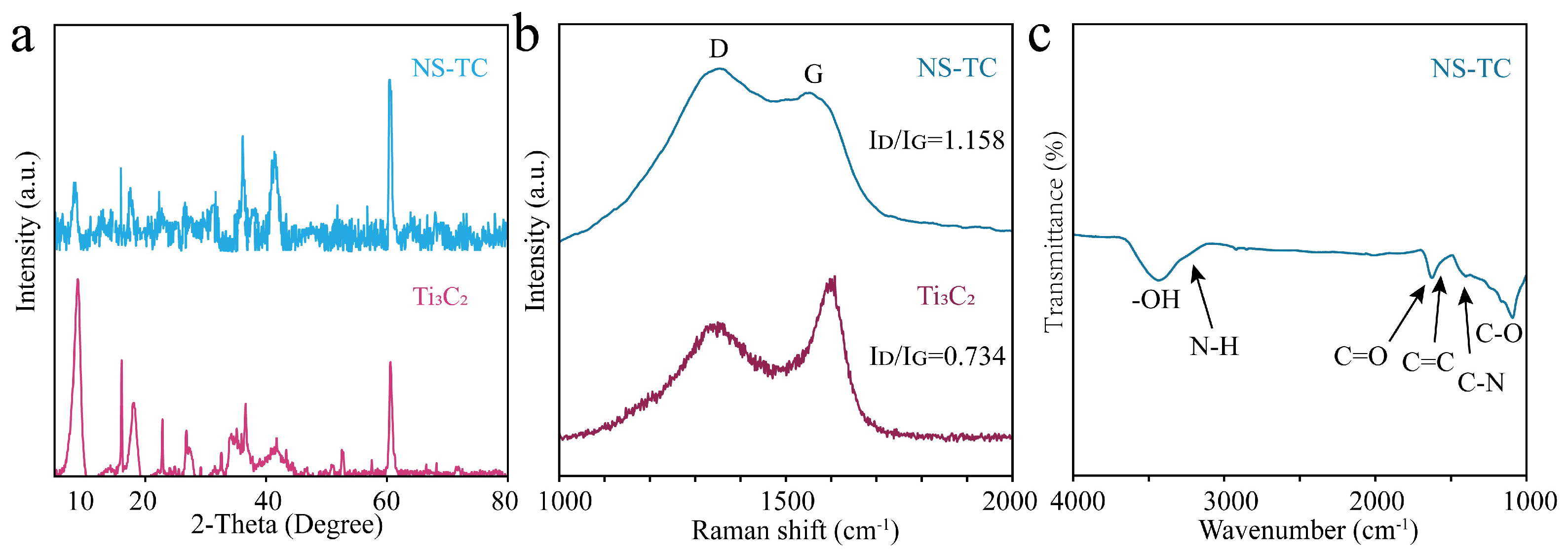
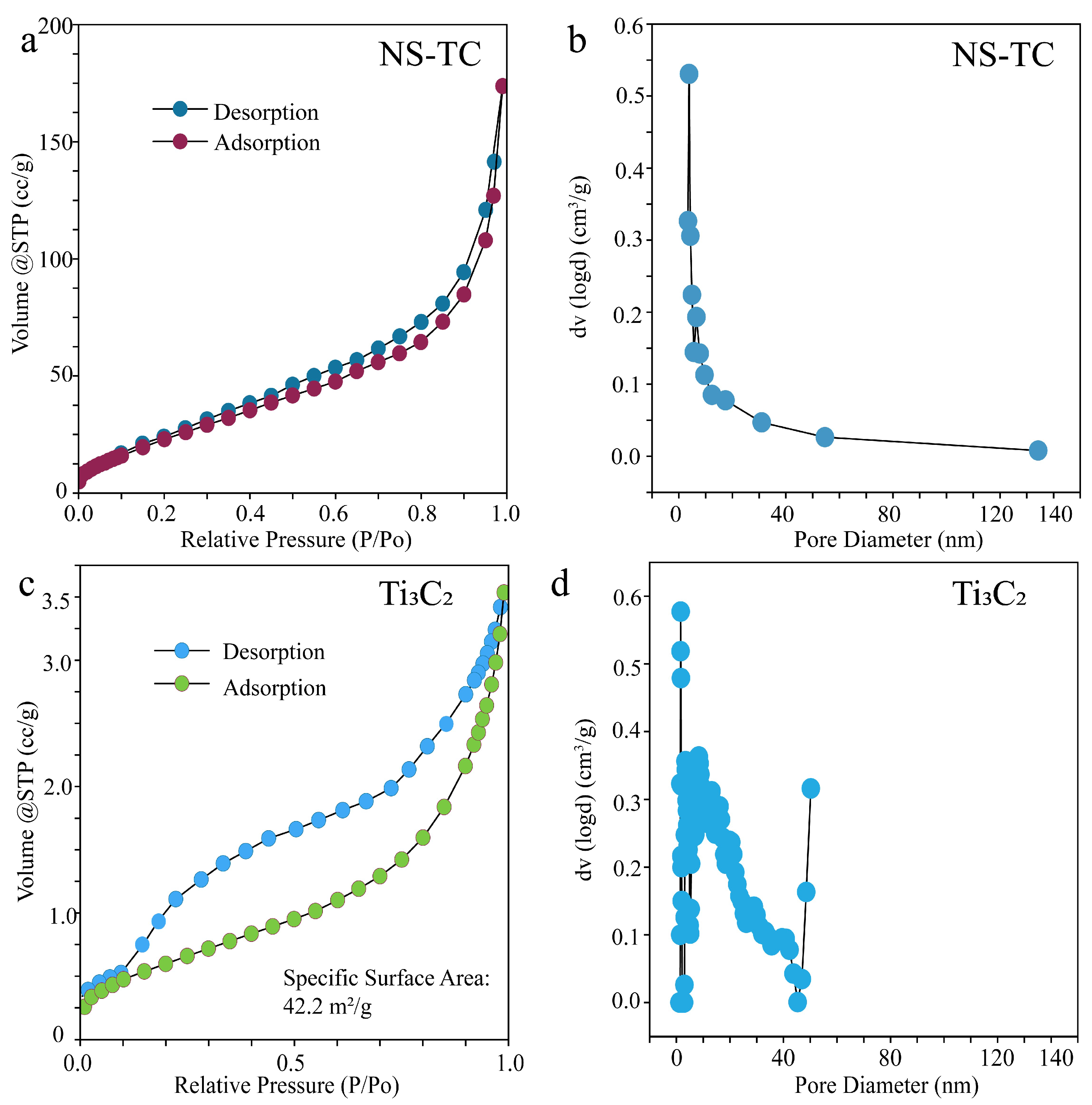
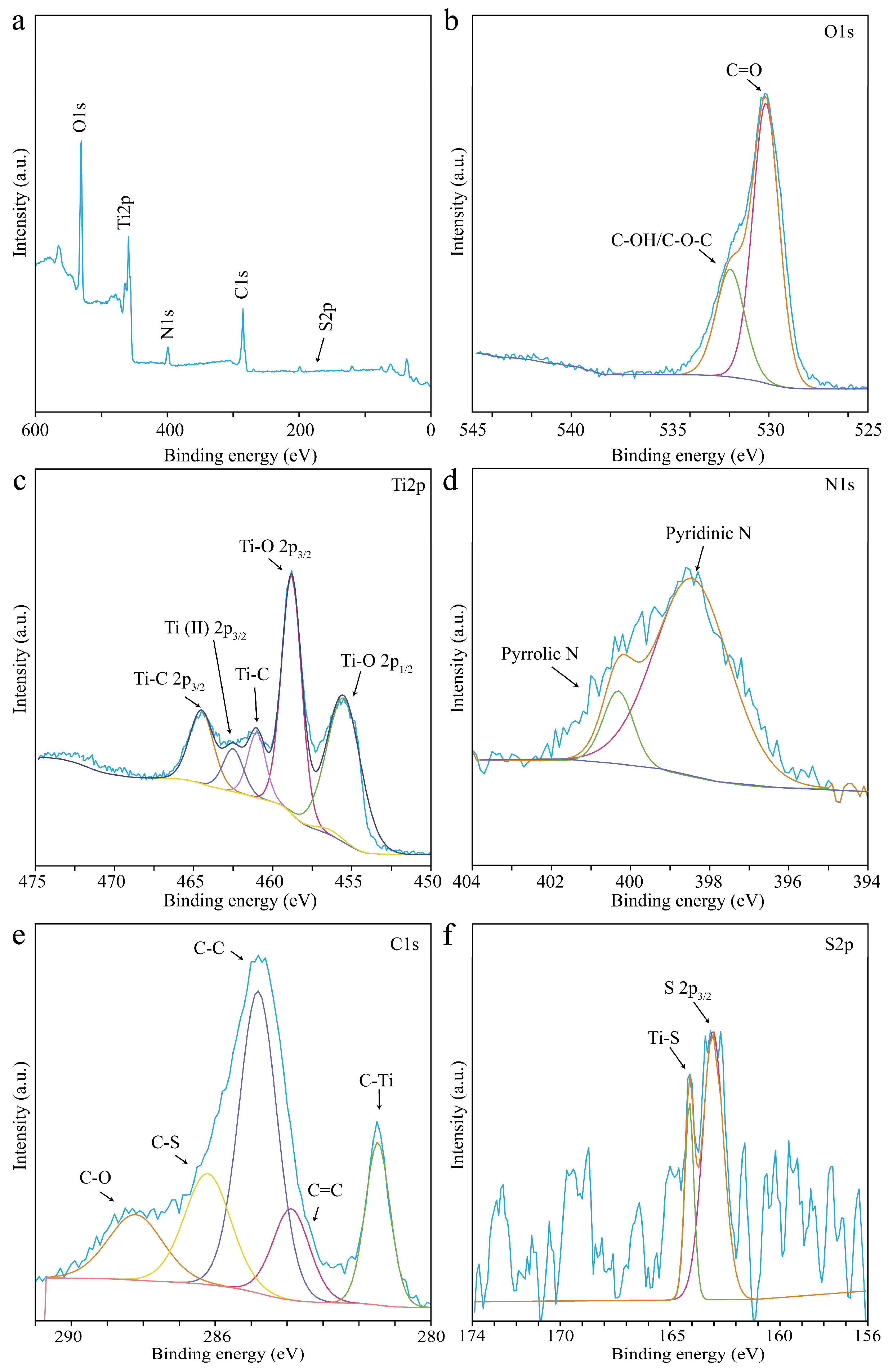
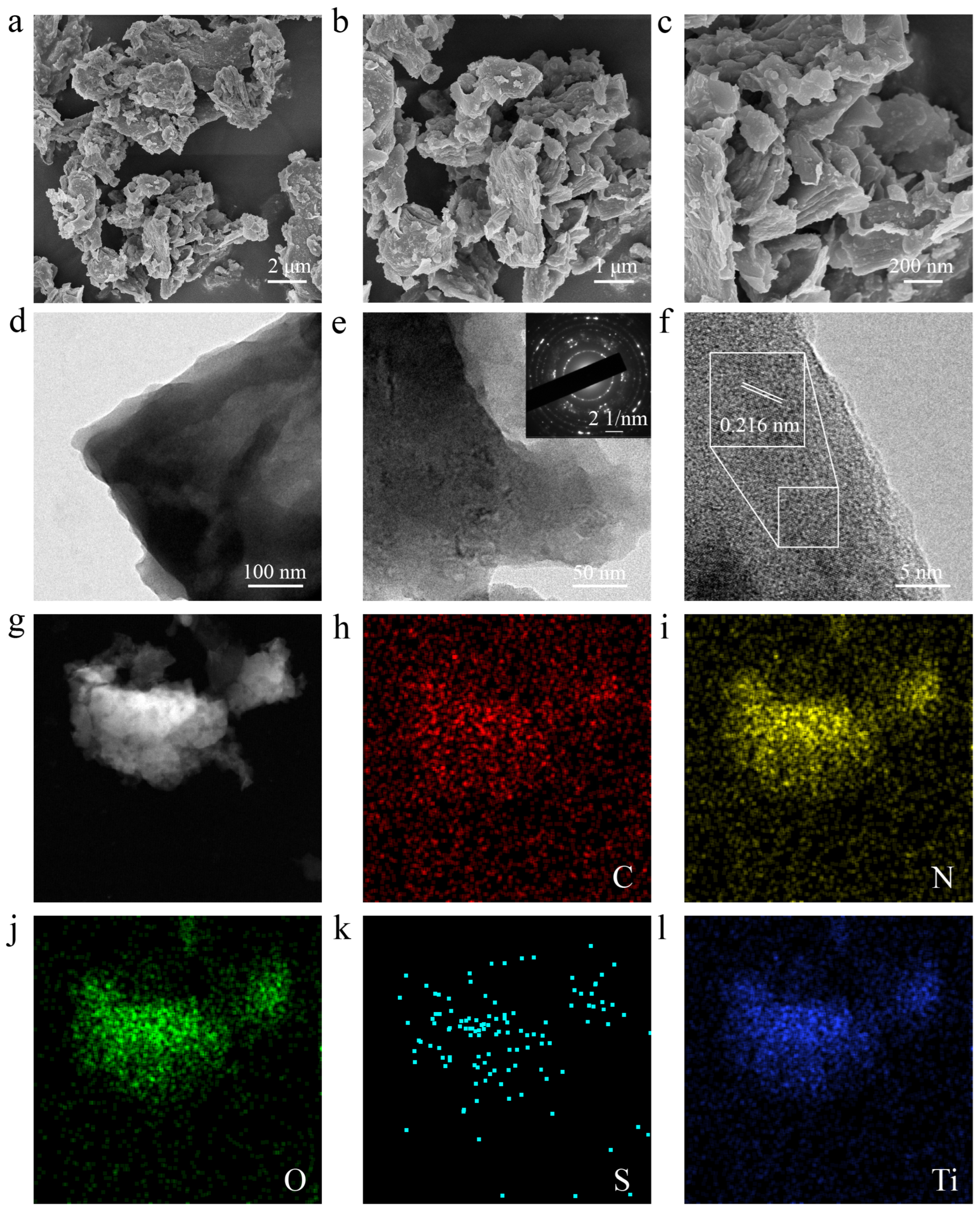
3.1. Structural and Morphology Characterization
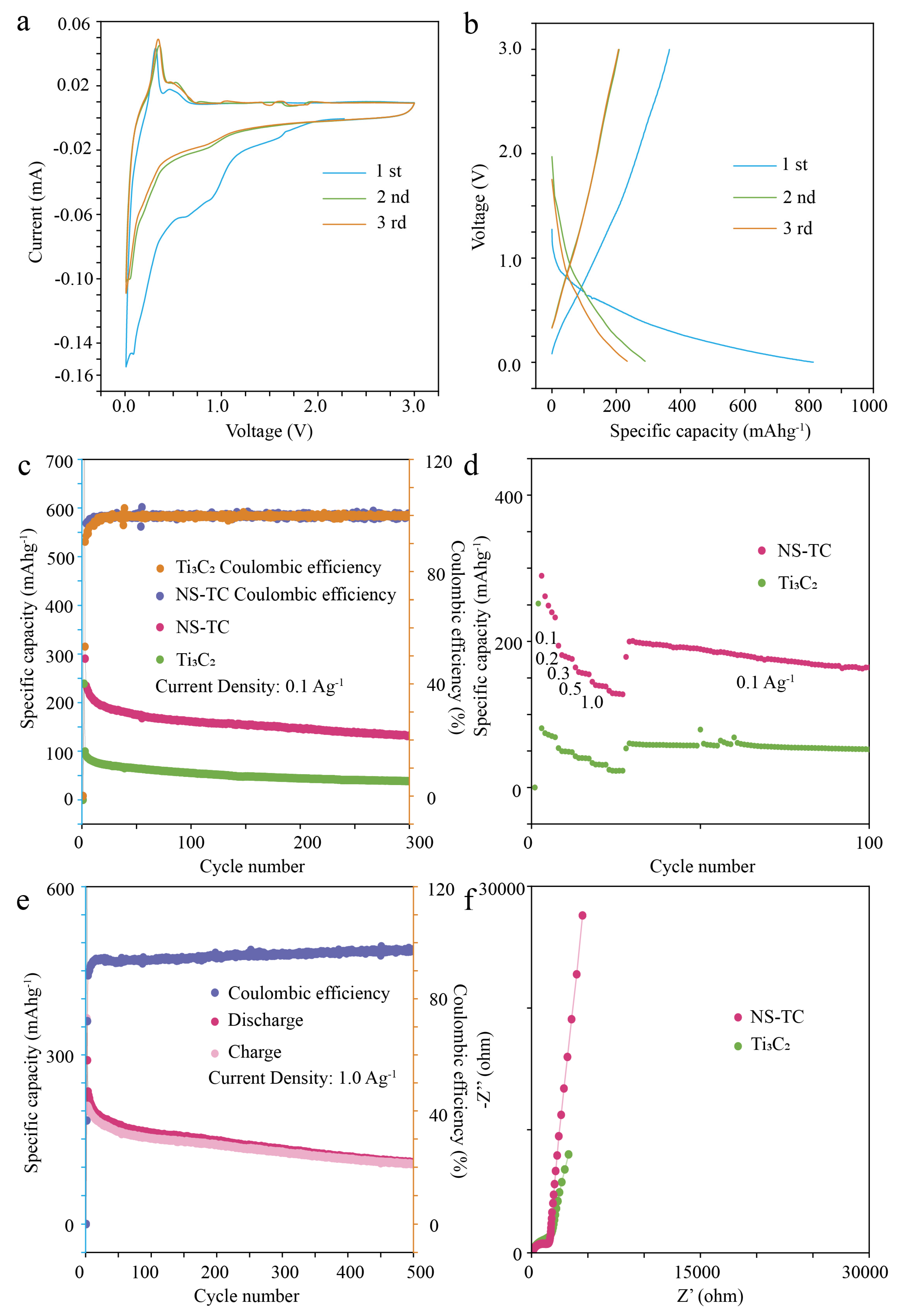
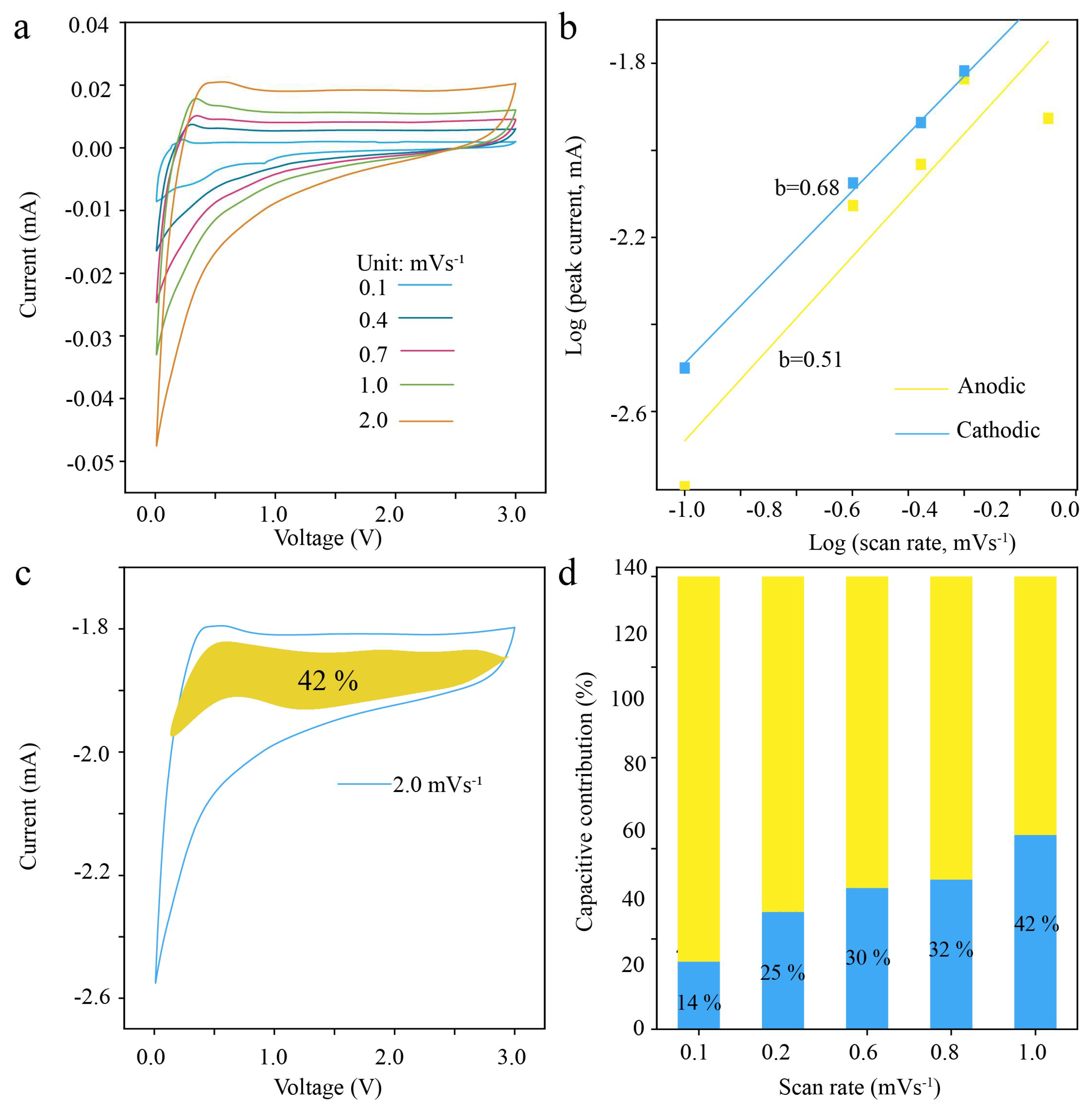
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NS-TC | Nitrogen–sulfur–Ti3C2 |
| NS-MXene | NS-MXene |
| PIBs | Potassium-ion batteries |
| PIB | Potassium-ion battery |
| 2D | Two-dimensional |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| XPS | X-ray photoelectron spectroscopy |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| SAED | Selected area electron diffraction |
| EDS | Energy-dispersive X-ray spectroscopy |
| AFM | Atomic force microscopy |
| CV | Cyclic voltammetry |
| SEI | Solid electrolyte interphase |
| EIS | Electrochemical impedance spectroscopy |
References
- Li, M.; Du, Z.; Khaleel, M.A.; Belharouak, I. Materials and engineering endeavors towards practical sodium-ion batteries. Energy Storage Mater. 2020, 25, 520–536. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, L.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H. Recent Advances in Sodium-Ion Battery Materials. Electrochem. Energy Rev. 2018, 1, 294–323. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 8, 1701785. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Liu, X.; Zhou, L.; Li, J.; Xiao, Y.; Peng, J.; Wang, J.; Chou, S.L. Long-Cycle-Life Cathode Materials for Sodium-Ion Batteries toward Large-Scale Energy Storage Systems. Adv. Energy Mater. 2023, 13, 2300149. [Google Scholar] [CrossRef]
- Bai, X.; Wu, N.; Yu, G.; Li, T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics 2023, 11, 289. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, X.; Tang, X.; Wu, Y.; Wang, Y.; Man, Z.; Qi, W.; Wang, J.; Lv, Z. A revolutionary design concept: Full-sealed lithium-oxygen batteries. Nano Energy 2024, 123, 109405. [Google Scholar] [CrossRef]
- Miyakawa, S.; Goto, M.; Ono, M.; Saito, T.; Yamaguchi, S.; Matsuda, S. Metal-Coated Polymer Fiber Mesh as an Ultralightweight Gas-Diffusible Current Collector for High-Energy-Density Rechargeable Lithium–Oxygen Batteries. ACS Appl. Energy Mater. 2023, 6, 1906–1912. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, X.; Gao, Y.; Yi, X.; Lou, G.; Wen, Z. Study on evolution process and electrochemical behavior of porous cathode in discharge process of the solid-state lithium-oxygen battery. J. Energy Storage 2023, 74, 109315. [Google Scholar] [CrossRef]
- Krammer, M.; Schmid, A.; Kubicek, M.; Fleig, J. Utilizing oxygen gas storage in rechargeable oxygen ion batteries. J. Power Sources 2023, 577, 233167. [Google Scholar] [CrossRef]
- Liu, J.; Shi, R.; Lang, X.; Wang, T.; Qu, T.; Li, L.; Yao, C.; Lai, Q.; Cai, K. Construction of a novel high electrochemical performance nanosheet Co3O4@Fe2O3 bifunctional catalytic material for lithium-oxygen batteries. Electrochim. Acta 2024, 474, 143569. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, S.; Yu, H.; Nie, M.; Feng, X.; Zuo, X.; Fu, L.; Liu, D.; Zhang, Y. CeO2 Supported on Reduced Graphene Oxide as Li-O2 Battery Cathode. Chem. Res. Chin. Univ. 2023, 39, 636–641. [Google Scholar] [CrossRef]
- Wang, B.; Liu, C.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. Defect-induced deposition of manganese oxides on hierarchical carbon nanocages for high-performance lithium-oxygen batteries. Nano Res. 2022, 15, 4132–4136. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. Two-Dimensional Materials: 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 982. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. In MXenes; Jenny Stanford Publishing: Singapore, 2023; pp. 677–722. [Google Scholar]
- Alli, Y.A.; Bamisaye, A.; Nancy, P.; Zachariah, S.M.; Oladoye, P.O.; Bankole, O.M.; Akamo, D.O.; Chkirida, S.; Anuar, H.; Thomas, S. MXene composites: Properties, synthesis and its emerging application in rechargeable batteries. J. Energy Storage 2024, 77, 109954. [Google Scholar] [CrossRef]
- Cao, J.-M.; Zatovsky, I.V.; Gu, Z.-Y.; Yang, J.-L.; Zhao, X.-X.; Guo, J.-Z.; Xu, H.; Wu, X.-L. Two-dimensional MXene with multidimensional carbonaceous matrix: A platform for general-purpose functional materials. Prog. Mater. Sci. 2023, 135, 101105. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. MXenes: Trends, growth, and future directions. Graphene 2D Mater. 2022, 7, 75–79. [Google Scholar] [CrossRef]
- Meng, W.; Liu, X.; Song, H.; Xie, Y.; Shi, X.; Dargusch, M.; Chen, Z.-G.; Tang, Z.; Lu, S. Advances and challenges in 2D MXenes: From structures to energy storage and conversions. Nano Today 2021, 40, 101273. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Amine-assisted delamination of Nb2C MXene for Li-ion energy storage devices. In MXenes; Jenny Stanford Publishing: Singapore, 2023; pp. 401–414. [Google Scholar]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Û.G. 2D Metal Carbides and Nitrides (MXenes); Springer: Berlin/Heidelberg, Germany, 2019; Volume 2549. [Google Scholar]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Q.; Miao, J.; Zhang, P.; Wan, P.; He, L.; Xu, B. Flexible 3D porous MXene foam for high-performance lithium-ion batteries. Small 2019, 15, 1904293. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Lamiel, C.; Javed, M.S.; Ahmad, M.; Sahoo, S.; Chen, X.; Qin, N.; Iqbal, S.; Gu, S.; Li, Y. MXene-based heterostructures: Current trend and development in electrochemical energy storage devices. Prog. Energy Combust. Sci. 2023, 97, 101097. [Google Scholar] [CrossRef]
- Bashir, T.; Zhou, S.; Yang, S.; Ismail, S.A.; Ali, T.; Wang, H.; Zhao, J.; Gao, L. Progress in 3D-MXene electrodes for lithium/sodium/potassium/magnesium/zinc/aluminum-ion batteries. Electrochem. Energy Rev. 2023, 6, 5. [Google Scholar] [CrossRef]
- Huang, W.; Ma, Z.; Zhong, L.; Luo, K.; Li, W.; Zhong, S.; Yan, D. Efficient Self-Assembly Preparation of 3D Carbon-Supported Ti3C2Tx Hollow Spheres for High-Performance Potassium Ion Batteries. Small 2024, 20, 2304690. [Google Scholar] [CrossRef]
- Shi, H.; Dong, Y.; Zheng, S.; Dong, C.; Wu, Z.-S. Three dimensional Ti3C2 MXene nanoribbon frameworks with uniform potassiophilic sites for the dendrite-free potassium metal anodes. Nanoscale Adv. 2020, 2, 4212–4219. [Google Scholar] [CrossRef]
- Bärmann, P.; Haneke, L.; Wrogemann, J.M.; Winter, M.; Guillon, O.; Placke, T.; Gonzalez-Julian, J. Scalable synthesis of MAX phase precursors toward titanium-based MXenes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 26074–26083. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, K.; Sudharshan, V.; Subramani, K.; Sathish, M. Insights into 2D/2D MXene heterostructures for improved synergy in structure toward next-generation supercapacitors: A review. Adv. Funct. Mater. 2022, 32, 2110267. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for sensing applications: Recent progress, design principles, and future perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef]
- Kumar, J.A.; Prakash, P.; Krithiga, T.; Amarnath, D.J.; Premkumar, J.; Rajamohan, N.; Vasseghian, Y.; Saravanan, P.; Rajasimman, M. Methods of synthesis, characteristics, and environmental applications of MXene: A comprehensive review. Chemosphere 2022, 286, 131607. [Google Scholar] [CrossRef]
- Oh, H.G.; Yang, S.H.; Kang, Y.C.; Park, S.-K. N-doped carbon-coated CoSe2 nanocrystals anchored on two-dimensional MXene nanosheets for efficient electrochemical sodium- and potassium-ion storage. Int. J. Energy Res. 2021, 45, 17738–17748. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Warner, J.H.; Zhang, K. Beyond Ti-based MXenes: A review of emerging non-Ti based metal-MXene structure, properties, and applications. Mater. Today 2023, 63, 313–338. [Google Scholar] [CrossRef]
- Song, P.; Liu, B.; Qiu, H.; Shi, X.; Cao, D.; Gu, J. MXenes for polymer matrix electromagnetic interference shielding composites: A review. Compos. Commun. 2021, 24, 100653. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Q.; Guan, X.; Liu, M.; Wang, F.; Li, R.; Xu, J. Ionically conductive self-healing polymer binders with poly (ether-thioureas) segments for high-performance silicon anodes in lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4934–4944. [Google Scholar] [CrossRef]
- Xiong, P.; Yin, H.; Chen, Z.; Zhao, C.; Yang, J.; Huang, S.; Xu, Y. Thiourea-based polyimide/RGO composite cathode: A comprehensive study of storage mechanism with alkali metal ions. Sci. China Mater. 2020, 63, 1929–1938. [Google Scholar] [CrossRef]
- Feng, J.; Liu, W.; Shi, C.; Zhang, C.; Zhao, X.; Wang, T.; Chen, S.; Li, Q.; Song, J. Enabling fast diffusion/conversion kinetics by thiourea-induced wrinkled N, S co-doped functional MXene for lithium-sulfur battery. Energy Storage Mater. 2024, 67, 103328. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef]
- Tao, M.; Du, G.; Zhang, Y.; Gao, W.; Liu, D.; Luo, Y.; Jiang, J.; Bao, S.; Xu, M. TiOxNy nanoparticles/C composites derived from MXene as anode material for potassium-ion batteries. Chem. Eng. J. 2019, 369, 828–833. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Feng, Y.; Peng, J.; Wu, K.; Feng, Z.; He, M.; Wen, K.; Xiong, D. Synthesis of MXene Composites Using Thiourea as a Nitrogen–Sulfur Precursor. Crystals 2025, 15, 353. https://doi.org/10.3390/cryst15040353
Zhang J, Feng Y, Peng J, Wu K, Feng Z, He M, Wen K, Xiong D. Synthesis of MXene Composites Using Thiourea as a Nitrogen–Sulfur Precursor. Crystals. 2025; 15(4):353. https://doi.org/10.3390/cryst15040353
Chicago/Turabian StyleZhang, Junming, Yefeng Feng, Junhao Peng, Kaidan Wu, Zuyong Feng, Miao He, Kunhua Wen, and Deping Xiong. 2025. "Synthesis of MXene Composites Using Thiourea as a Nitrogen–Sulfur Precursor" Crystals 15, no. 4: 353. https://doi.org/10.3390/cryst15040353
APA StyleZhang, J., Feng, Y., Peng, J., Wu, K., Feng, Z., He, M., Wen, K., & Xiong, D. (2025). Synthesis of MXene Composites Using Thiourea as a Nitrogen–Sulfur Precursor. Crystals, 15(4), 353. https://doi.org/10.3390/cryst15040353






