Influence of Milling Energy and Precursors on CaKFe4As4 Polycrystalline Superconductor Morphology
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamihara, Y.; Hiramatsu, H.; Hirano, M.; Kawamura, R.; Yanagi, H.; Kamiya, T.; Hosono, H. Iron-Based Layered Superconductor: LaOFeP. J. Am. Chem. Soc. 2006, 128, 10012–10013. [Google Scholar] [CrossRef]
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-Based Layered Superconductor La[O1−xFx]FeAs (x = 0.05−0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297. [Google Scholar] [CrossRef]
- Iyo, A.; Kawashima, K.; Kinjo, T.; Nishio, T.; Ishida, S.; Fujihisa, H.; Gotoh, Y.; Kihou, K.; Eisaki, H.; Yoshida, Y. New-Structure-Type Fe-Based Superconductors: CaAFe4As4 (A = K, Rb, Cs) and SrAFe4As4 (A = Rb, Cs). J. Am. Chem. Soc. 2016, 138, 3410–3415. [Google Scholar] [CrossRef]
- Rotter, M.; Pangerl, M.; Tegel, M.; Johrendt, D. Superconductivity and Crystal Structures of (Ba1−XKx)Fe2As2 (X=0–1). Angew. Chem.—Int. Ed. 2008, 47, 7949–7952. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.R.; Kong, T.; Kaluarachchi, U.S.; Taufour, V.; Jo, N.H.; Drachuck, G.; Böhmer, A.E.; Saunders, S.M.; Sapkota, A.; Kreyssig, A.; et al. Anisotropic Thermodynamic and Transport Properties of Single-Crystalline CaKFe4As4. Phys. Rev. B 2016, 94, 064501. [Google Scholar] [CrossRef]
- Pyon, S.; Takahashi, A.; Veshchunov, I.; Tamegai, T.; Ishida, S.; Iyo, A.; Eisaki, H.; Imai, M.; Abe, H.; Terashima, T.; et al. Large and Significantly Anisotropic Critical Current Density Induced by Planar Defects in CaKFe4 As4 Single Crystals. Phys. Rev. B 2019, 99, 104506. [Google Scholar] [CrossRef]
- Singh, S.J.; Bristow, M.; Meier, W.; Taylor, P.; Blundell, S.J.; Canfield, P.C.; Coldea, A.I. Ultra-High Critical Current Densities, the Vortex Phase Diagram and the Effect of Granularity of the Stoichiometric High-Tc Superconductor, CaKFe4As4. Phys. Rev. Mater. 2018, 2, 074802. [Google Scholar] [CrossRef]
- Pyon, S.; Miyawaki, D.; Veshchunov, I.; Tamegai, T.; Takano, K.; Kajitani, H.; Koizumi, N.; Awaji, S. Fabrication and Characterization of CaKFe4As4 Round Wires Sintered at High Pressure. Appl. Phys. Express 2018, 11, 123101. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, S.; Dong, C.; Huang, H.; Li, L.; Zhu, Y.; Awaji, S.; Ma, Y. Effects of Core Density and Impurities on the Critical Current Density of CaKFe4As4 Superconducting Tapes. Supercond. Sci. Technol. 2019, 32, 105014. [Google Scholar] [CrossRef]
- Dong, C.; Xu, Q.; Ma, Y. Towards High-Field Applications: High-Performance, Low-Cost Iron-Based Superconductors. Natl. Sci. Rev. 2024, 11, nwae122. [Google Scholar] [CrossRef]
- Yao, C.; Ma, Y. Recent Breakthrough Development in Iron-Based Superconducting Wires for Practical Applications. Supercond. Sci. Technol. 2019, 32, 023002. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Liu, C.; Huang, H.; Yao, C.; Zhang, X.; Wang, D.; Liu, F.; Liu, H.; Ma, Y. Effects of Precursor Powder Particle Size on the Powder-in-Tube Ba1−xKxFe2As2 Superconducting Tapes. Supercond. Sci. Technol. 2022, 35, 055008. [Google Scholar] [CrossRef]
- Traverso, A.; Bordonaro, M.; Hassan, H.M.U.; Leveratto, A.; Loria, F.; Bellingeri, E.; Bernini, C.; Braccini, V.; Ballarino, A.; Malagoli, A. Impact of Powder Granulometry on the Transport Properties of Ba0.6K0.4Fe2As2 Superconducting Tapes. IEEE Trans. Appl. Supercond. 2025, 35, 7300405. [Google Scholar] [CrossRef]
- Dong, C.; Han, M.; Guo, W.; Zhang, X.; Liu, C.; Huang, H.; Yao, C.; Wang, D.; Liu, H.; Ma, Y. Modulation of Superconducting Grain Structure to Achieve High Critical Current in Ba0.6K0.4Fe2As2 Multifilament Round Wires. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Singh, S.J.; Cassidy, S.J.; Bristow, M.; Blundell, S.J.; Clarke, S.J.; Coldea, A.I. Optimization of Superconducting Properties of the Stoichiometric CaKFe4As4. Supercond. Sci. Technol. 2020, 33, 025003. [Google Scholar] [CrossRef]
- Ishida, S.; Pavan Kumar Naik, S.; Tsuchiya, Y.; Mawatari, Y.; Yoshida, Y.; Iyo, A.; Eisaki, H.; Kamiya, Y.; Kawashima, K.; Ogino, H. Synthesis of CaKFe4As4 Bulk Samples with High Critical Current Density Using a Spark Plasma Sintering Technique. Supercond. Sci. Technol. 2020, 33, 094005. [Google Scholar] [CrossRef]
- Masi, A.; Angrisani Armenio, A.; Celentano, G.; La Barbera, A.; Rufoloni, A.; Silva, E.; Vannozzi, A.; Varsano, F. Mechanochemically Assisted Low Temperature Synthesis Route of the 1144 Ca-K Iron Based Superconductor. Supercond. Sci. Technol. 2020, 33, 074003. [Google Scholar] [CrossRef]
- Weiss, J.D.; Jiang, J.; Polyanskii, A.A.; Hellstrom, E.E. Mechanochemical Synthesis of Pnictide Compounds and Superconducting Ba0.6K0.4Fe2As2 Bulks with High Critical Current Density. Supercond. Sci. Technol. 2013, 26, 074003. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571. [Google Scholar] [CrossRef]
- Masi, A.; Angrisani Armenio, A.; Celentano, G.; La Barbera, A.; Rufoloni, A.; Silva, E.; Vannozzi, A.; Varsano, F. The Role of Chemical Composition in the Synthesis of Ca/K-1144 Iron Based Superconductors. J. Alloys Compd. 2021, 869, 159202. [Google Scholar] [CrossRef]
- Zaikina, J.V.; Batuk, M.; Abakumov, A.M.; Navrotsky, A.; Kauzlarich, S.M. Facile Synthesis of Ba1–xKxFe2As2 Superconductors via Hydride Route. J. Am. Chem. Soc. 2014, 136, 16932–16939. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Duchenko, A.; Celentano, G.; Varsano, F. Tailoring the Critical Temperature of Ca/K-1144 Superconductors: The Effect of Aliovalent Substitution on Tetragonality. Supercond. Sci. Technol. 2022, 35, 065015. [Google Scholar] [CrossRef]
- Delogu, F.; Mulas, G.; Schiffini, L.; Cocco, G. Mechanical Work and Conversion Degree in Mechanically Induced Processes. Mater. Sci. Eng. A 2004, 382, 280–287. [Google Scholar] [CrossRef]
- Poole, C.P.; Prozorov, R.; Farach, H.A.; Creswick, R.J. Superconductivity; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124095090. [Google Scholar]
- Bean, C.P. Magnetization of High-Field Superconductors. Rev. Mod. Phys. 1964, 36, 31–39. [Google Scholar] [CrossRef]
- Tokuta, S.; Shimada, Y.; Yamamoto, A. Evolution of Intergranular Microstructure and Critical Current Properties of Polycrystalline Co-Doped BaFe2As2 through High-Energy Milling. Supercond. Sci. Technol. 2020, 33, 094010. [Google Scholar] [CrossRef]
- Naik, S.P.K.; Ishida, S.; Kamiya, Y.; Tsuchiya, Y.; Kawashima, K.; Eisaki, H.; Iyo, A.; Ogino, H. Sn Addition Effects on CaKFe4As4 Superconductors. Supercond. Sci. Technol. 2020, 33, 104004. [Google Scholar] [CrossRef]
- Manasa, M.; Azam, M.; Zajarniuk, T.; Diduszko, R.; Cetner, T.; Morawski, A.; Wiśniewski, A.; Singh, S.J. Effect of Impurity Phase and High-Pressure Synthesis on the Superconducting Properties of CaKFe4As4. J. Phys. Chem. Solids 2024, 190, 111996. [Google Scholar] [CrossRef]
- Bellingeri, E.; Bernini, C.; Loria, F.; Traverso, A.; Leveratto, A.; Braccini, V.; Ballarino, A.; Malagoli, A. Effects of K Excess in Microstructure of (Ba0.6K0.4)Fe2As2 Superconducting Powders. Supercond. Sci. Technol. 2024, 37, 095014. [Google Scholar] [CrossRef]
- Bonura, M.; Huang, H.; Yao, C.; Ma, Y.; Senatore, C. Current Transport, Magnetic and Elemental Properties of Densified Ag-Sheathed Ba1–xKxFe2As2 Tapes. Supercond. Sci. Technol. 2020, 33, 095008. [Google Scholar] [CrossRef]
- Augieri, A.; Duchenko, A.; De Marzi, G.; Varsano, F.; Celentano, G.; Pompeo, N.; Masi, A. Pinning Properties of 1144 Polycrystalline Samples with Aliovalent Doping. IEEE Trans. Appl. Supercond. 2024, 34, 7300805. [Google Scholar] [CrossRef]
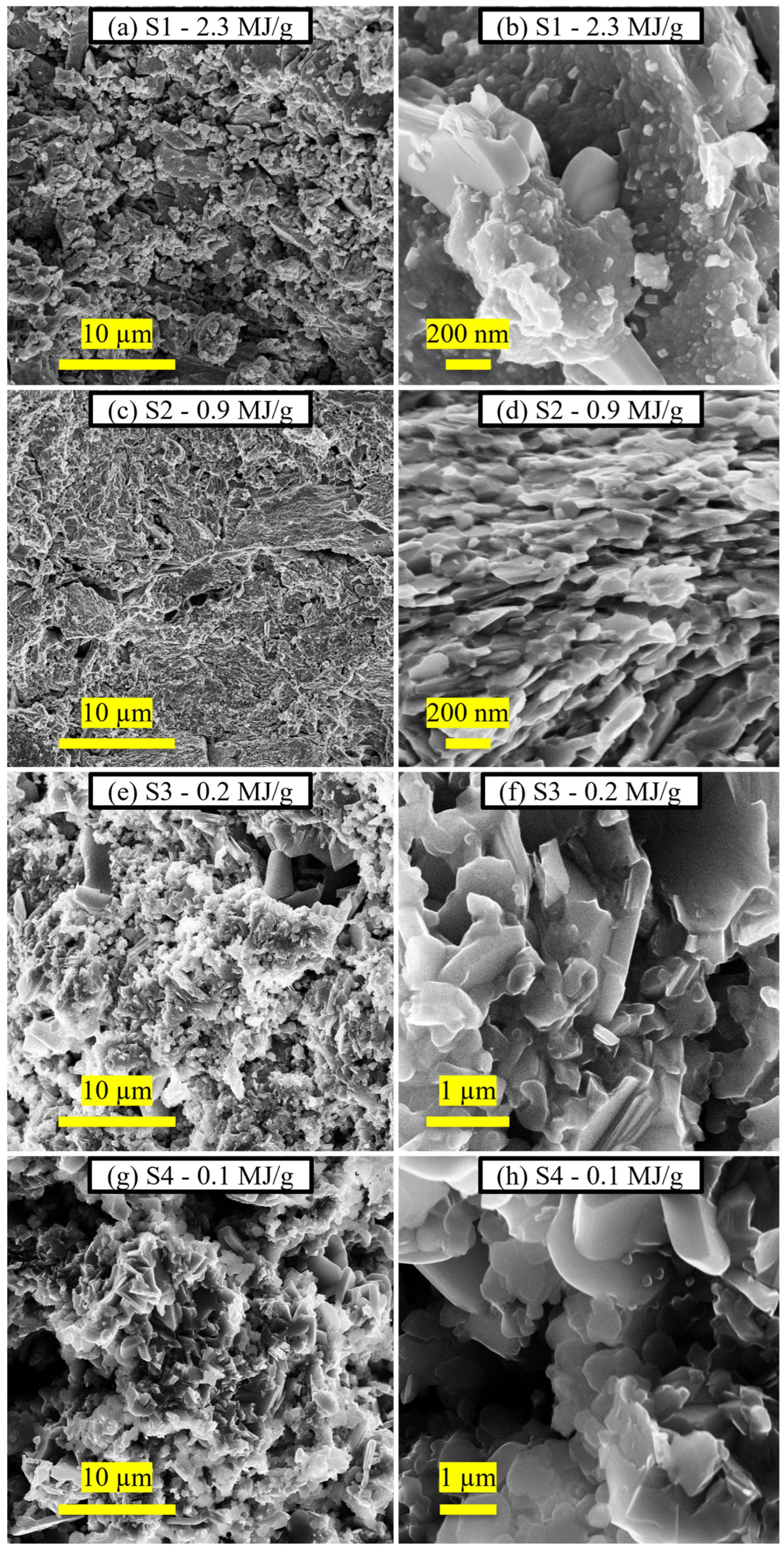
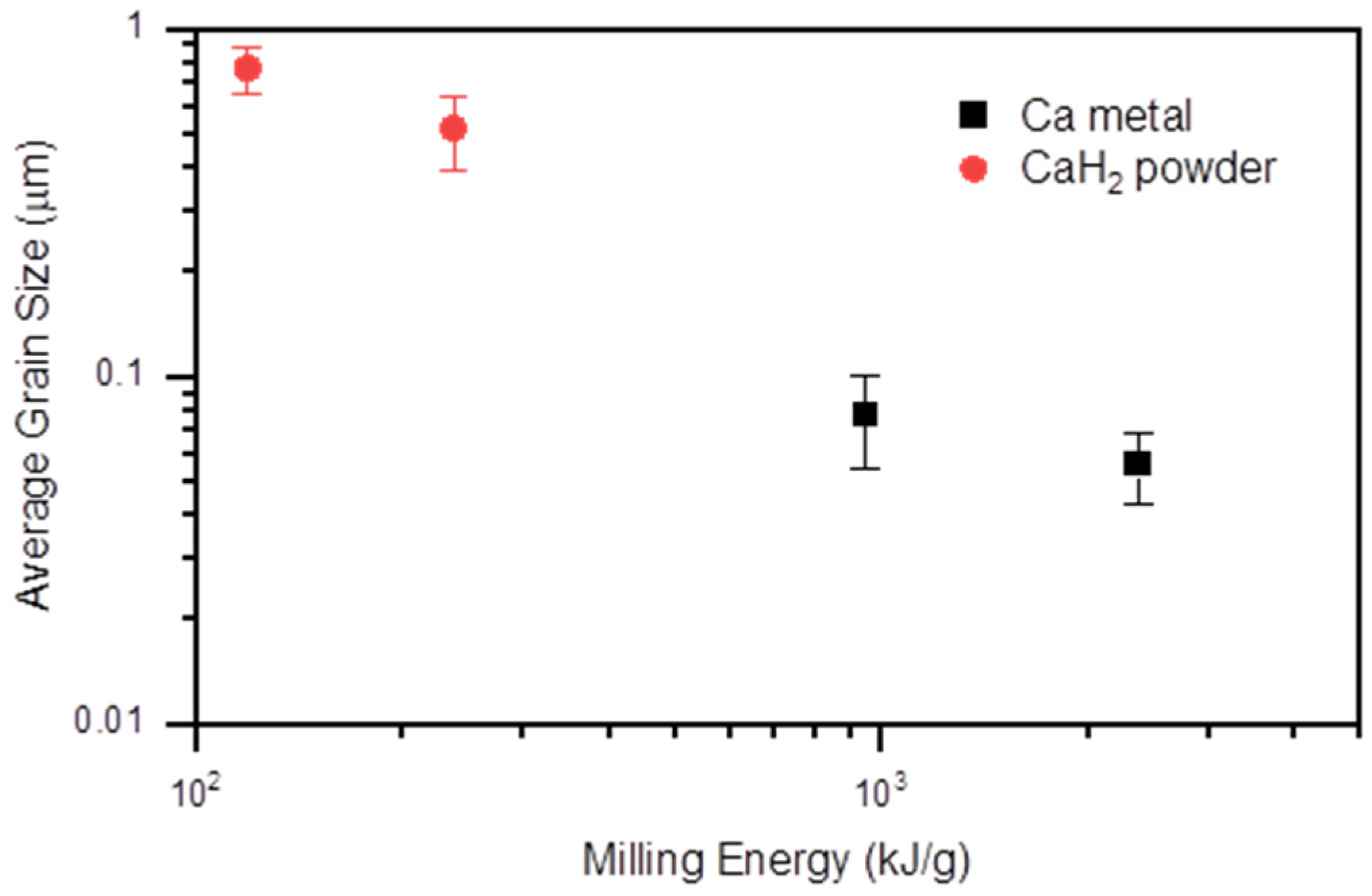
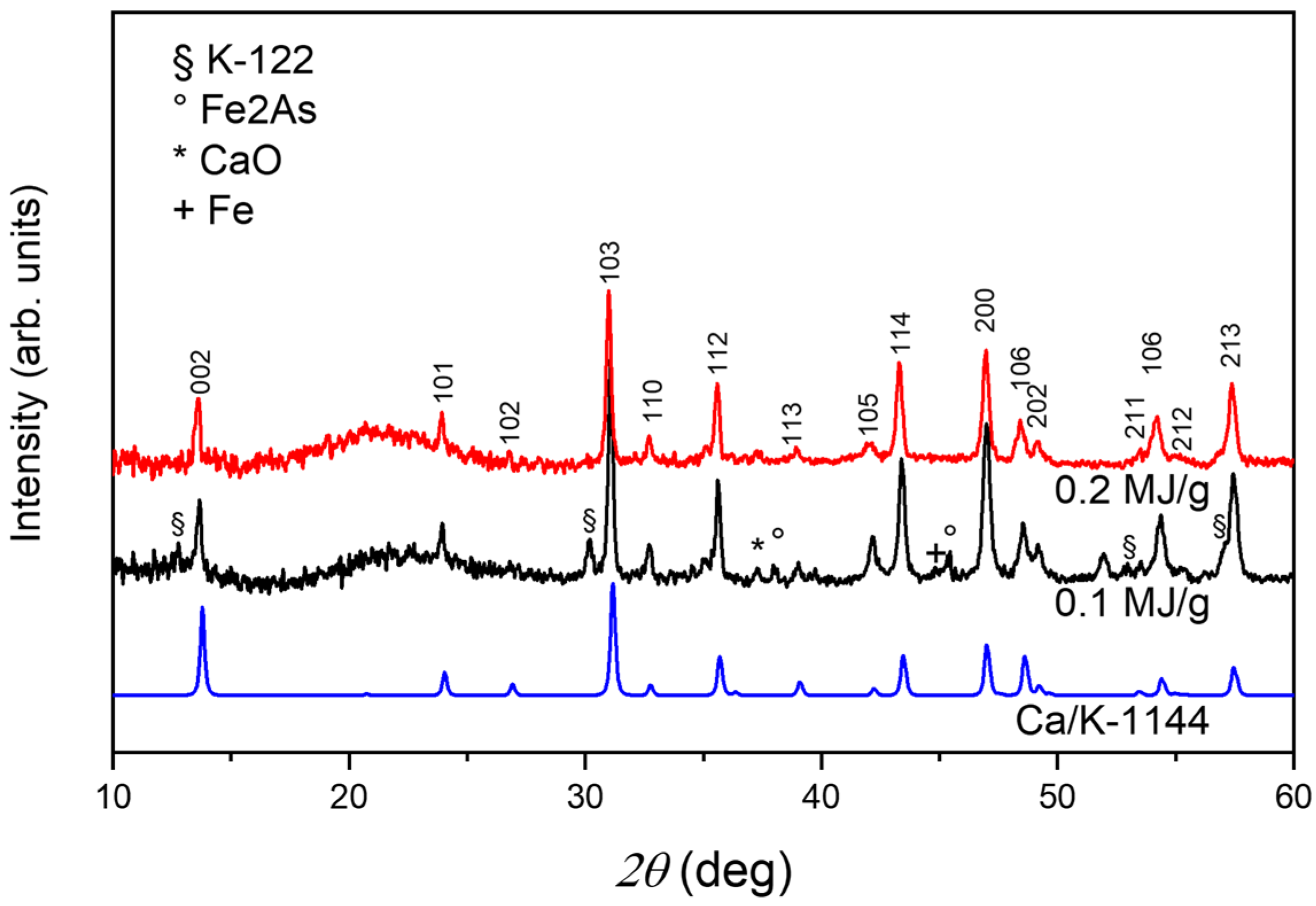
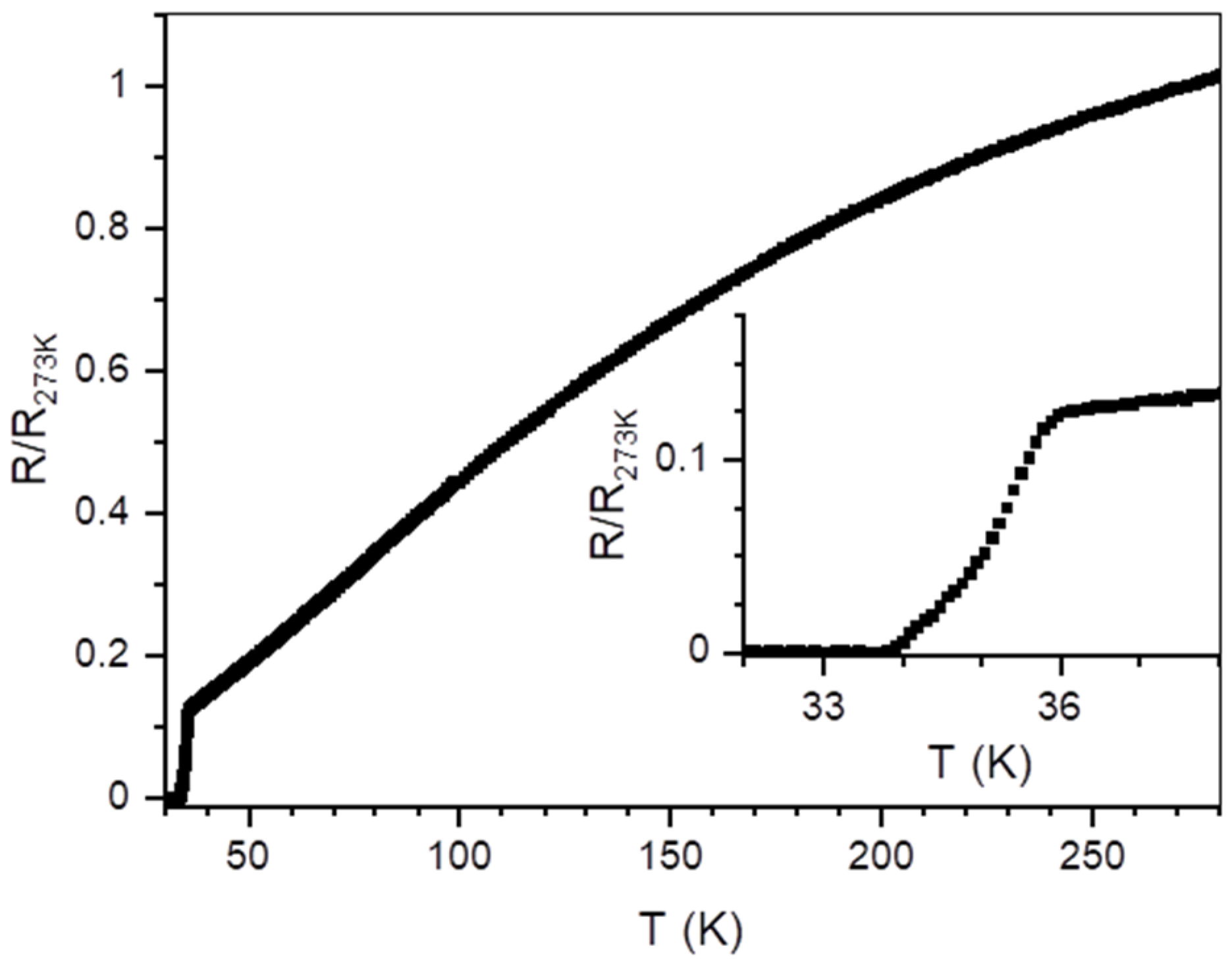

| Sample | Milling Energy | Nominal Composition Ca:K:Fe:As | Ca Source | Ball Diameter (mm) | Ball to Powder Ratio | Milling Time (h) |
|---|---|---|---|---|---|---|
| S1 | 2.3 MJ/g | 1.27:1.18:3.8:4 | Ca metal | 12 | 30 | 5 |
| S2 | 0.9 MJ/g | 2 | ||||
| S3 | 0.2 MJ/g | 1.35:1.05:3.5:4 | CaH2 powder | 8 | 15 | 1 |
| S4 | 0.1 MJ/g | 0.5 |
| Element | At % |
|---|---|
| Ca | 10 ± 1 |
| K | 11 ± 1 |
| Fe | 41 ± 2 |
| As | 38 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchenko, A.; Angrisani Armenio, A.; Celentano, G.; Fava, A.; Mirabile Gattia, D.; Pompeo, N.; Silva, E.; Varsano, F.; Masi, A. Influence of Milling Energy and Precursors on CaKFe4As4 Polycrystalline Superconductor Morphology. Crystals 2025, 15, 276. https://doi.org/10.3390/cryst15030276
Duchenko A, Angrisani Armenio A, Celentano G, Fava A, Mirabile Gattia D, Pompeo N, Silva E, Varsano F, Masi A. Influence of Milling Energy and Precursors on CaKFe4As4 Polycrystalline Superconductor Morphology. Crystals. 2025; 15(3):276. https://doi.org/10.3390/cryst15030276
Chicago/Turabian StyleDuchenko, Anastasiya, Achille Angrisani Armenio, Giuseppe Celentano, Alessandra Fava, Daniele Mirabile Gattia, Nicola Pompeo, Enrico Silva, Francesca Varsano, and Andrea Masi. 2025. "Influence of Milling Energy and Precursors on CaKFe4As4 Polycrystalline Superconductor Morphology" Crystals 15, no. 3: 276. https://doi.org/10.3390/cryst15030276
APA StyleDuchenko, A., Angrisani Armenio, A., Celentano, G., Fava, A., Mirabile Gattia, D., Pompeo, N., Silva, E., Varsano, F., & Masi, A. (2025). Influence of Milling Energy and Precursors on CaKFe4As4 Polycrystalline Superconductor Morphology. Crystals, 15(3), 276. https://doi.org/10.3390/cryst15030276






