Emission Wavelength Control via Molecular Structure Design of Dinuclear Pt(II) Complexes: Optimizing Optical Properties for Red- and Near-Infrared Emissions
Abstract
1. Introduction
2. Emission Wavelength Control
2.1. Cyclometalating Ligand Control
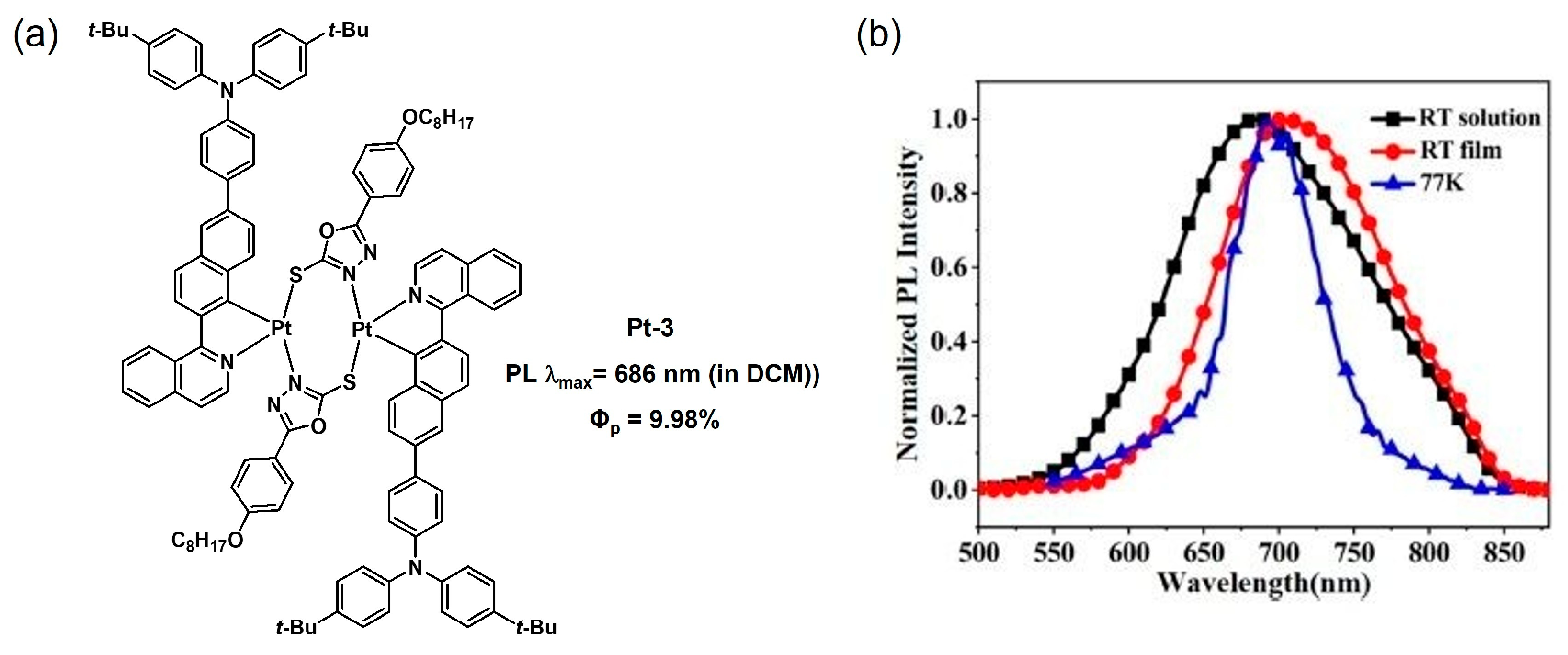
2.1.1. π-Conjugation Length Extension
2.1.2. Electron Density Modulation
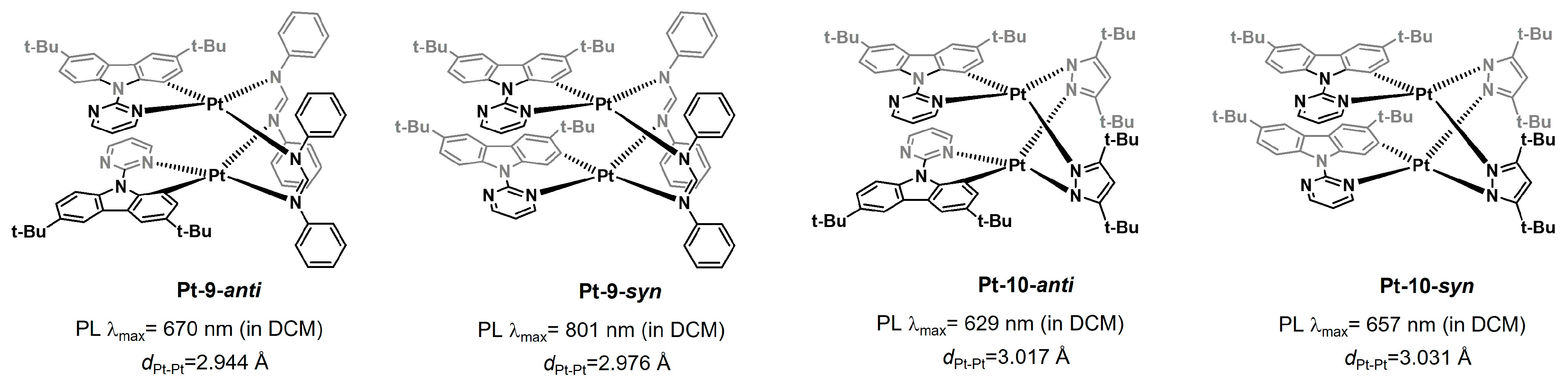
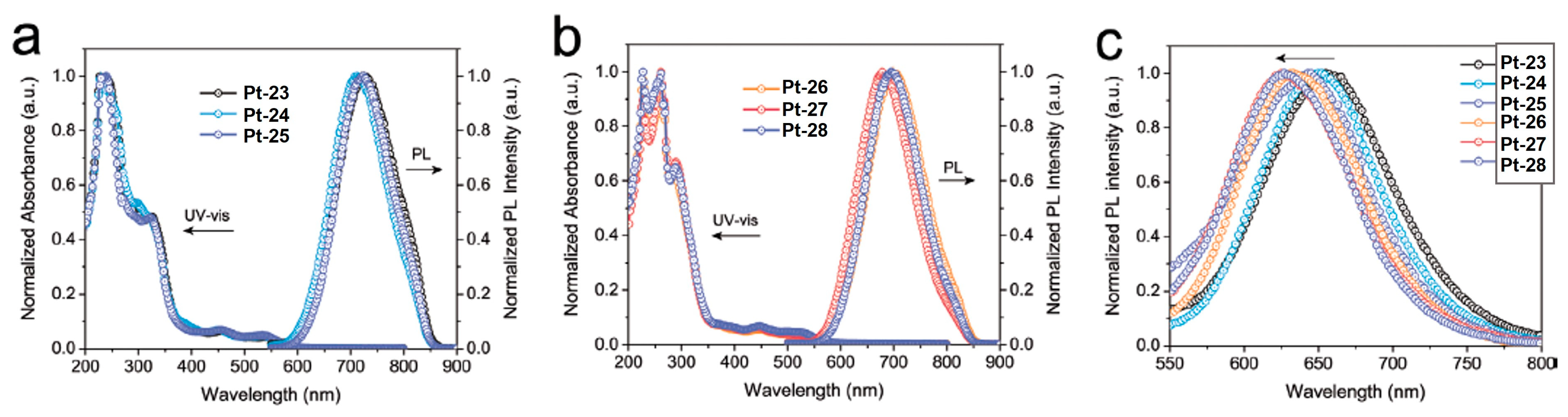
2.1.3. Isomeric Control
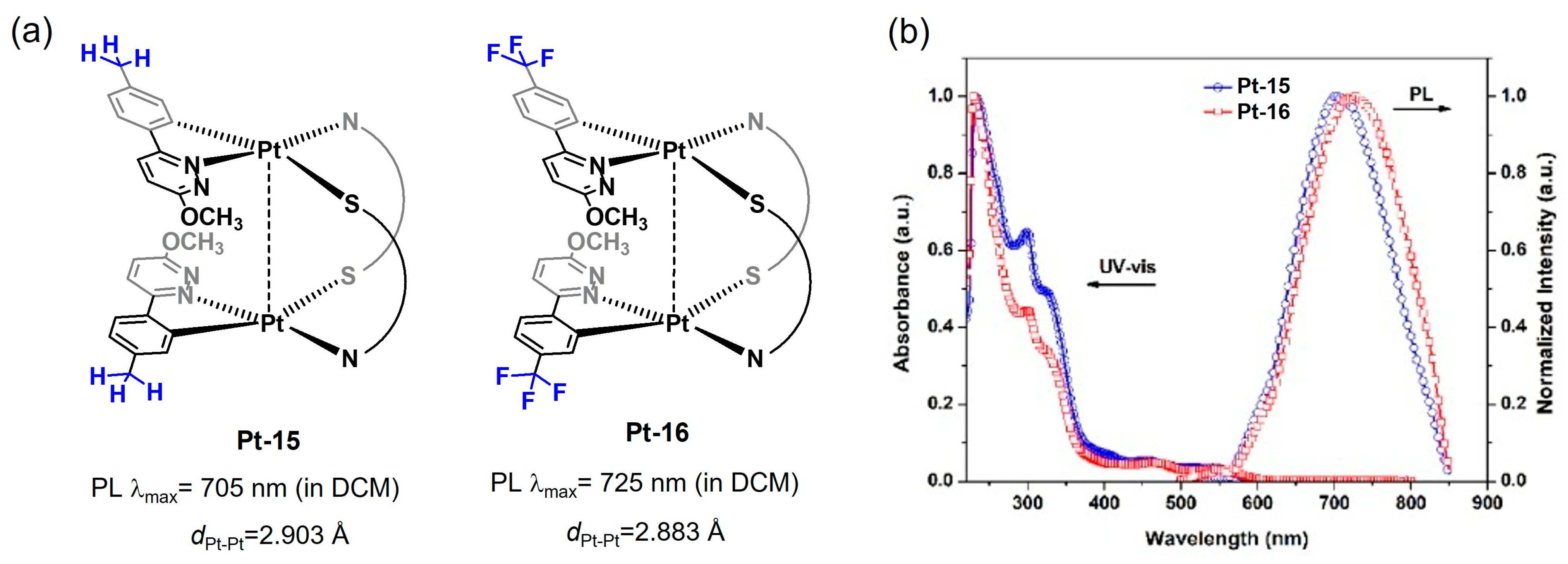
2.1.4. Substituent Effects
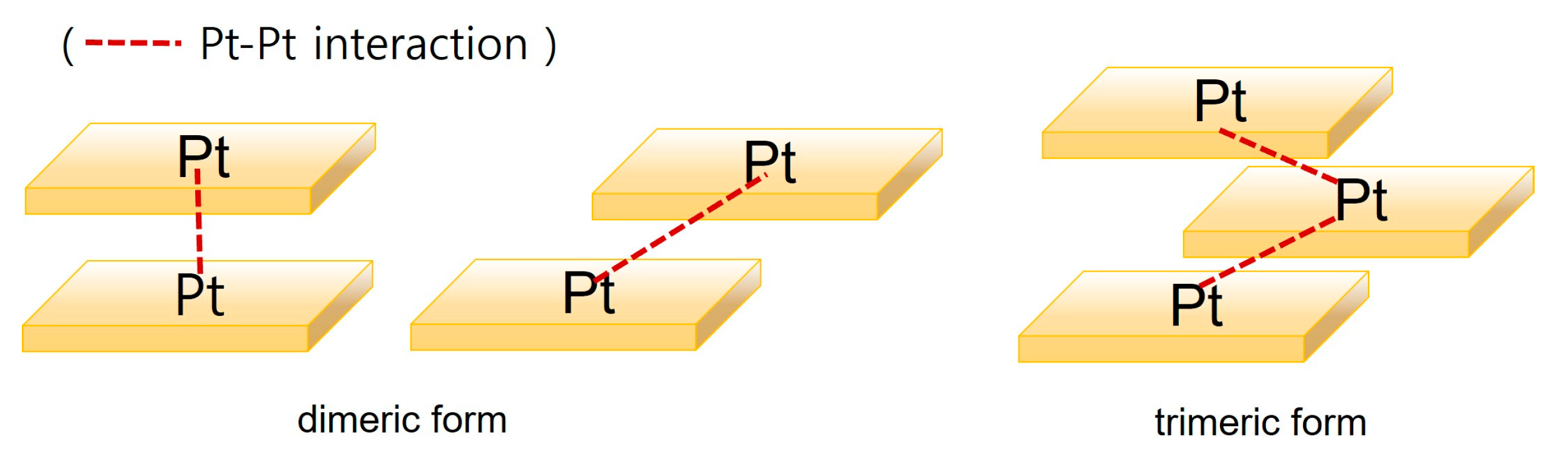
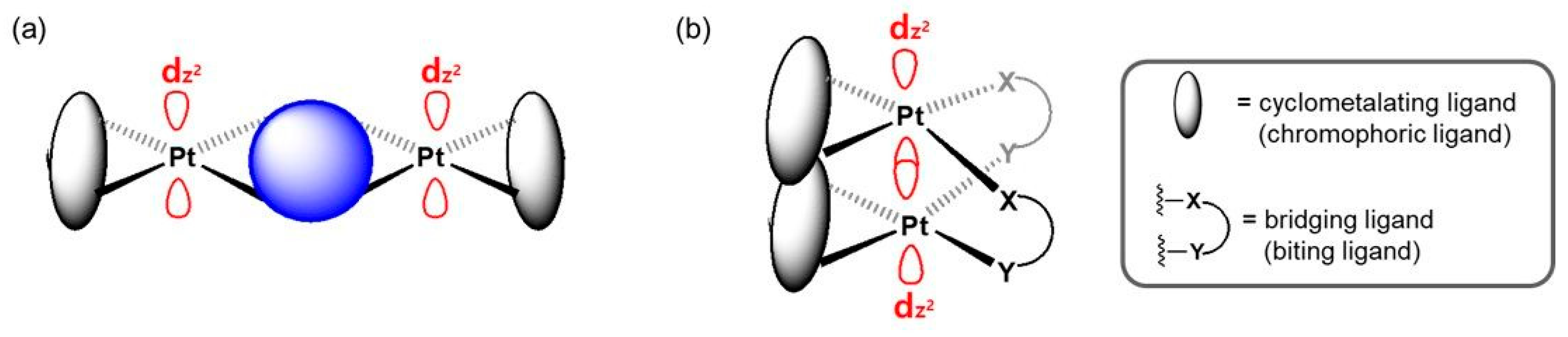
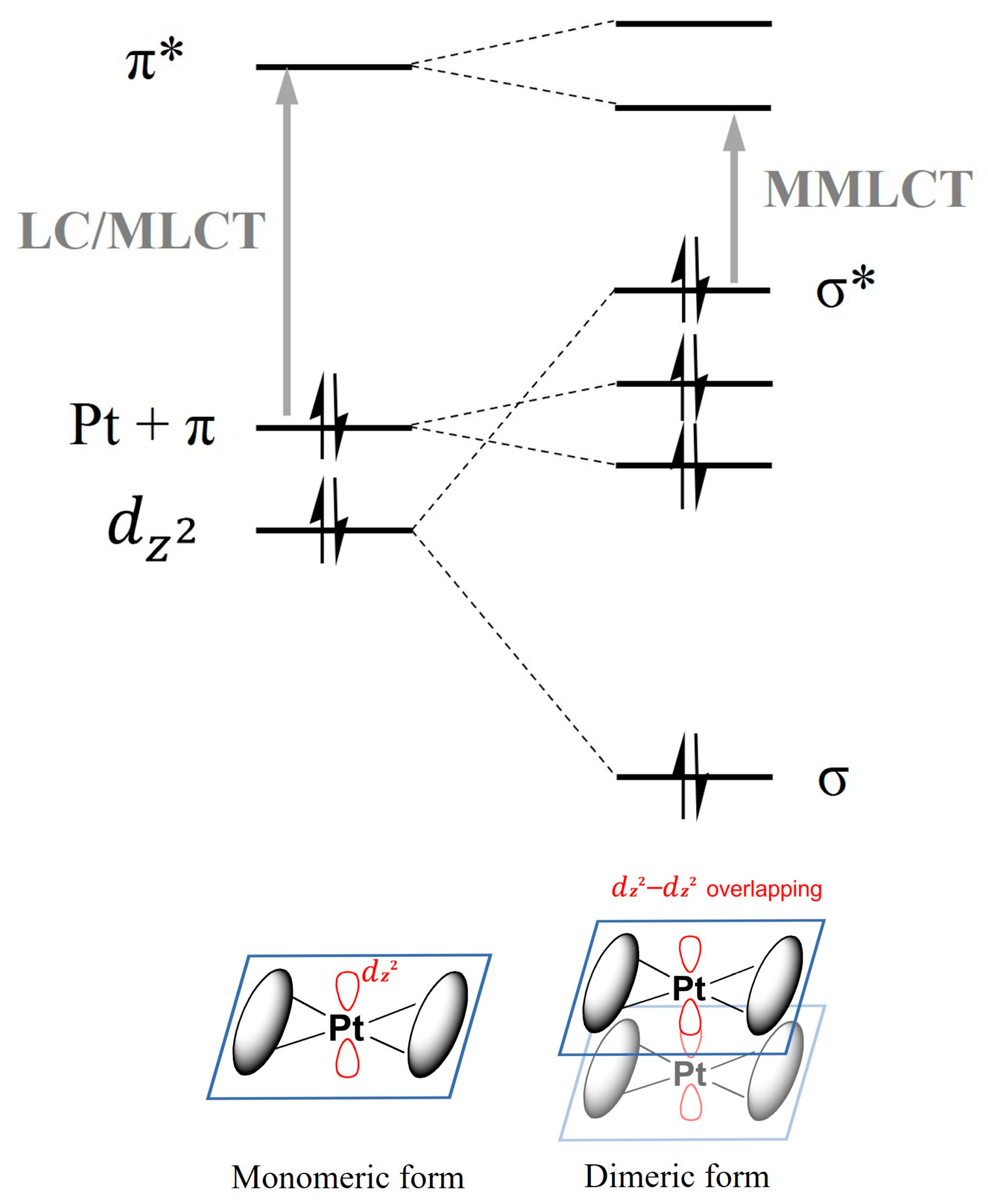
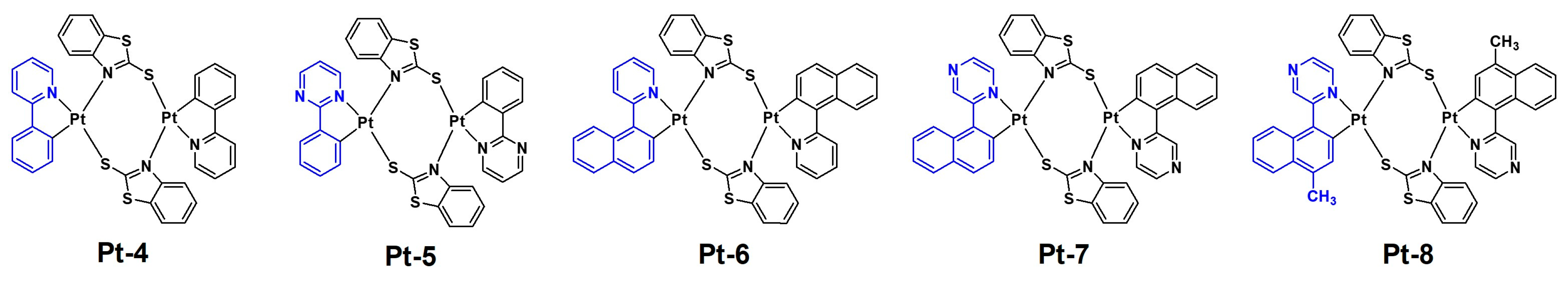
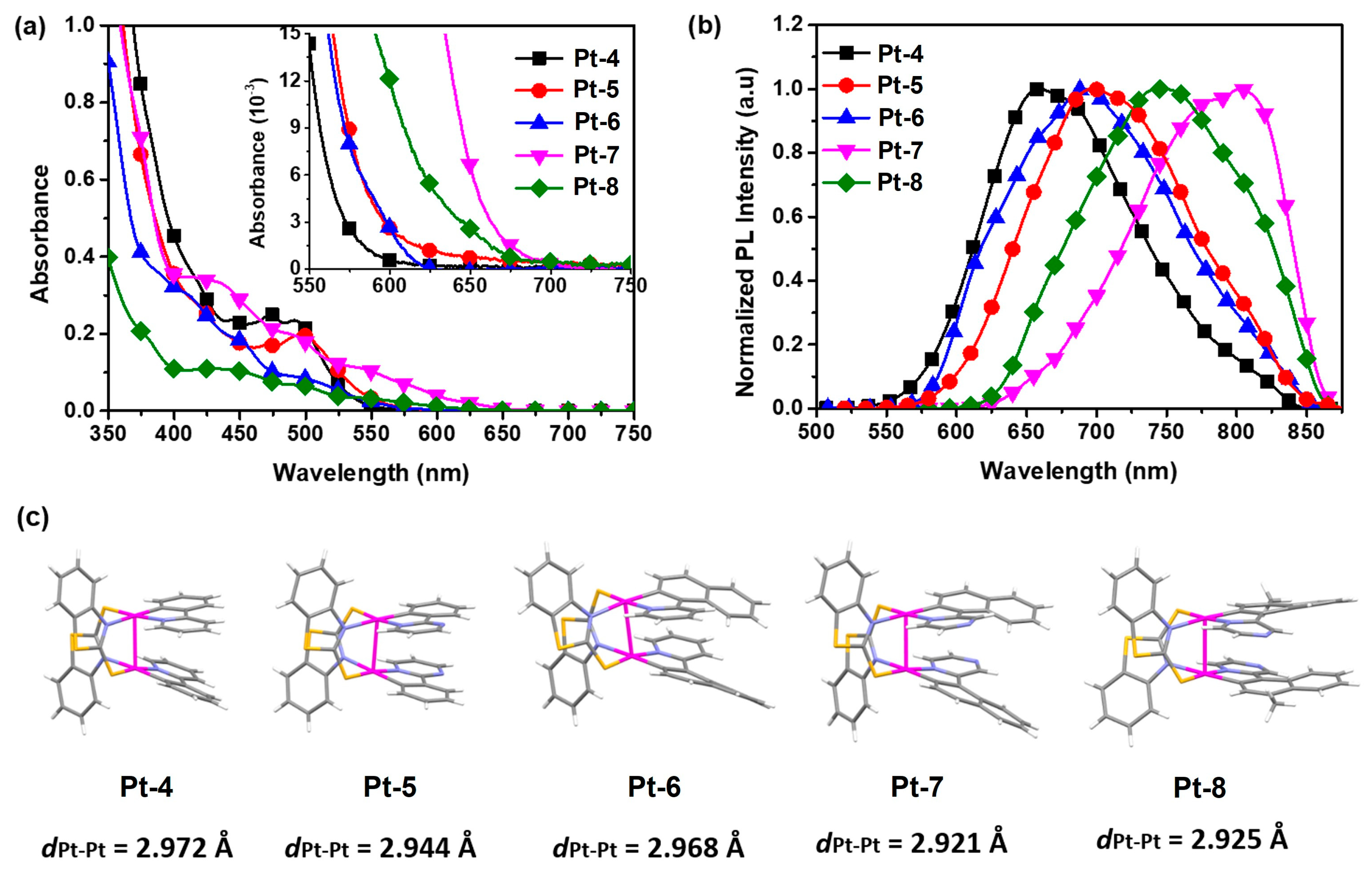
2.2. Bridging Ligand Control
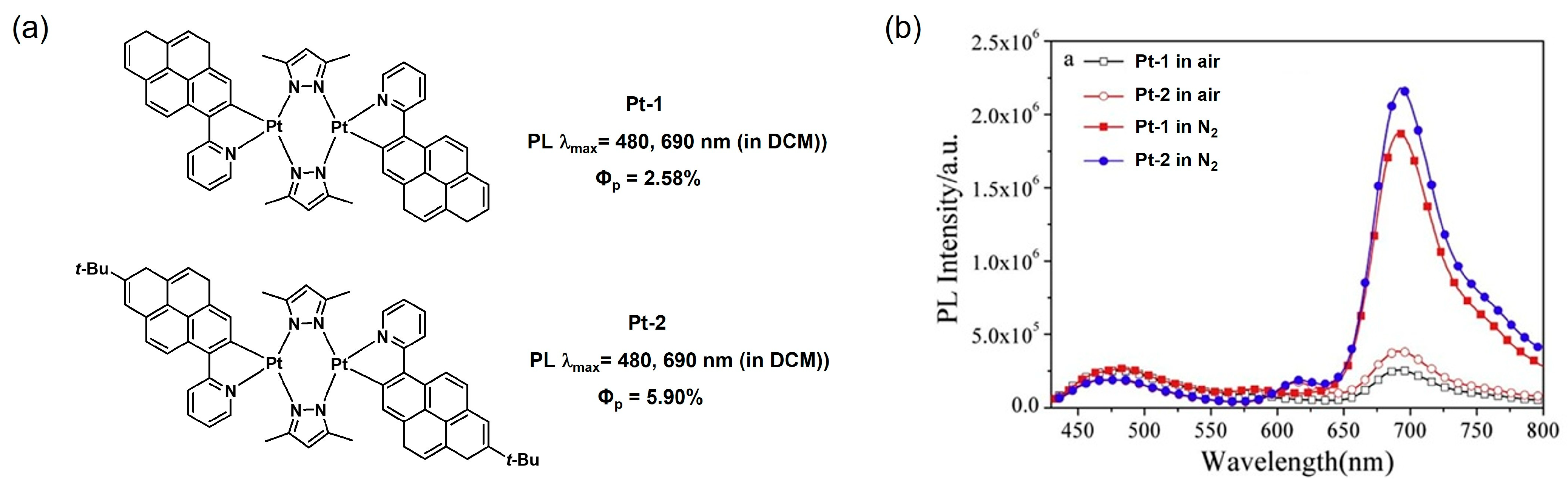
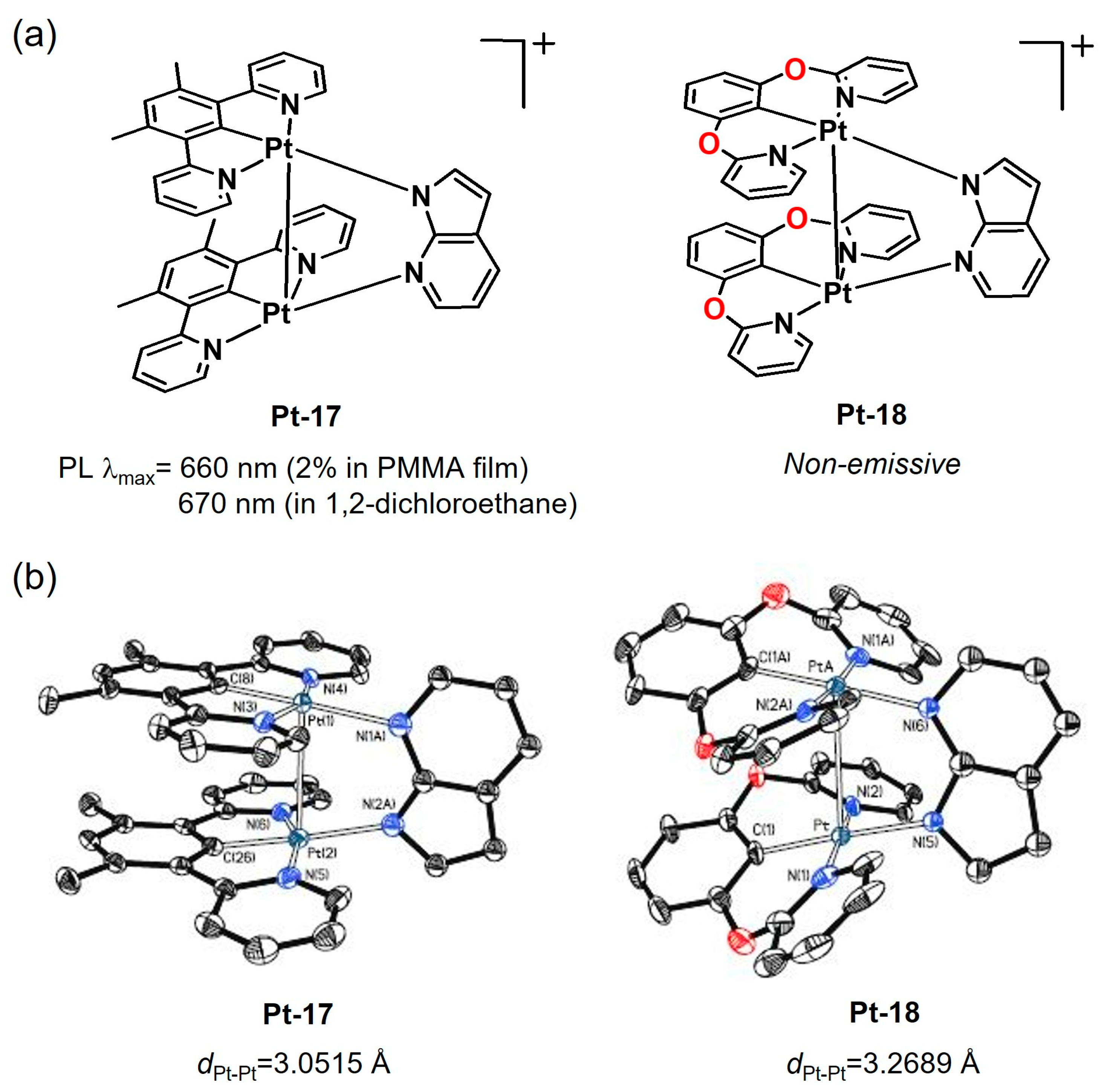
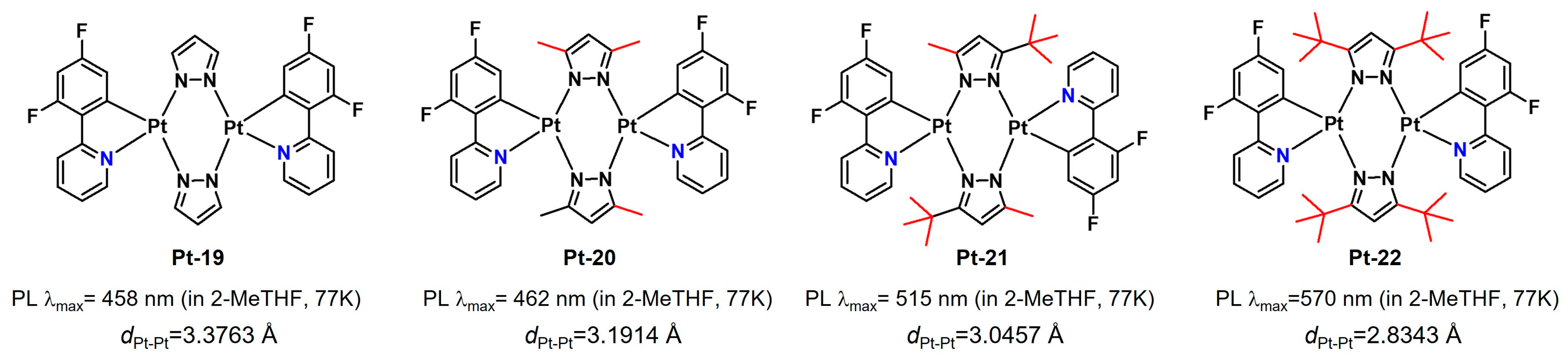
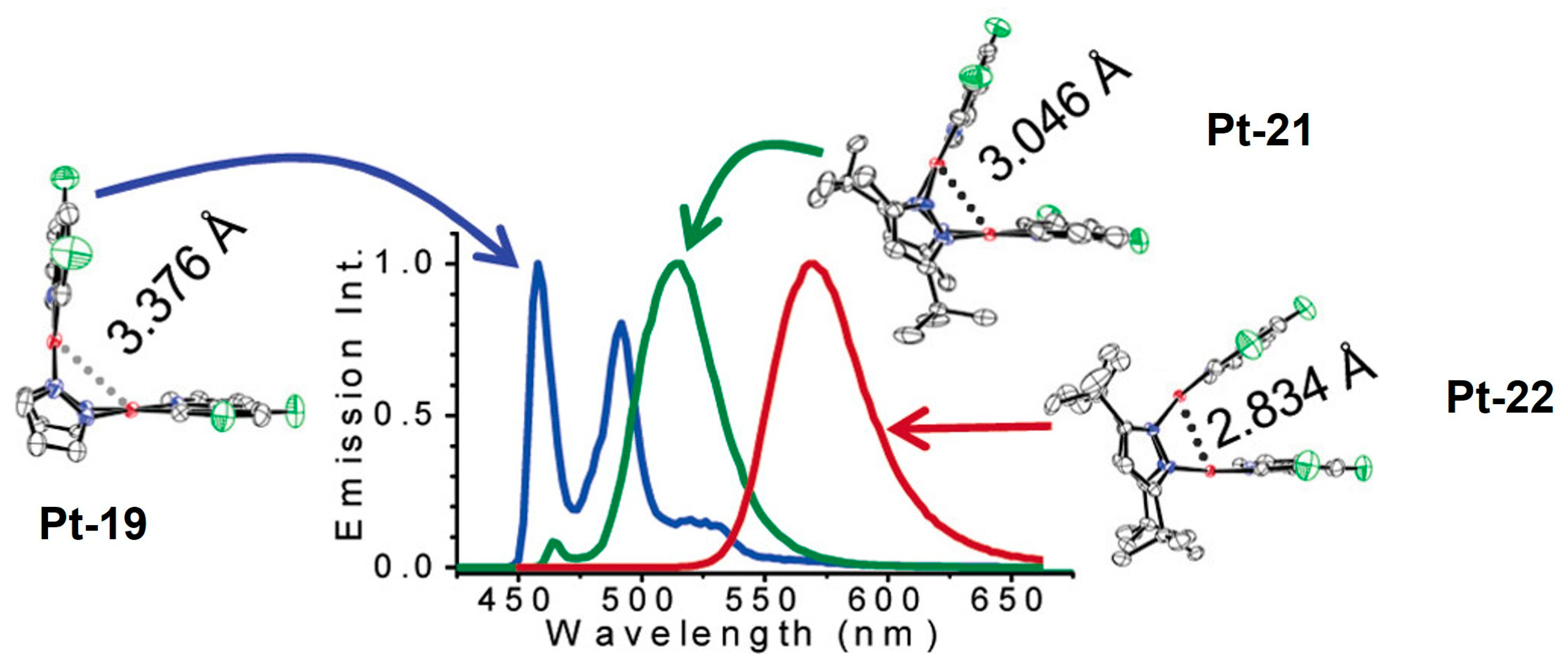
2.2.1. Biting Angle Effect
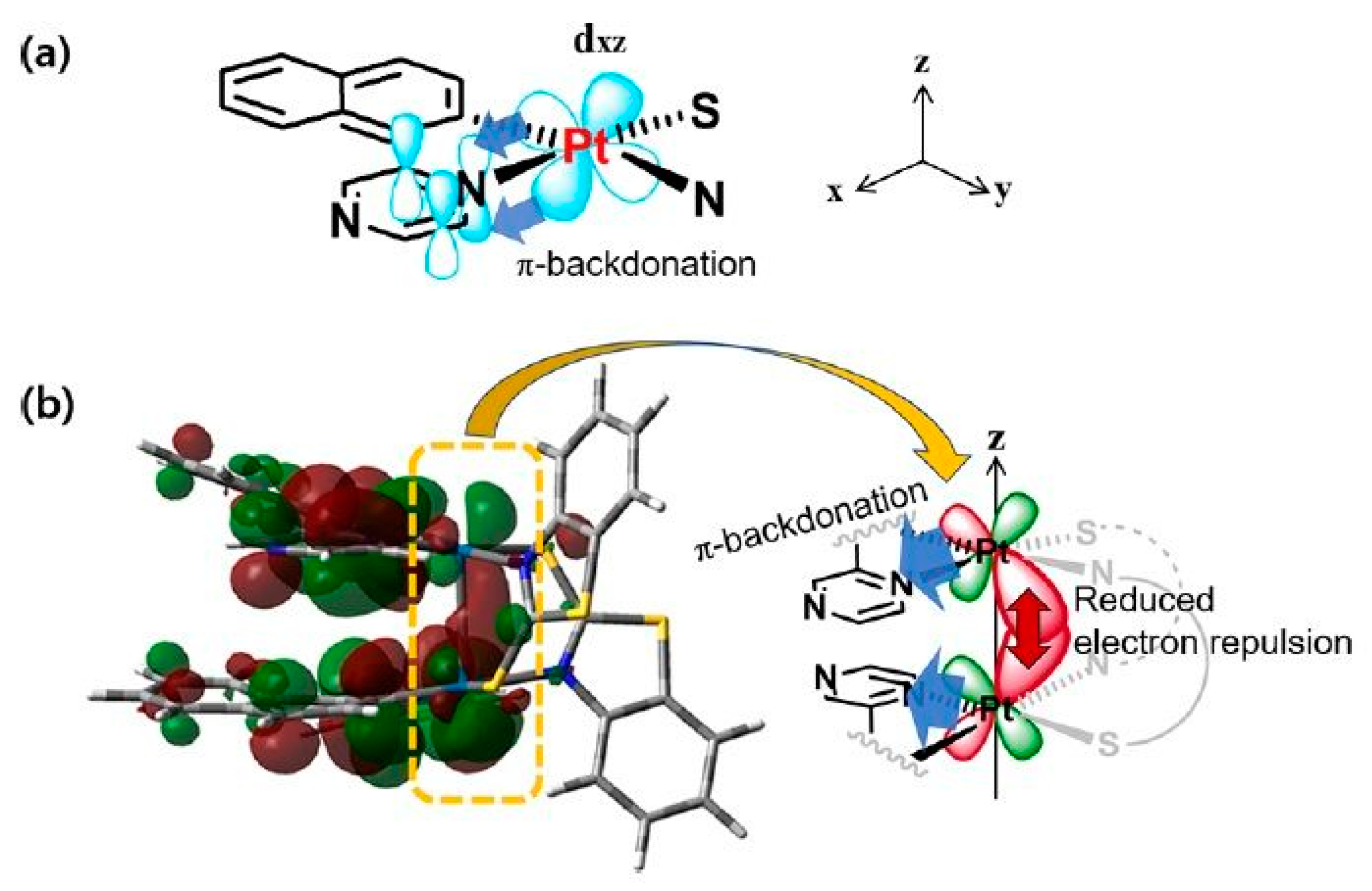
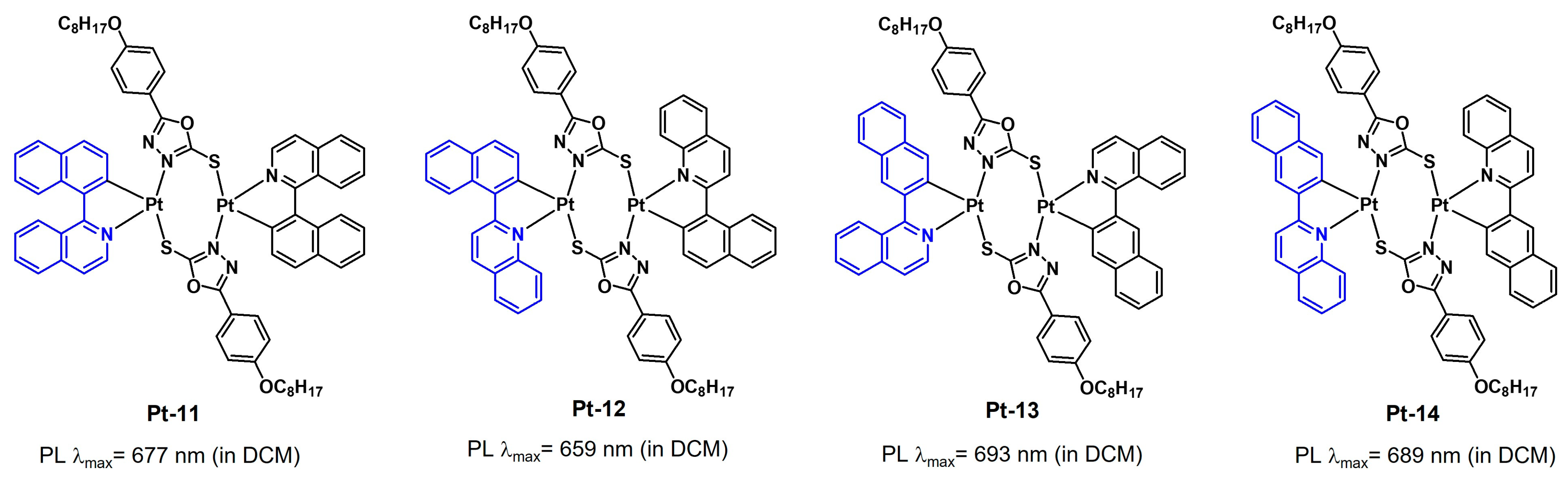
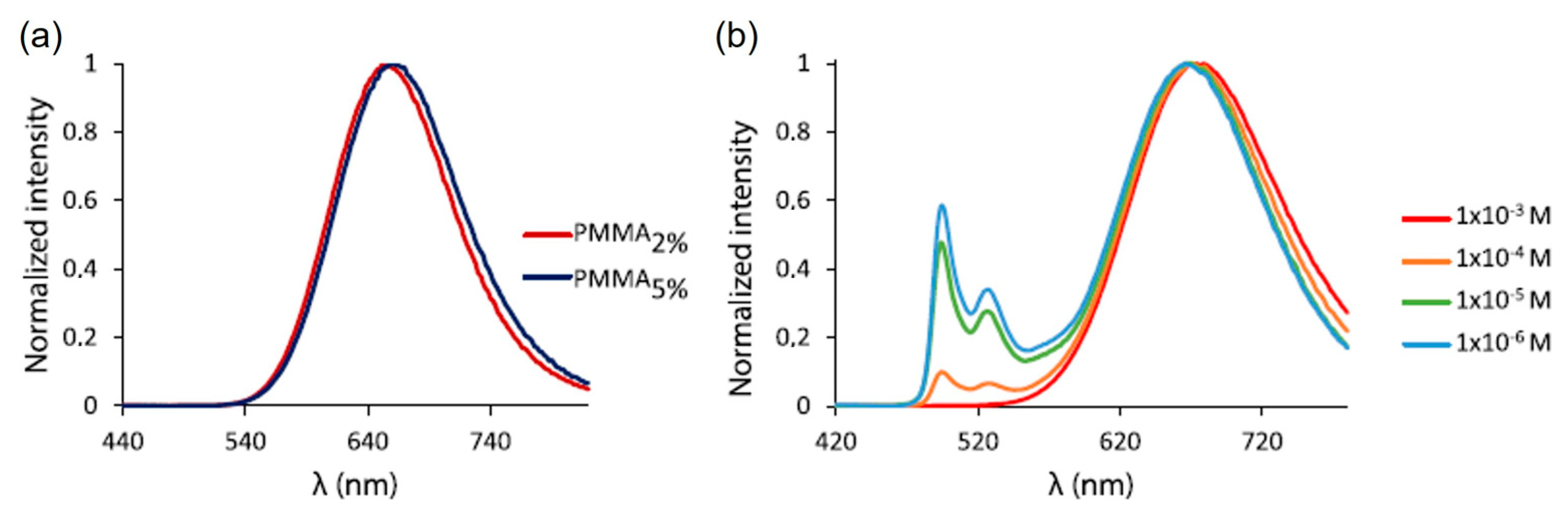
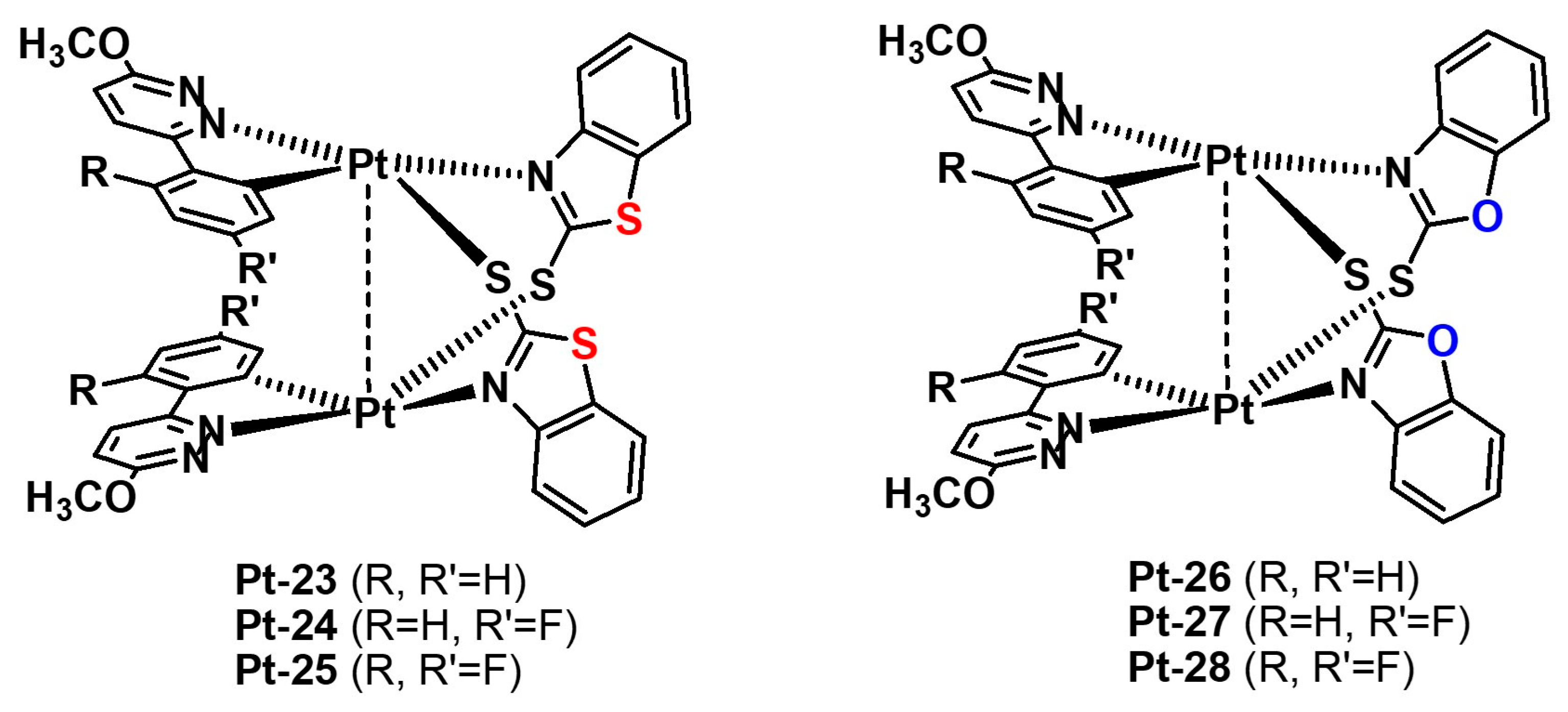
2.2.2. Electronic Effects
3. Summary and Perspectives
Funding
Conflicts of Interest
References
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and opportunities. Adv. Funct. Mater. 2019, 21, 1807623. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Tan, J.H.; Chen, W.C.; Yuan, Y.; Fu, L.W.; Cao, C.; You, C.L.; Ni, S.F.; Chi, Y.; Lee, C.S. High performance NIR OLEDs with low efficiency roll-off by leveraging Os (II) phosphors and exciplex co-host. Adv. Funct. Mater. 2021, 31, 2102787. [Google Scholar] [CrossRef]
- Wang, S.-F.; Su, B.-K.; Wang, X.-Q.; Wei, Y.-C.; Kuo, K.-H.; Wang, C.-H.; Liu, S.-H.; Liao, L.-S.; Hung, W.-Y.; Fu, L.-W.; et al. Polyatomic molecules with emission quantum yields>20% enable efficient organic light-emitting diodes in the NIR (II) window. Nat. Photonics 2022, 16, 843. [Google Scholar] [CrossRef]
- Yuan, Y.; Liao, J.L.; Ni, S.F.; Jen, A.K.J.; Lee, C.S.; Chi, Y. Boosting efficiency of nearinfrared organic light-emitting diodes with Os(II)-based pyrazinyl azolate emitters. Adv. Funct. Mater. 2020, 30, 1906738. [Google Scholar] [CrossRef]
- Bai, G.; Yang, Z.; Lin, H.; Jie, W.; Hao, J. Lanthanide Yb/Er co-doped semiconductor layered WSe2 nanosheets with near-infrared luminescence at telecommunication wavelengths. Nanoscale 2018, 10, 9261–9267. [Google Scholar] [CrossRef]
- Martinic, I.; Eliseeva, S.V.; Nguyen, T.N.; Pecoraro, V.L.; Petoud, S. Near-Infrared Optical Imaging of Necrotic Cells by Photostable Lanthanide-Based Metallacrowns. J. Am. Chem. Soc. 2017, 139, 8388–8391. [Google Scholar] [CrossRef]
- Huang, T.; Yu, Q.; Liu, S.; Huang, W.; Zhao, Q. Phosphorescent Iridium(III) complexes: A versatile tool for bio sensing and photodynamic therapy. Dalton Trans. 2018, 47, 7628. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of platinum complexes for biomedical applications. Acc. Chem. Res. 2015, 48, 2622. [Google Scholar] [CrossRef]
- Tan, C.P.; Zhong, Y.M.; Ji, L.N.; Mao, Z.W. Phosphorescent metal complexes as theranostic anticancer agents: Combining imaging and therapy in a single molecule. Chem. Sci. 2021, 12, 2357. [Google Scholar] [CrossRef]
- Baskaran, S.; Thangaraj, K.; Palanichamy, V.; Shankar, B.; Rajakannu, P. Effect of substituents in tuning the inter- and intra-molecular interactions in the dinuclear Pt(II) complexes. J. Mol. Struct. 2024, 1296, 136900. [Google Scholar] [CrossRef]
- Wei, Y.C.; Chen, B.H.; Ye, R.S.; Huang, H.W.; Su, J.X.; Lin, C.Y.; Hodgkiss, J.; Hsu, L.Y.; Chi, Y.; Chen, K.; et al. Excited-State THz vibrations in aggregates of PtII complexes contribute to the enhancement of near-infrared emission efficiencies. Angew. Chem. Int. Ed. 2023, 62, e202300815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wan, Q.; Ang, W.H.; Kwong, C.L.; To, W.P.; Chow, P.K.; Kwok, C.C.; Che, C.M. High-Performance deep-red/near-infrared OLEDs with tetradentate [Pt(O^N^C^N)] emitters. Adv. Opt. Mater. 2019, 7, 1801452. [Google Scholar] [CrossRef]
- Miskowski, V.M.; Houlding, V.H. Electronic Spectra and Photophysics of Platinum(II) Complexes with α-Diimine Ligands. Solid-State Effects. 2. Metal-Metal Interaction in Double Salts and Linear Chains. Inorg. Chem. 1992, 30, 4446–4452. [Google Scholar] [CrossRef]
- Houlding, V.H.; Miskowski, V.M. The Effect of Linear Chain Structure on the Electronic Structure of Pt(II) Diimine Complexes. Coord. Chem. Rev. 1991, 111, 145–152. [Google Scholar] [CrossRef]
- Li, G.; Shen, G.; Fang, X.; Yang, Y.-F.; Zhan, F.; Zheng, J.; Lou, W.; Zhang, Q.; She, Y. Phosphorescent Tetradentate Platinum(II) Complexes Containing Fused 6/5/5 or 6/5/6 Metallocycles. Inorg. Chem. 2020, 59, 18109–18121. [Google Scholar] [CrossRef] [PubMed]
- Díez, Á.; Forniés, J.; Larraz, C.; Lalinde, E.; López, J.A.; Martín, A.; Moreno, M.T.; Sicilia, V. Structural and Luminescence Studies on π···π and Pt···Pt Interactions in Mixed Chloro-Isocyanide Cyclometalated Platinum(II) Complexes. Inorg. Chem. 2010, 49, 3239–3251. [Google Scholar] [CrossRef]
- Kong, X.; Shen, Y.; Bian, H.; Zhang, Y. Platinum(ii) Diimine Complexes Containing Phenylpyridine Ligands Decorated with Anionic Closo-monocarborane Clusters [CB11H12]−. Dalton Trans. 2023, 52, 3249–3253. [Google Scholar] [CrossRef]
- Navarro-Ranniger, C.; Lopez-Solera, I.; Perez, J.M.; Rodriguez, J.; Garcia-Ruano, J.L.; Raithby, P.R.; Masaguer, J.; Alonso, C. Analysis of Two Cycloplatinated Compounds Derived from N-(4-Methoxyphenyl)-α-benzoylbenzylidenamine. Comparison of the Activity of These Compounds with other Isostructural Cyclopalladated Compounds. J. Med. Chem. 1993, 36, 3795–3801. [Google Scholar] [CrossRef]
- Sakai, K.; Takeshita, M.; Tanaka, Y.; Ue, T.; Yanagisawa, M.; Kosaka, M.; Tsubomura, T.; Ato, M.; Nakano, T. A New One-Dimensional Platinum System Consisting of Carboxylate-Bridged cis-Diammineplatinum Dimers. J. Am. Chem. Soc. 1998, 120, 11353–11363. [Google Scholar] [CrossRef]
- Lu, W.; Chan, M.C.W.; Zhu, N.; Che, C.-M.; Li, C.; Hui, Z. Structural and Spectroscopic Studies on Pt-Pt and π-π Interactions in Luminescent Multinuclear Cyclometalated Platinum(II) Homologues Tethered by Oligophosphine Auxiliaries. J. Am. Chem. Soc. 2004, 126, 7639–7651. [Google Scholar] [CrossRef]
- Jain, V.K.; Jain, L. The Chemistry of Binuclear Palladium(II) and Platinum(II) Complexes. Coord. Chem. Rev. 2005, 249, 3075–3197. [Google Scholar]
- Hendon, C.H.; Walsh, A.; Akiyama, N.; Konno, Y.; Kajiwara, T.; Ito, T.; Kitagawa, H.; Sakai, K. One-dimensional Magnus-type Platinum Double Salts. Nat. Commun. 2016, 7, 11950–11956. [Google Scholar] [CrossRef] [PubMed]
- Tuong-Ly, K.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Nearinfrared Organic Light-emitting Diodes with very High External Quantum Efficiency and Radiance. Nat. Photonics 2017, 11, 63–68. [Google Scholar] [CrossRef]
- Fang, B.; Zhu, Y.; Hu, L.; Shen, Y.; Jiang, G.; Zhang, Q.; Tian, X.; Li, S.; Zhou, H.; Wu, J.; et al. Series of C^N^C Cyclometalated Pt(II) Complexes: Synthesis, Crystal Structures, and Nonlinear Optical Properties in the Near-Infrared Region. Inorg. Chem. 2018, 57, 14134–14143. [Google Scholar] [CrossRef]
- Yang, X.; Guo, H.; Xu, X.; Sun, Y.; Zhou, G.; Ma, W.; Wu, Z. Enhancing Molecular Aggregations by Intermolecular Hydrogen Bonds to Develop Phosphorescent Emitters for High-Performance Near-Infrared OLEDs. Adv. Sci. 2019, 6, 1801930. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, M. Regulation of metal–metal interactions and chromic phenomena of multi-decker platinum complexes having π-systems. Coord. Chem. Rev. 2018, 355, 101–115. [Google Scholar] [CrossRef]
- Yao, H.; Qiao, L.; Yuan, L.; Luan, X.; Shen, Y.; Zhang, Y.; Zhou, L.; Bian, H. Synthesis of phosphorescent syn, anti-isomeric clamshell platinum(II) dimers for OLED applications. J. Mater. Chem. C 2024, 12, 4473–4483. [Google Scholar] [CrossRef]
- Xiong, W.; Meng, F.; Tan, H.; Wang, Y.; Wang, P.; Zhang, Y.; Tao, Q.; Su, S.; Zhu, W. Dinuclear platinum complexes containing aryl-isoquinoline and oxadiazole-thiol with an efficiency of over 8.8%: In-depth investigation of the relationship between their molecular structure and near-infrared electroluminescent properties in PLEDs. J. Mater. Chem. C 2016, 4, 6007–6015. [Google Scholar] [CrossRef]
- Aghakhanpour, R.B.; Nabavizadeh, S.M.; Rashidi, M. Newly Designed Luminescent Di- and Tetranuclear Double Rollover Cycloplatinated(II) Complexes. J. Organomet. Chem. 2016, 819, 216–227. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Zolotarev, A.A.; Balashev, K.P. Binuclear Complexes of Pt(II) with Platinated 2-Phenylbenzothiazole and Bridged Derivatives of Pyridin- and Benzothiazol-2-thiolsRussian. J. Gen. Chem. 2014, 84, 1593–1598. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Yuan, Z.; Han, M.; Wang, J.; Zhu, L.; Tameh, M.S.; Huang, C.; Ma, B. Precise Design of Phosphorescent Molecular Butterflies with Tunable Photoinduced Structural Change and Dual Emission. Angew. Chem. Int. Ed. 2015, 54, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.E.; Chakraborty, A.; Castellano, F.N. Excited State Equilibrium Induced Lifetime Extension in a Dinuclear Platinum(II) Complex. J. Phys. Chem. A 2014, 118, 10391–10399. [Google Scholar] [CrossRef]
- Han, M.; Tian, Y.; Yuan, Z.; Zhu, L.; Ma, B. A Phosphorescent Molecular “Butterfly” that undergoes a Photoinduced Structural Change allowing Temperature Sensing and White Emission. Angew. Chem. Int. Ed. 2014, 53, 1090–10912. [Google Scholar] [CrossRef]
- Chakraborty, A.; Deaton, J.C.; Haefele, A.; Castellano, F.N. Charge-Transfer and Ligand-Localized Photophysics in Luminescent Cyclometalated Pyrazolate-Bridged Dinuclear Platinum(II) Complexes. Organometallics 2013, 32, 3819–3829. [Google Scholar] [CrossRef]
- Sicilia, V.; Borja, P.; Casas, J.M.; Fuertes, S.; Martín, A. Selective synthesis of new half-lantern benzoquinolate platinum complexes. DFT and photophysical studies on the platinum (II,II) derivative. J. Organomet. Chem. 2013, 731, 10–17. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chen, J.-L.; Chi, Y.; Cheng, Y.-M.; Lee, G.-H.; Jiang, C.-M.; Chou, P.-T. Blue-Emitting Platinum(II) Complexes Bearing both Pyridylpyrazolate Chelate and Bridging Pyrazolate Ligands: Synthesis, Structures, and Photophysical Properties. Inorg. Chem. 2007, 46, 11202–11212. [Google Scholar] [CrossRef]
- Utsuno, M.; Yutaka, T.; Murata, M.; Kurihara, M.; Tamai, M.; Nishihara, H. Synthesis of Pendant-Type Anthraquinone-Bridged Cofacial Dinuclear Platinum(II) Complexes and Their Emission Properties. Inorg. Chem. 2007, 46, 11291–11296. [Google Scholar] [CrossRef]
- Rachford, A.A.; Castellano, F.N. Thermochromic Absorption and Photoluminescence in [Pt(ppy)(μ-Ph2pz)]2. Inorg. Chem. 2009, 48, 10865–10867. [Google Scholar] [CrossRef]
- Jamali, S.; Czerwieniec, R.; Kia, R.; Jamshidid, Z.; Zabel, M. Synthesis, structure and photophysical properties of binuclear methylplatinum complexes containing cyclometalating 2-phenylpyridine or benzo{h}quinolineligands: A comparison of intramolecular Pt–Pt and π–π interactions. Dalton Trans. 2011, 40, 9123–9130. [Google Scholar] [CrossRef]
- Aoki, R.; Kobayashi, A.; Chang, H.-C.; Kato, M. Structures and Luminescence Properties of Cyclometalated Dinuclear Platinum(II) Complexes Bridged by Pyridinethiolate Ions. Bull. Chem. Soc. Jpn. 2011, 84, 218–225. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Wang, Y.; Wang, L.; Tan, H.; Zhu, M.; Zhu, W.; Cao, Y. Highly efficient near-infrared emission from binuclear cyclo-metalated platinum complexes bridged with 5-(4-octyloxyphenyl)-1,3,4-oxadiazole-2-thiol in PLEDs. Org. Electron. 2012, 13, 932–937. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Kato, M.; Sasaki, Y.; Ohba, T.; Ito, H.; Kobayashi, A.; Chang, H.-C.; Uosaki, K. Terpyridine platinum(II) complexes containing triazine di- or tri-thiolate bridges: Structures, luminescence, electrochemistry, and aggregation. Dalton Trans. 2012, 41, 11497–11506. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, L.; Liu, Z.-P.; Gan, C.R.R.; Liu, Z.; Zhang, X.-H.; Zhao, J.; Hor, T.S.A. Facile formation and redox of benzoxazole-2-thiolate-bridged dinuclear Pt(II/III) complexes. Dalton Trans. 2012, 41, 12568–12576. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, V.; Forniés, J.; Casas, J.; Martín, A.; López, J.A.; Larraz, C.; Borja, P.; Ovejero, C.; Tordera, D.; Bolink, H. Highly Luminescent Half-Lantern Cyclometalated Platinum(II) Complex: Synthesis, Structure, Luminescence Studies, and Reactivity. Inorg. Chem. 2012, 51, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Yashiro, N.; Shitama, H.; Kobayashi, A.; Kato, M. A Redox-Active Dinuclear Platinum Complex Exhibiting Multicolored Electrochromism and Luminescence. Chem. Eur. J. 2016, 22, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Leopold, H.; Tenne, M.; Tronnier, A.; Metz, S.; Mgnster, I.; Wagenblast, G.; Strassner, T. Binuclear C^C* Cyclometalated Platinum(II) NHC Complexes with Bridging Amidinate Ligands. Angew. Chem. Int. Ed. 2016, 55, 15779–15782. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhong, Y.-W.; Yao, J. Regulation of intra- and intermolecular Pt–Pt and π–π interactions of a U-shaped diplatinum complex to achieve pseudo-polymorphic emissions in solution and crystalline states. J. Mater. Chem. C 2017, 5, 7222–7229. [Google Scholar] [CrossRef]
- Su, N.; Meng, F.; Wang, P.; Liu, X.; Zhu, M.; Zhu, W.; Su, S.; Yu, J. Near-infrared emission from binuclear platinum (II) complexes containing pyrenylpyridine and pyridylthiolate units: Synthesis, photo-physical and electroluminescent properties. Dyes Pigment. 2017, 138, 162–168. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Wang, L.-L.; Qi, X.-W.; Zhang, D.-S.; Yang, Q.-Y.; Shi, Z.-F.; Lina, Q.; Wu, T. Pt…Pt interaction triggered tuning of circularly polarized luminescence activity in chiral dinuclear platinum(II) complexes. Dalton Trans. 2018, 47, 10179–10186. [Google Scholar] [CrossRef]
- Radler, J.J.; Lingerfelt, D.B.; Castellano, F.N.; Chen, L.X.; Li, X. Role of Vibrational Dynamics on Excited-State Electronic Coherence in a Binuclear Platinum Complex. J. Phys. Chem. A 2018, 122, 5071–5077. [Google Scholar] [CrossRef]
- Deaton, J.C.; Chakraborty, A.; Czerwieniec, R.; Yersin, H.; Castellano, F.N. Temperature dependence of photophysical properties of a dinuclear C^N-cyclometalated Pt(II) complex with an intimate Pt–Pt contact. Zero-field splitting and sub-state decay rates of the lowest triplet. Phys. Chem. Chem. Phys. 2018, 20, 25096–25104. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, K.; Li, X.; Wang, H.; Yang, C.; Ni, H.; Li, Q. Four new binuclear platinum (II) complexes with 2-(1H)-quinolinone as bridging ligands: Synthesis, crystal structure and photophysical properties. J. Lumin. 2018, 204, 296–302. [Google Scholar] [CrossRef]
- Chakraborty, A.; Yarnell, J.E.; Sommer, R.D.; Roy, S.; Castellano, F.N. Excited-State Processes of Cyclometalated Platinum(II) Charge-Transfer Dimers Bridged by Hydroxypyridines. Inorg. Chem. 2018, 57, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Fu, L.-W.; Wei, Y.-C.; Liu, S.-H.; Lin, J.-A.; Lee, G.-H.; Chou, P.-T.; Huang, J.-Z.; Wu, C.-I.; Yuan, Y.; et al. Near-Infrared Emission Induced by Shortened Pt−Pt Contact: Diplatinum(II) Complexes with Pyridyl Pyrimidinato Cyclometalates. Inorg. Chem. 2019, 58, 13892–13901. [Google Scholar] [CrossRef] [PubMed]
- Ikeshita, M.; Ito, M.; Naota, T. Variations in the Solid-State Emissions of Clothespin-Shaped Binuclear trans-Bis(salicylaldiminato)platinum(II) with Halogen Functionalities. Eur. J. Inorg. Chem. 2019, 31, 3561–3571. [Google Scholar] [CrossRef]
- Adachi, J.; Mori, T.; Inoue, R.; Naito, M.; Le, N.H.-T.; Kawamorita, S.; Hill, J.P.; Naota, T.; Ariga, K. Emission Control by Molecular Manipulation of Double-Paddled Binuclear PtII Complexes at the Air-Water Interface. Chem. Asian J. 2020, 15, 406–414. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Yuan, C.; Su, F.; Wu, Y.-B.; Lu, L.; Zhu, M.; Xing, S.; Fu, X. Syntheses, crystal structures, and biological evaluations of new dinuclear platinum(II) complexes with 1,2,4-triazole derivatives as bridging ligands. Dalton Trans. 2021, 50, 4527–4538. [Google Scholar] [CrossRef]
- Pinter, P.; Soellner, J.; Strassner, T. Metallophilic Interactions in Bimetallic Cyclometalated Platinum(II) N-Heterocyclic Carbene Complexes. Eur. J. Inorg. Chem. 2021, 30, 3104–3310. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Y.; Chan, M.H.-Y.; Xiao, G.; Zou, B.; Yam, V.W.-W. Realization of Distinct Mechano- and Piezochromic Behaviors via Alkoxy Chain Length-Modulated Phosphorescent Properties and Multidimensional Self-Assembly Structures of Dinuclear Platinum(II) Complexes. J. Am. Chem. Soc. 2021, 143, 10659–10667. [Google Scholar] [CrossRef]
- Lo, K.-W.; Tong, G.S.M.; Cheng, G.; Low, K.-H.; Che, C.-M. Dinuclear PtII Complexes with Strong Blue Phosphorescence for Operationally Stable Organic Light-Emitting Diodes with EQE up to 23 % at 1000 cd m−2. Angew. Chem. Int. Ed. 2022, 61, e202115515. [Google Scholar] [CrossRef]
- Stipurin, S.; Strassner, T. Phosphorescent Bimetallic C^C* Platinum(ii) Complexes with Bridging Substituted Diphenylformamidinates. Chem. Eur. J. 2022, 28, e202202227. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Sun, Y.; Lou, W.; Yang, Y.-F.; She, Y. N-Heterocyclic carbene-based tetradentate platinum(II) complexes for phosphorescent OLEDs with high brightness. J. Mater. Chem. C 2022, 10, 210–218. [Google Scholar] [CrossRef]
- Shi, Z.; Li, F.; Zhao, H.; Chakraborty, I.; Chen, Z.; Raphael, G. Raptis, Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study. Chemistry 2023, 5, 187–200. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Kryukov, D.M.; Kurtsevich, A.E.; Degtyarenko, K.M.; Valiev, R.R.; Levin, O.V.; Kukushkin, V.Y.; Rozhkov, A.V. Incorporation of a Fluorine Atom in a Bridging Ligand of Half-Lantern PtII2 Complexes Provides up to 10-Fold Enhancement of Electroluminescence Brightness. Inorg. Chem. 2023, 62, 11080–11094. [Google Scholar] [CrossRef] [PubMed]
- Pander, P.; Walden, M.T.; Salthouse, R.J.; Sil, A.; Yufit, D.S.; Dias, F.B.; Williams, J.A.G. Rigidly linked dinuclear platinum(II) complexes showing intense, excimer-like, near-infrared luminescence. J. Mater. Chem. C 2023, 11, 15335–15346. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Z.; Xu, Y.; Zhang, Y.; Miao, J.; Chen, Z.; Li, K. High-efficiency and stable red to near-infrared organic light-emitting diodes using dinuclear platinum(II) complexes. Mater. Chem. Front. 2023, 7, 873–880. [Google Scholar] [CrossRef]
- Segura, D.Z.; Corral-Zorzano, A.; Alcolea, E.; Moreno, M.T.; Lalinde, E. Phenylbenzothiazole-Based Platinum(II) and Diplatinum(II) and (III) Complexes with Pyrazolate Groups: Optical Properties and Photocatalysis. Inorg. Chem. 2024, 63, 1589–1606. [Google Scholar] [CrossRef]
- Melendo, I.; Fuertes, S.; Martín, A.; Sicilia, V. NIR-II Emission from Cyclometalated Dinuclear Pt(III) Complexes. Inorg. Chem. 2024, 63, 5470–5480. [Google Scholar] [CrossRef]
- Pander, P.; Dikova, Y.M.; Puttock, E.V.; Williams, J.A.G. Dinuclear platinum(II) complexes emitting through TADF: New ligand design to minimise aggregation and the S1–T1 energy gap. Inorg. Chem. Front. 2024, 11, 7545–7551. [Google Scholar] [CrossRef]
- Salthouse, R.J.; Dikova, Y.M.; Etherington, M.K.; Williams, J.A.G. Dinuclear platinum(II) complexes featuring rigidly linked Pt(NCN)X units: The effect of X = SCN- in favouring low-energy, excimer-like luminescence. New J. Chem. 2024, 48, 18865–18872. [Google Scholar] [CrossRef]
- Hay, P.J. Theoretical Studies of the Ground and Excited Electronic States in Cyclometalated Phenylpyridine Ir(III) Complexes Using Density Functional Theory. J. Phys. Chem. A 2002, 106, 1634. [Google Scholar] [CrossRef]
- Takizawa, S.; Echizen, H.; Nishida, J.; Tsuzuki, T.; Tokito, S.; Yamashita, Y. Finely-tuned Blue-phosphorescent Iridium Complexes based on 2-Phenylpyridine Derivatives and Application to Polymer Organic Light-Emitting Device. Chem. Lett. 2006, 35, 748–749. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.N.; Yoo, H.-J.; Wee, K.-R.; Kang, S.O.; Cho, D.W.; Yoon, U.C. Rational Design, Synthesis, and Characterization of Deep Blue Phosphorescent Ir(III) Complexes Containing (4′-Substituted-2′-pyridyl)-1,2,4-triazole Ancillary Ligands. J. Org. Chem. 2013, 78, 8054–8064. [Google Scholar] [CrossRef]
- Jiang, B.; Gu, Y.; Qin, J.; Ning, X.; Gong, S.; Xie, G.; Yang, C. Deep-red iridium (III) complexes cyclometalated by phenanthridine derivatives for highly efficient solution-processed organic light- emitting diodes. J. Mater. Chem. C 2016, 4, 3492–3498. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Meng, F.; Tang, J.H.; Wang, Y.; You, C.; Tan, H.; Liu, Y.; Zhong, Y.-W.; Su, S.; Zhu, W. Achieving near-infrared emission in platinum (II) complexes by using an extended donor–acceptor-type ligand. Dalton Trans. 2016, 45, 5071–5080. [Google Scholar] [CrossRef]
- Yu, J.; Tan, H.; Meng, F.; Lv, K.; Zhu, W.; Su, S. Benzotriazole-containing donor–acceptor–acceptor type cyclometalated iridium (III) complex for solution-processed near-infrared polymer light emitting diodes. Dyes Pigment. 2016, 131, 231–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.; You, C.; Yang, S.; Xiong, W.; Wang, Y.; Su, S.; Zhu, W. Achieving NIR emission for tetradentate platinum (II) salophen complexes by attaching dual donor-accepter frameworks in the heads of salophen. Dyes Pigment. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wang, X.; He, J.; Wu, J.; Liu, H.; Song, J.; Qu, J.; Chan, W.T.-K.; Wong, W.-Y. Achieving NIR emission for donor–acceptor type platinum (II) complexes by adjusting coordination position with isomeric ligands. Inorg. Chem. 2018, 57, 14208–14217. [Google Scholar] [CrossRef]
- Sommer, J.R.; Shelton, A.H.; Parthasarathy, A.; Ghiviriga, I.; Reynolds, J.R.; Schanze, K.S. Photophysical Properties of Near-Infrared Phosphorescent π-Extended Platinum Porphyrins. Chem. Mater. 2011, 23, 5296–5304. [Google Scholar] [CrossRef]
- Su, N.; Meng, F.; Chen, J.; Wang, Y.; Tan, H.; Su, S.; Zhu, W. Near-Infrared Emitting Pyrazole-Bridged Binuclear Platinum Complexes:Synthesis, Photophysical and Electroluminescent Properties in PLEDs. Dyes Pigment. 2016, 128, 68–74. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, Y.; He, D.; Xu, K.; Bian, Y.; Li, Y.; Sun, W.; Peng, M.; Xiong, W. Achieving deep red to near-infrared emission in dinuclear platinum (II) complexes with the donor-π-acceptor-type cyclometalated ligand. J. Organomet. Chem. 2024, 1021, 123372. [Google Scholar] [CrossRef]
- Park, H.J.; Boelke, C.L.; Cheong, P.H.-Y.; Hwang, D.-H. Dinuclear Pt(II) Complexes with Red and NIR Emission Governed by Ligand Control of the Intramolecular Pt–Pt Distance. Inorg. Chem. 2022, 61, 5178–5183. [Google Scholar] [CrossRef]
- Tzeng, B.-C.; Chiu, T.-H.; Lin, S.-Y.; Yang, C.-M.; Chang, T.-Y.; Huang, C.-H.; Chang, A.H.-H.; Lee, G.-H. Structural Isomerism of Luminescent Dinuclear Pt(II)-Thiolate Diimines. Cryst. Growth Des. 2009, 9, 5356–5362. [Google Scholar] [CrossRef]
- Xue, M.; Lam, T.L.; Cheng, G.; Liu, W.; Low, K.H.; Du, L.; Xu, S.; Hung, F.F.; Phillips, D.L.; Che, C.M. Exceedingly Stable Luminescent Dinuclear Pt(II) Complexes with Ditopic Formamidinate Bridging Ligands for High-Performance Red and Deep-Red OLEDs with LT97 up to 2446 h at 1000 cd m−2. Adv. Opt. Mater. 2022, 10, 2200741. [Google Scholar] [CrossRef]
- Kato, M.; Omura, A.; Toshikawa, A.; Kishi, S.; Sugimoto, Y. Vapor-Induced Luminescence Switching in Crystals of the Syn Isomer of a Dinuclear (Bipyridine)platinum(II) Complex Bridged with Pyridine-2-Thiolate Ions. Angew. Chem. Int. Ed. 2002, 41, 3183–3185. [Google Scholar] [CrossRef]
- Xiong, W.; Meng, F.; You, C.; Wang, P.; Yu, J.; Wu, X.; Pei, Y.; Zhu, W.; Wang, Y.; Su, S. Molecular isomeric engineering of naphthyl-quinoline-containing dinuclear platinum complexes to tune emission from deep red to near infrared. J. Mater. Chem. C 2019, 7, 630–638. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Moreno-Blázquez, S.; Oliván, M.; Oñate, E. N,C,N-Pincers in Platinum Bimetallic Complexes: Influence of the Pincer and Bridging Ligands on the Metal–Metal Bond and the Photophysical Properties. Inorg. Chem. 2024, 63, 14482–14494. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, J.; Xiong, J.; Li, K.; Yang, C. Rigid Bridge-Confined Double-Decker Platinum(II) Complexes Towards High-Performance Red and Near-Infrared Electroluminescence. Angew. Chem. Int. Ed. 2022, 61, e202113718. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, Y.; Song, X.-F.; Miao, J.; Zhang, Y.; Li, K.; Yang, C. Approaching the Shortest Intermetallic Distance of Half-Lantern Diplatinum(II) Complexes for Efficient and Stable Deep-Red Organic Light-Emitting Diodes. Adv. Optical. Mater. 2023, 11, 2300201. [Google Scholar] [CrossRef]
- Koshiyama, T.; Omura, A.; Kato, M. Redox-controlled Luminescence of a Cyclometalated Dinuclear Platinum Complex Bridged with Pyridine-2-thiolate Ions. Chem. Lett. 2004, 33, 1386–1387. [Google Scholar] [CrossRef]
- Roy, S.; Lopez, A.A.; Yarnell, J.E.; Castellano, F.N. Metal-Metal-to-Ligand Charge Transfer in Pt(II) Dimers Bridged by Pyridyl and Quinoline Thiols. Inorg. Chem. 2022, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, M.; Chi, Y.-C.; Worku, M.; Zhou, C.; Lin, H.; Lee, S.; Ben-Akacha, A.; Lin, X.; Huang, C.; Ma, B. Thiazol-2-thiolate-Bridged Binuclear Platinum(II) Complexes with High Photoluminescence Quantum Efficiencies of up to Near Unity. Inorg. Chem. 2020, 59, 13109–13116. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, K.; Zhao, L.; Ni, H.; Li, Q. Binuclear platinum(II) complexes based on 2-mercaptobenzothiazole 2-mercaptobenzimidazole and 2-hydroxpyridine as brigding ligands: Red and near-infrared luminescence originated from MMLCT transition. Dyes Pigment. 2017, 145, 144–151. [Google Scholar] [CrossRef]
- Rajakannu, P.; Lee, W.; Park, S.; Kim, H.S.; Mubarok, H.; Lee, M.H.; Yoo, S. Molecular Engineering for Shortening the Pt···Pt Distances in Pt(II) Dinuclear Complexes and Enhancing the Efficiencies of these Complexes for Application in Deep-Red and Near-IR OLEDs. Adv. Funct. Mater. 2023, 33, 2211853. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J. Emission Wavelength Control via Molecular Structure Design of Dinuclear Pt(II) Complexes: Optimizing Optical Properties for Red- and Near-Infrared Emissions. Crystals 2025, 15, 273. https://doi.org/10.3390/cryst15030273
Park HJ. Emission Wavelength Control via Molecular Structure Design of Dinuclear Pt(II) Complexes: Optimizing Optical Properties for Red- and Near-Infrared Emissions. Crystals. 2025; 15(3):273. https://doi.org/10.3390/cryst15030273
Chicago/Turabian StylePark, Hea Jung. 2025. "Emission Wavelength Control via Molecular Structure Design of Dinuclear Pt(II) Complexes: Optimizing Optical Properties for Red- and Near-Infrared Emissions" Crystals 15, no. 3: 273. https://doi.org/10.3390/cryst15030273
APA StylePark, H. J. (2025). Emission Wavelength Control via Molecular Structure Design of Dinuclear Pt(II) Complexes: Optimizing Optical Properties for Red- and Near-Infrared Emissions. Crystals, 15(3), 273. https://doi.org/10.3390/cryst15030273





