Study on the Chromogenic Mechanism of Blue Kyanite from Coronel Murta, Minas Gerais, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. X-Rite SP62
2.3. CIE 1976 L*a*b* Uniform Color Space
2.4. X-Ray Fluorescence Spectrometer
2.5. UV–Vis Spectrum
2.6. Fourier-Transform Infrared Spectroscopy
3. Results
3.1. Gemological Characteristics
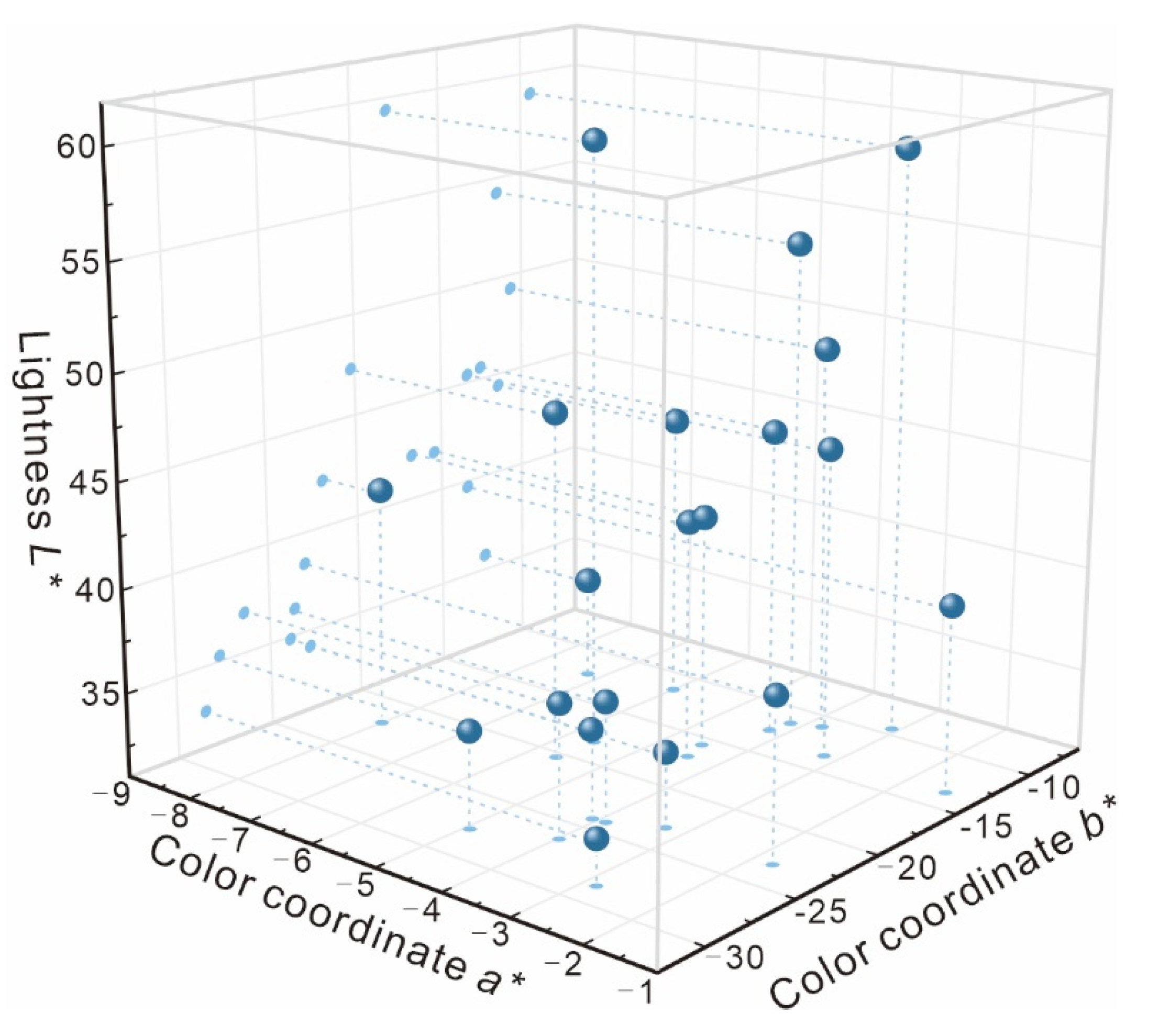
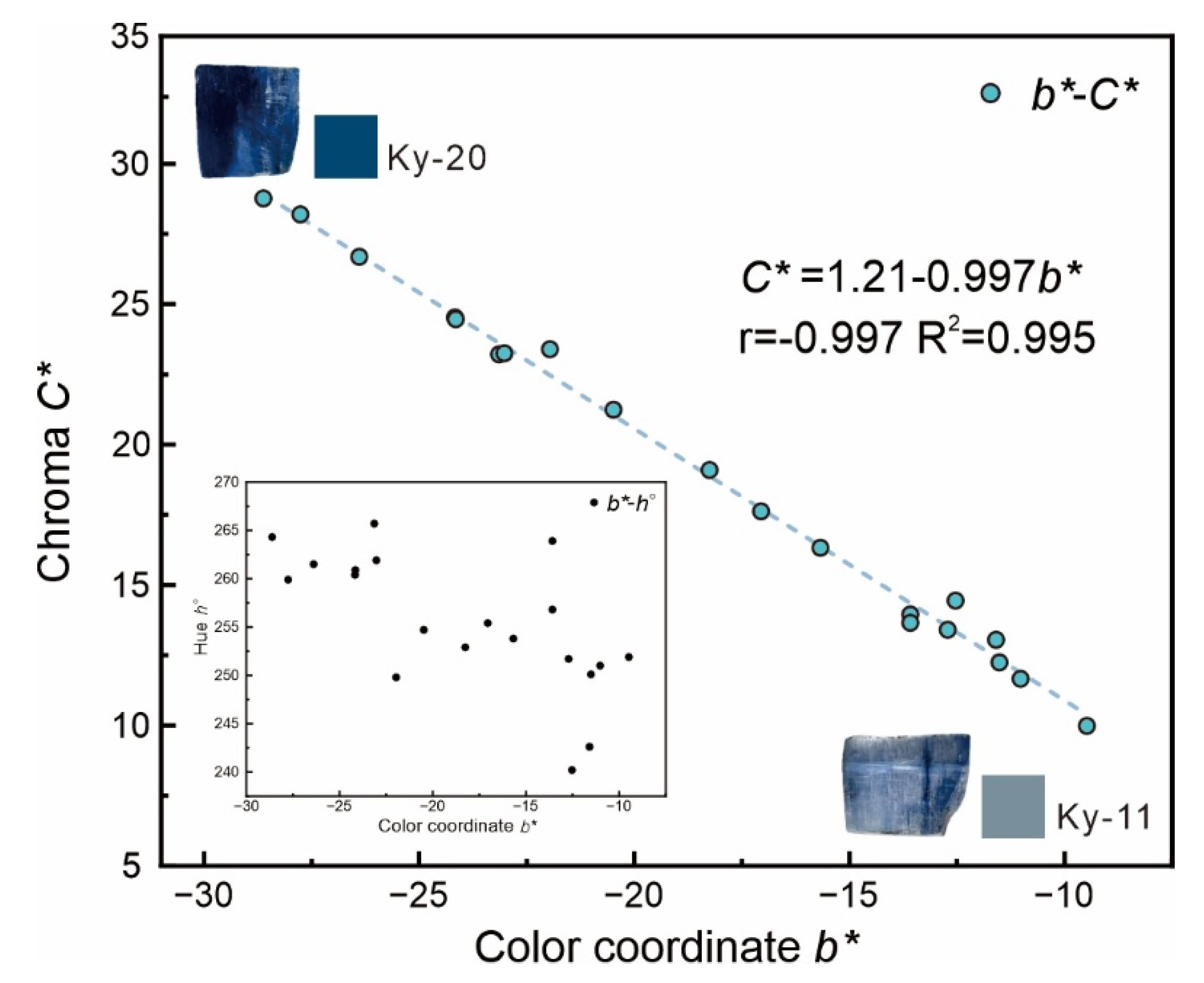
3.2. Color Analysis
3.3. Chemical Analysis
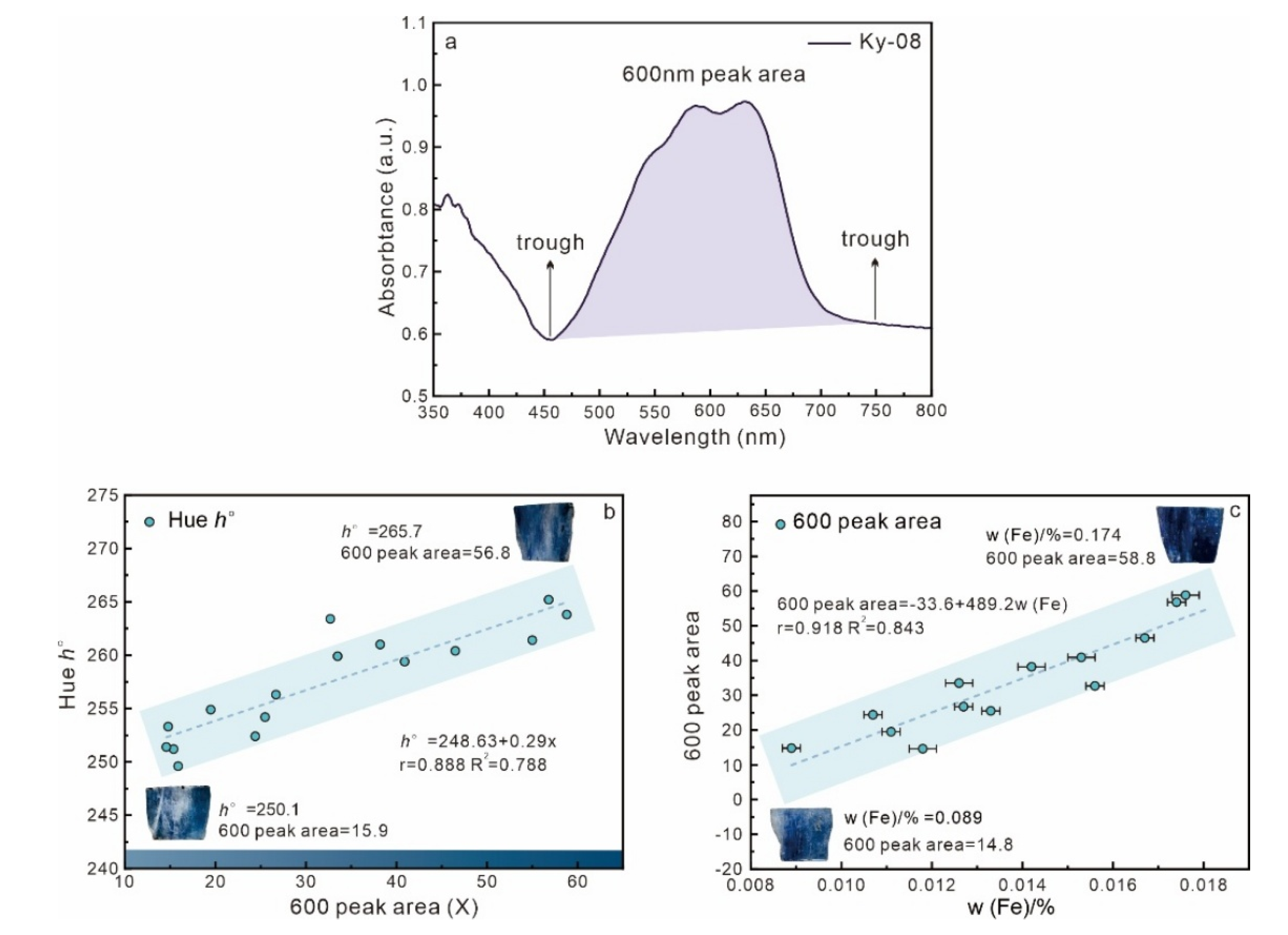
3.4. UV–Vis Spectrum Analysis
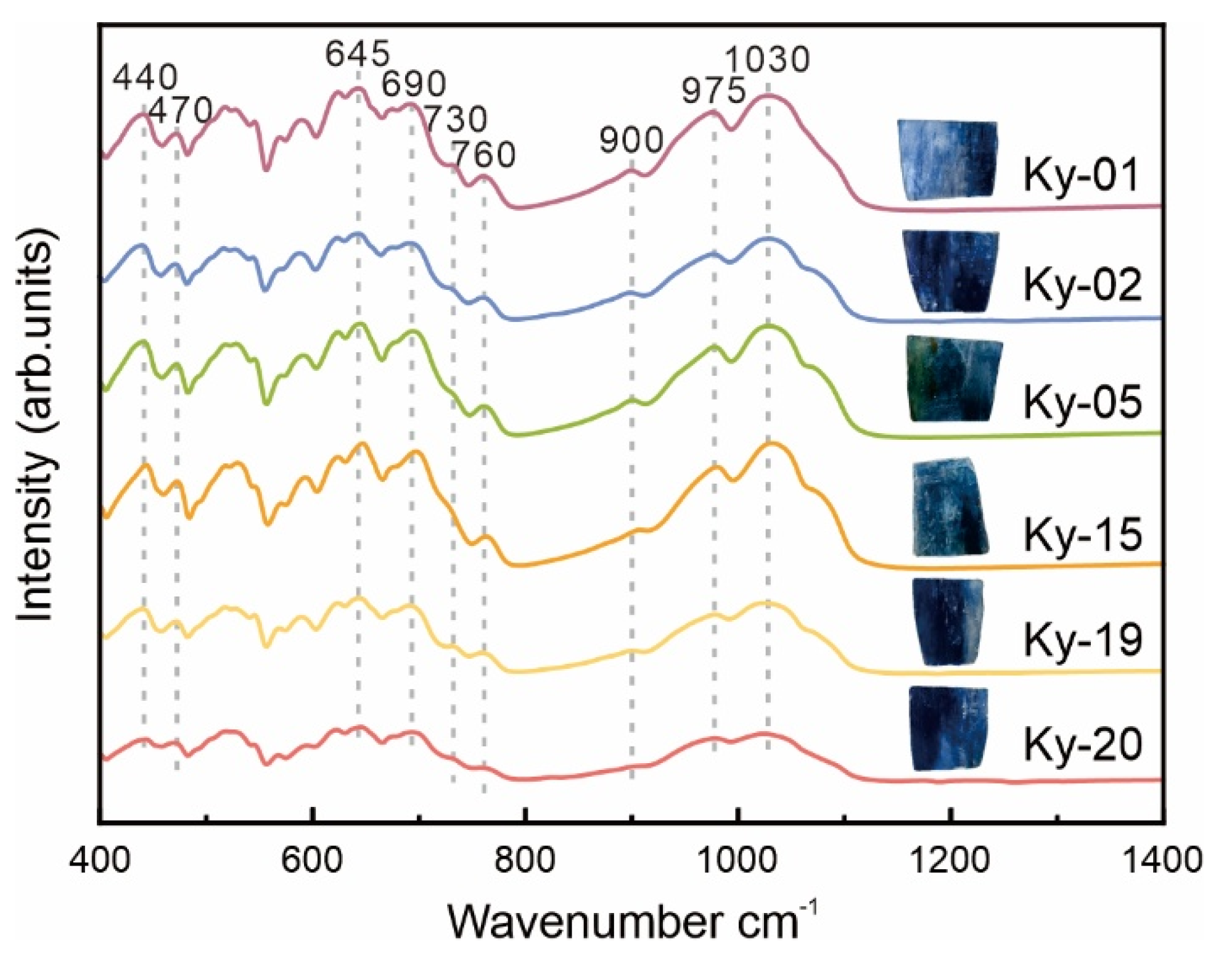
3.5. Infrared Spectral
4. Discussion
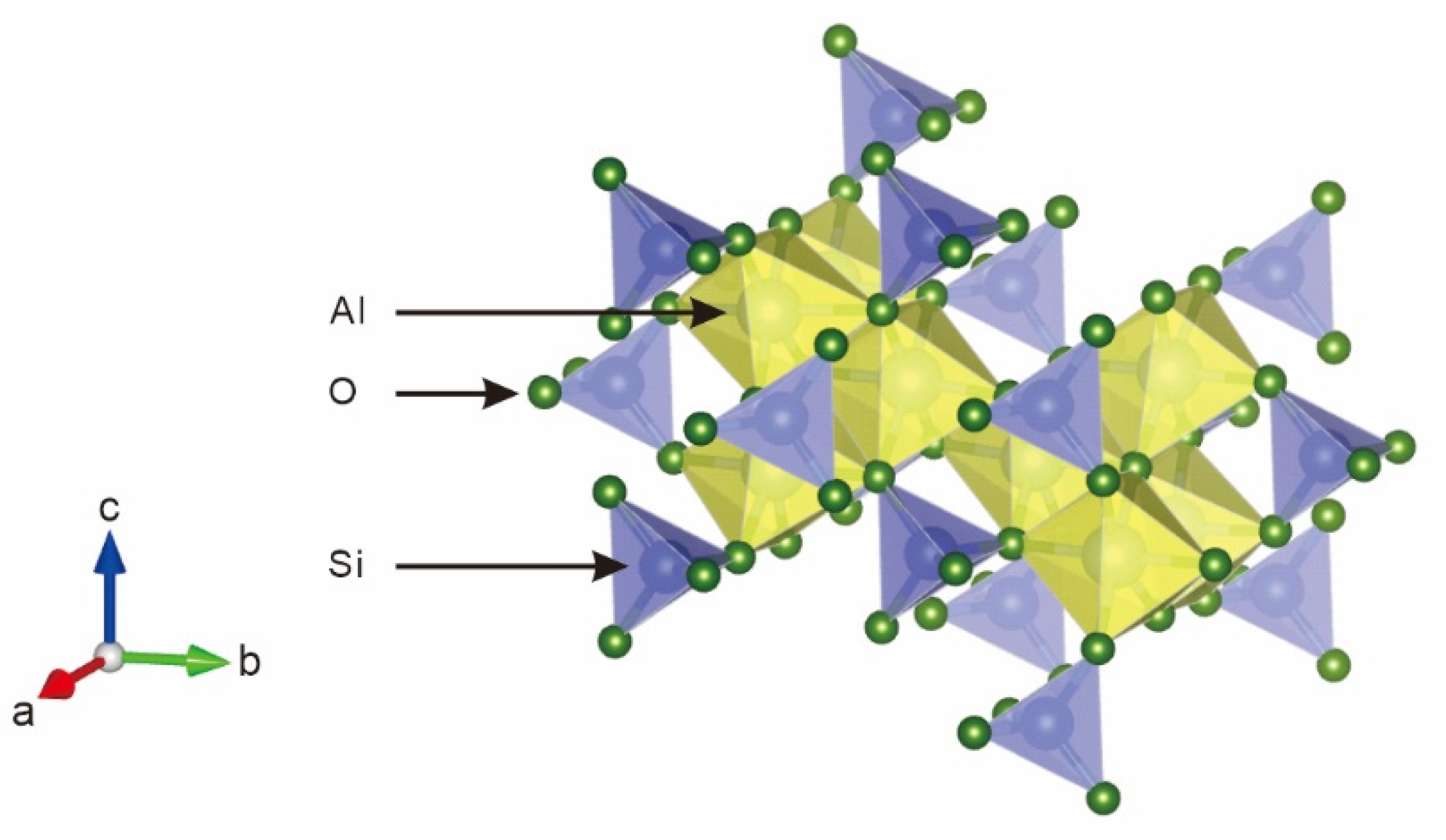
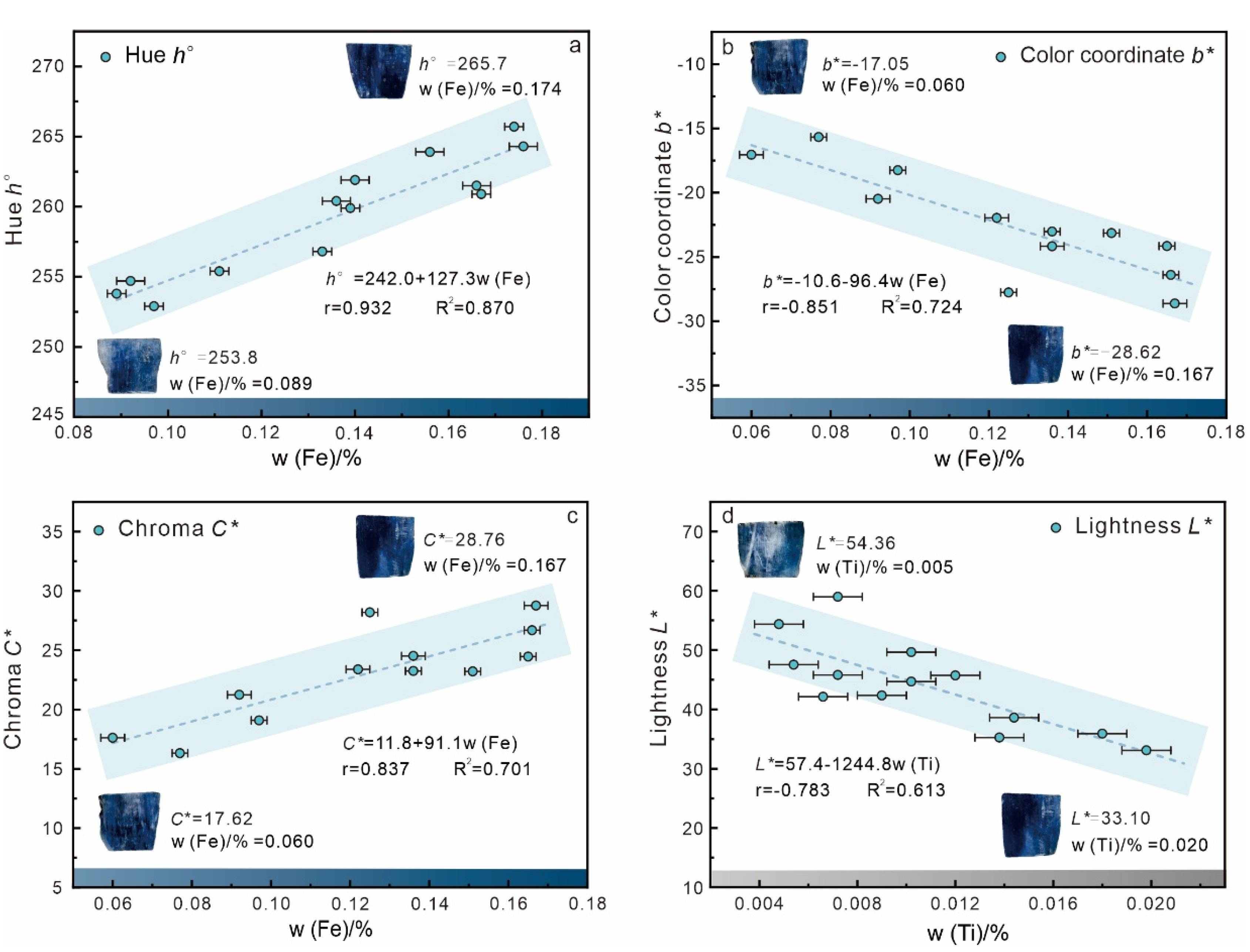
4.1. Relationship Between Composition and Color
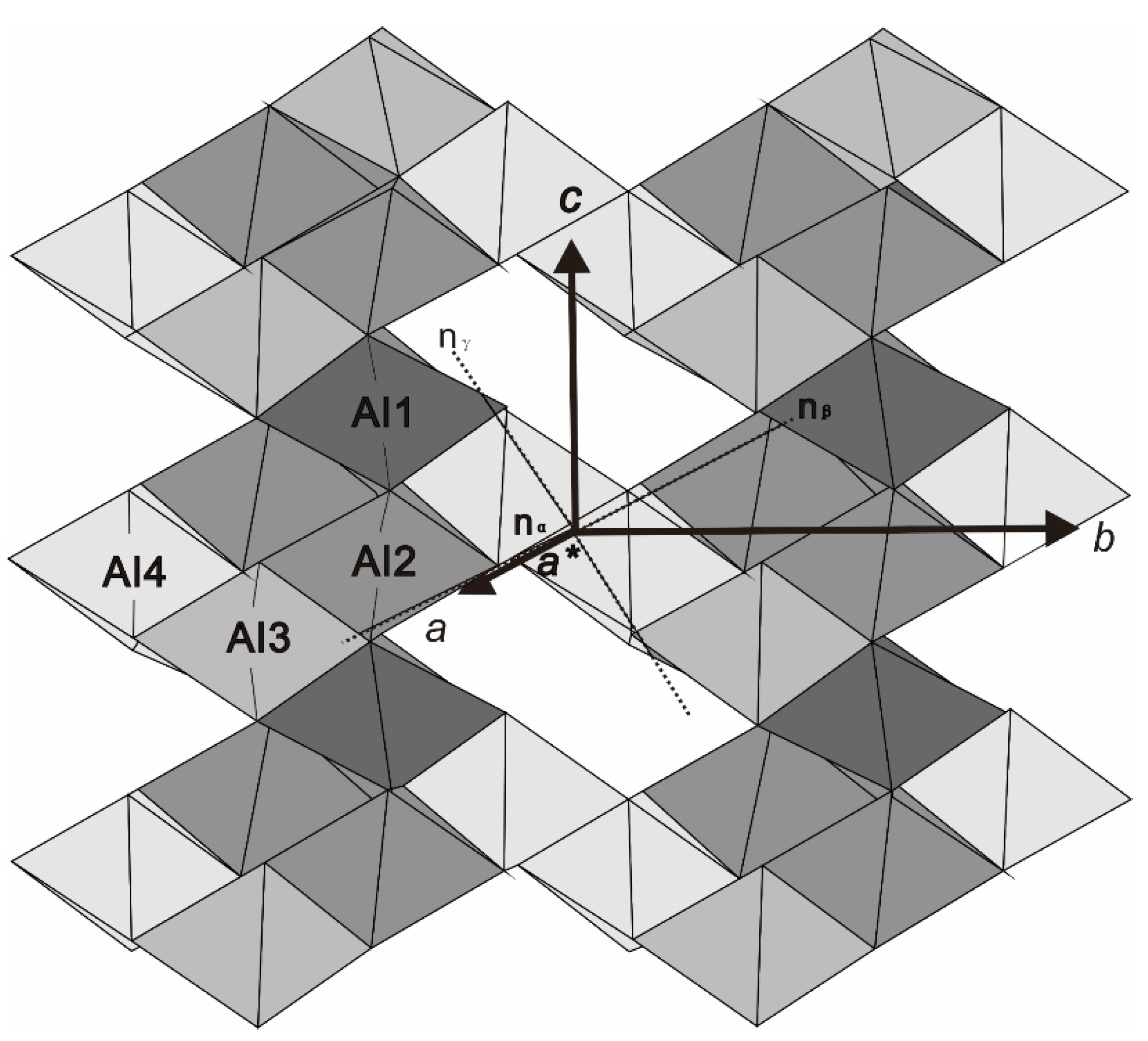
4.2. Causes of the Color of Blue Kyanite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, Y. Geological characteristics and genetic analysis of Tongbai kyanite deposit in Henan province. Sichuan Build. Mater. 2011, 37, 175–176. [Google Scholar]
- Chadwick, K.M.; Rossman, G.R. Orange kyanite from Tanzania. Gems Gemol. 2009, 45, 146–147. [Google Scholar]
- Zhang, J.; Hao, Y.; Xuan, Z. Study on crystal chemistry characteristics and flotation behavior of kyanite mineral. China Min. Mag. 2016, 25, 139–143. [Google Scholar]
- Jin, J.; Gao, H.; Ren, Z.; Chen, Z. The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector. Minerals 2016, 6, 90. [Google Scholar] [CrossRef]
- Wildner, M.; Beran, A.; Koller, F. Spectroscopic characterisation and crystal field calculations of varicoloured kyanites from Loliondo, Tanzania. Mineral. Petrol. 2012, 107, 289–310. [Google Scholar] [CrossRef]
- Pradat, T.; Choudhary, G. Gem-quality Cr-rich kyanite from India. Gems Gemol. 2014, 50, 87–88. [Google Scholar]
- Platonov, A.N.; Tarashchan, A.N.; Langer, K.; Andrut, M.; Partzsch, G.; Matsyuk, S.S. Electronic absorption and luminescence spectroscopic studies of kyanite single crystals: Differentiation between excitation of FeTi charge transfer and Cr3+ dd transitions. Phys. Chem. Miner. 1998, 25, 203–212. [Google Scholar]
- Qiu, L.; Guo, Y.; Yuan, B.; Su, Y.-S.; Qi, Y.-H. Chromatographic Study of Blue-Violet Tanzanite’s Color Appearance. Sci. Adv. Mater. 2022, 14, 1032–1040. [Google Scholar] [CrossRef]
- Deng, S.; Guo, Y. Investigation of Factors Affecting the Color of Serpentine Jade from Shandong, China. Crystals 2023, 13, 431. [Google Scholar] [CrossRef]
- Yuan, B.; Guo, Y.; Chen, J.; Liu, Z. Study of Natural and Synthetic Yellow–Orange Sapphires Based on the CIE 1976 L*a*b* Uniform Colour Space. Sci. Adv. Mater. 2022, 14, 1570–1581. [Google Scholar] [CrossRef]
- Kirillova, N.P.; Vodyanitskii, Y.N.; Sileva, T.M. Conversion of soil color parameters from the Munsell system to the CIE-L*a*b* system. Eurasian Soil Sci. 2015, 48, 468–475. [Google Scholar]
- Araujo, N.J.F.; Barreto, S.B.; Carrino, T.A.; Souza, I.; Santos, G.L. Geochemical and reflectance spectroscopy data integration to characterize emerald deposits: The case of the Parana deposit, Brazil. Acad. Bras. Cienc. 2021, 93, e20200236. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Marsman, M.; Wagenmakers, E.J. Analytic posteriors for Pearson’s correlation coefficient. Stat. Neerl. 2017, 72, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guo, Y.; Tang, J.; Yang, Y. Spectroscopy Characteristics and Color-Influencing Factors of Green Iron-Bearing Elbaite. Crystals 2023, 13, 1461. [Google Scholar] [CrossRef]
- Fiamegos, Y.; Calle, G.; Maria, B. Validation strategy for an ED-XRF method to determine trace elements in a wide range of organic and inorganic matrices based on fulfilment of performance criteria. Spectrochim. Acta Part B At. Spectrosc. 2018, 150, 59–66. [Google Scholar] [CrossRef]
- Phillips, S.E.; Argles, T.W.; Warren, C.J.; Harris, N.B.W.; Kunz, B.E. Kyanite petrogenesis in migmatites: Resolving melting and metamorphic signatures. Contrib. Mineral. Petrol. 2023, 178, 10. [Google Scholar] [CrossRef]
- Deng, M.; Liao, C.; Wang, X.; Chen, S.; Qi, F.; Zhao, X.; Yu, P. A paper-based colorimetric microfluidic sensor fabricated by a novel spray painting prototyping process for iron analysis. Can. J. Chem. 2019, 97, 373–377. [Google Scholar] [CrossRef]
- Dong, J.; Huang, F.; Wang, D. Geochemical characteristics and geological significance of beryl in different types of berylium deposits. Acta Petrol. Sin. 2023, 39, 2153–2166. [Google Scholar] [CrossRef]
- Harte, B. Kyanite and Cordierite-Andalusite occurrences in parts of Barrow’s Staurolite zones—The effects of high ferric iron. J. Metamorph. Geol. 2024, 42, 1131–1158. [Google Scholar] [CrossRef]
- Filiz, G. Study of Geobarometers in Pelitic Schists from The Pütürge Metamorphites. Earth Environ. Sci. 2016, 44, 042010. [Google Scholar] [CrossRef]
- O’Bannon, E.F., III; Williams, Q. High-pressure Cr3+ R-line luminescence of zoisite andkyanite: A probe ofoctahedral site distortion. Phys. Chem. Miner. 2022, 49, 35. [Google Scholar]
- Ruihua, W.; Qionglin, L. Study on the role of Fe3+ in sapphire. J. Chang. Univ. Sci. Technol. 2000, 30, 38–41. [Google Scholar]
- Niamjan, N.; T-Thienprasert, J.; Kim, H.-S.; Na-Phattalung, S. Intervalence charge transfer of Ti and Fe defects in blue kyanite. J. Korean Phys. Soc. 2021, 78, 671–678. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; Liu, Y. Gem characteristics and color genesis of orange kyanite. China Sci. 2018, 13, 2442–2446. [Google Scholar]
- Guo, K.; Zhou, Z.; Zhong, Q.; Lai, M.; Wang, H.; Li, Y.; Qiao, X.; Nong, P. A comparative study of element content and UV-Vis spectroscopy characteristics of rubies from Burma and Mozambique. Acta Petrol. Sin. 2018, 37, 1002–1010. [Google Scholar]
- Chen, X. Gemological Properties and Color Mechanism of Green Kyanite. Master’s Thesis, University of Geosciences, Beijing, China, 2019; pp. 31–50. [Google Scholar]
- Blereau, E.; Piazolo, S.; Trimby, P.; Skrzypek, E. Kyanite microstructural and microchemical characteristics reveal differences in growth, deformation and chemical modification: A case study from the Paleoproterozoic suture zone of South Harris, NW Scotland. Lithos 2024, 484–485, 107748. [Google Scholar] [CrossRef]
- Taran, M.N. Electronic intervalence Fe2+ + Ti4+ → Fe3+ + Ti3+ charge-transfer transition in ilmenite. Phys. Chem. Miner. 2019, 46, 839–843. [Google Scholar] [CrossRef]
- Kalita, J.M.; Wary, G. Trap spectroscopy and dosimetric aspects of natural kyanite mineral. Appl. Phys. A Mater. Sci. Process. 2015, 119, 1555–1560. [Google Scholar]
- Xiaolian, H. The chromatics of Fe and Ti in Shandong Changle blue sapphire. J. Ceram. 2018, 46, 1483–1488. [Google Scholar] [CrossRef]
- Gao, J.; Wu, W.; Jia, L.; Wang, C.P.; Liu, Y.; Xu, C.; Chen, F.; Fei, C.; Su, W. Raman and infrared spectra to monitor the phase transition of natural kyanite under static compression. J. Raman Spectrosc. 2020, 51, 2102–2111. [Google Scholar] [CrossRef]
- Xunhai, M. Study on color genesis of Hanshan cyanite in Jiangsu Province. J. Xi ’an Min. Inst. 1991, 9, 34–37. [Google Scholar]
- Somakumar, A.K.; Bulyk, L.-l.; Tsiumra, V.; Barzowska, J.; Xiong, P.; Lysak, A.; Zhydachevskyy, Y.; Suchocki, A. High-Pressure Near-Infrared Luminescence Studies of Fe3+-ActivatediGaO2. Inorg. Chem. 2023, 62, 12434–12444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Ding, H.; Lu, A.; Li, Y.; Ji, X.; Zhang, Y.; Wang, C. Vibrational and structural insight into silicate minerals by mid-infrared absorption and emission spectroscopies. Phys. Chem. Miner. 2022, 49, 6. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Stojanceska, S. Minerals from Macedonia XIII: Vibrational spectra of some commonly appearing nesosilicate minerals. J. Mol. Struct. 2005, 744, 79–92. [Google Scholar]
- Keqin, W.; Mingkai, L.; Guozhi, Y.; Huimin, F. Study on the typomorphic characteristics of kyanite in the kyanite deposits in the Yu and Su areas. Geol. Prospect. Agent 1994, 30, 37–42. [Google Scholar]
- Frost, R.L.; Xi, Y.; Scholz, R.; Lima, R.M.F.; Horta, L.F.C.; Lopez, A. Thermal analysis and vibrational spectroscopic characterization of the boro silicate mineral datolite—CaBSiO4(OH). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 376–381. [Google Scholar] [CrossRef]
- Dyar, M.D.; Sklute, E.C.; Post, J.E. Infrared Spectroscopy of Fe-Bearing Minerals: Insights into Crystal Chemistry and Environmental Applications. Am. Mineral. 2009, 94, 1–17. [Google Scholar] [CrossRef]
- Karabulut, M.; Metwalli, E.; Brow, R.K. Influence of Cr³⁺ ions on the infrared spectra of phosphate glasses. J. Non Cryst. Solids 2001, 283, 211–219. [Google Scholar]
- Mekki, A.; Khattak, G.D.; Wenger, L.E. Effect of TiO₂ on the structural, thermal, and optical properties of phosphate glasses. J. Non Cryst. Solids 2004, 344, 128–134. [Google Scholar]
- Yan, C.; Wang, L.; Lin, J.; Xu, J.; Zhang, T.; Qi, J.; Li, X.; Ni, W.; Wu, G.; Huang, J.; et al. A Fully Automatic AI-based CT Image Analysis System for Accurate Detection, Diagnosis, and Quantitative Severity Evaluation of Pulmonary Tuberculosis. Res. Sq. 2021, 1–17. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Rodríguez-Marín, F.; Anera, R.G.; Barco, L.J.d. Deviations of Lambert-Beer’s law affect corneal refractive parameters after refractive surgery. Opt. Express 2006, 14, 5411–5417. [Google Scholar] [PubMed]
- Zobel-Roos, S.; Mouellef, M.; Siemers, C.; Strube, J. Process Analytical Approach towards Quality Controlled Process Automation for the Downstream of Protein Mixtures by Inline Concentration Measurements Based on Ultraviolet/Visible Light (UV/VIS) Spectral Analysis. Antibodies 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.D. Thermal expansion of the Al2SiO5 polymorphs, kyanite, andalusite and sillimanite, between 10 and 1573 K determined using time-of-flight neutron powder diffraction. Phys. Chem. Miner. 2019, 46, 687–704. [Google Scholar] [CrossRef]
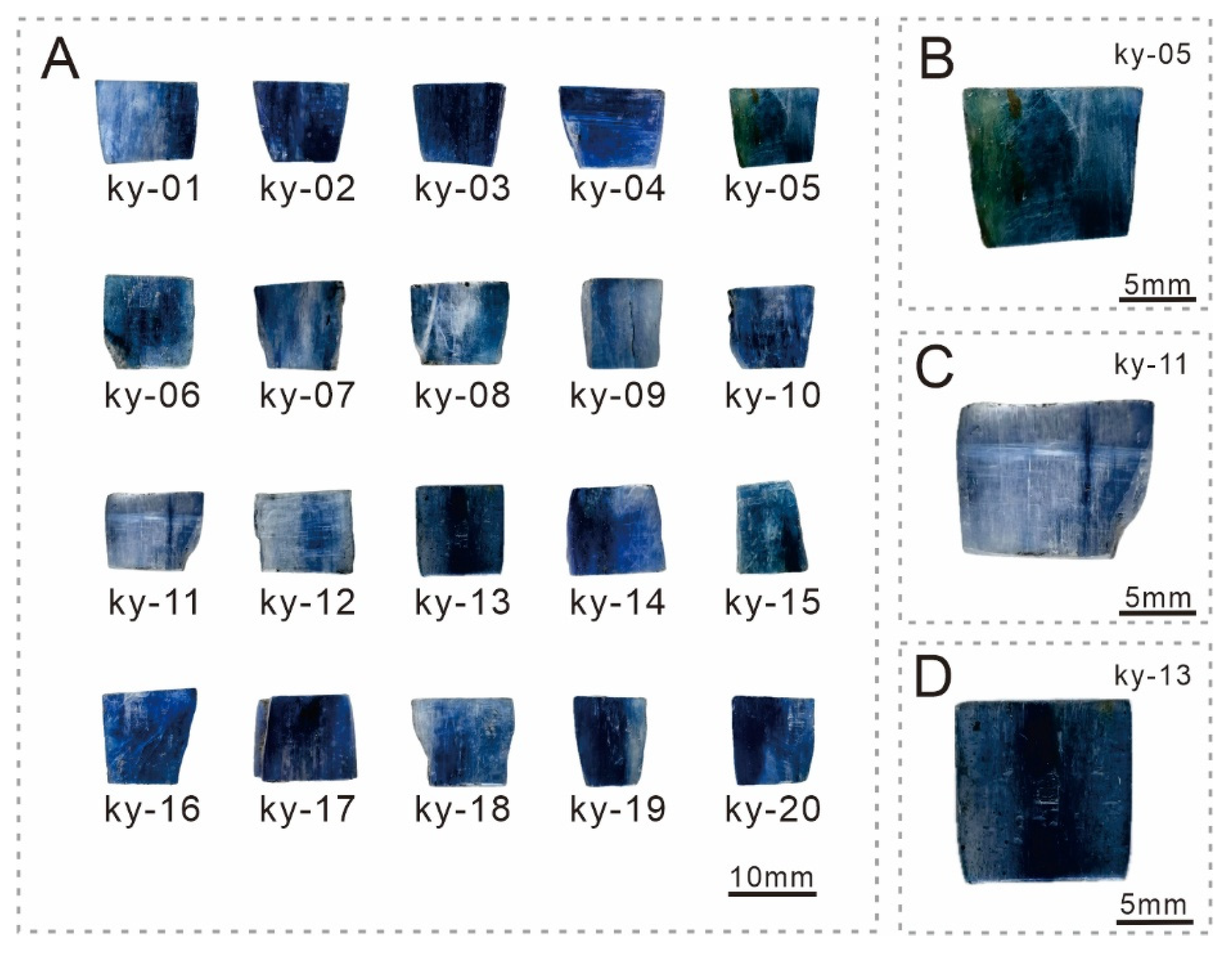



| Characteristics | Results |
|---|---|
| Color | Dark blue-light blue |
| Weight | 3.26~6.02 ct |
| SG | 3.62~3.70 |
| RI | 1.706~1.732 |
| Birefringence | 0.012~0.016 |
| Diaphaneity | Translucent to opaque |
| Inclusions | Small subhedral inclusions |
| Samples | Ky-05 | Ky-01 | Ky-14 | Ky-08 | Ky-18 | Ky-13 | Ky-11 | Ky-02 |
|---|---|---|---|---|---|---|---|---|
| Simulated Color |  |  |  |  |  |  |  |  |
| Al | 33.07 (±0.199) | 33.1 1(±0.200) | 33.03 (±0.201) | 32.25 (±0.202) | 33.39 (±0.202) | 32.09 (±0.209) | 32.09 (±0.201) | 32.04 (±0.111) |
| Si | 16.84 (±0.110) | 16.95 (±0.111) | 16.96 (±0.112) | 17.64 (±0.112) | 17.04 (±0.111) | 17.64 (±0.115) | 17.64 (±0.111) | 17.71 (±0.026) |
| S | 0.38 (±0.015) | 0.28 (±0.026) | 0.35 (±0.014) | 0.30 (±0.015) | 0.00 (+0.017) | 0.39 (±0.008) | 0.39 (±0.027) | 0.43 (±0.001) |
| Fe | 0.12 (±0.002) | 0.10 (±0.002) | 0.13 (±0.003) | 0.17 (±0.003) | 0.09 (±0.002) | 0.14 (±0.003) | 0.14 (±0.003) | 0.18 (±0.003) |
| K | 0.04 (±0.003) | 0.09 (±0.003) | 0.10 (±0.003) | 0.02 (±0.003) | 0.13 (±0.003) | 0.05 (±0.003) | 0.00 | 0.03(±0.002) |
| Ca | 0.06 (±0.002) | 0.03 (±0.002) | 0.02 (±0.002) | 0.01 (±0.003) | 0.02 (±0.002) | 0.03 (±0.002) | 0.03 (±0.002) | 0.01 (±0.002) |
| Ti | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) |
| V | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) | 0.00 | 0.00 | 0.01 (±0.001) | 0.01 (±0.001) | 0.01 (±0.001) |
| Cr | 0.00 | 0.01 (±0.001) | 0.00 | 0.00 | 0.00 | 0.13 (±0.001) | 0.00 | 0.02 (±0.001) |
| O | 49.34 | 49.33 | 49.37 | 49.39 | 49.26 | 49.45 | 49.45 | 49.50 |
| Other | 0.12 | 0.08 | 0.05 | 0.22 | 0.06 | 0.06 | 0.06 | 0.06 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Oxides (wt.%) | L* | C* | h° | b* | a* | |
|---|---|---|---|---|---|---|
| Fe | Pearson’s r | −0.420 | 0.837 ** | 0.932 ** | −0.832 ** | 0.338 |
| Sig. | 0.300 | 0.014 | 0.007 | 0.011 | 0.321 | |
| Ti | Pearson’s r | −0.783 ** | −0.101 | 0.467 | 0.049 | 0.449 |
| Sig. | 0.023 | 0.679 | 0.243 | 0.724 | 0.287 | |
| Fe+ Ti | Pearson’s r | −0.149 | −0.248 | 0.555 | −0.589 | 0.553 |
| Sig. | 0.725 | 0.554 | 0.330 | 0.260 | 0.279 | |
| Fe+ Cr | Pearson’s r | −0.632 * | 0.453 | 0.225 | −0.607 | 0.139 |
| Sig. | 0.189 | 0.260 | 0.340 | 0.213 | 0.789 | |
| Fe/Ti | Pearson’s r | 0.280 | −0.132 | −0.227 | −0.137 | −0.157 |
| Sig | 0.232 | 0.579 | 0.337 | 0.565 | 0.508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Guo, Y.; Gao, M. Study on the Chromogenic Mechanism of Blue Kyanite from Coronel Murta, Minas Gerais, Brazil. Crystals 2025, 15, 302. https://doi.org/10.3390/cryst15040302
Chen X, Guo Y, Gao M. Study on the Chromogenic Mechanism of Blue Kyanite from Coronel Murta, Minas Gerais, Brazil. Crystals. 2025; 15(4):302. https://doi.org/10.3390/cryst15040302
Chicago/Turabian StyleChen, Xiaorui, Ying Guo, and Min Gao. 2025. "Study on the Chromogenic Mechanism of Blue Kyanite from Coronel Murta, Minas Gerais, Brazil" Crystals 15, no. 4: 302. https://doi.org/10.3390/cryst15040302
APA StyleChen, X., Guo, Y., & Gao, M. (2025). Study on the Chromogenic Mechanism of Blue Kyanite from Coronel Murta, Minas Gerais, Brazil. Crystals, 15(4), 302. https://doi.org/10.3390/cryst15040302







