Crystallography and Morphology of (Gd,Y)H2 Hydride in a Mg-Gd-Y-Al Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
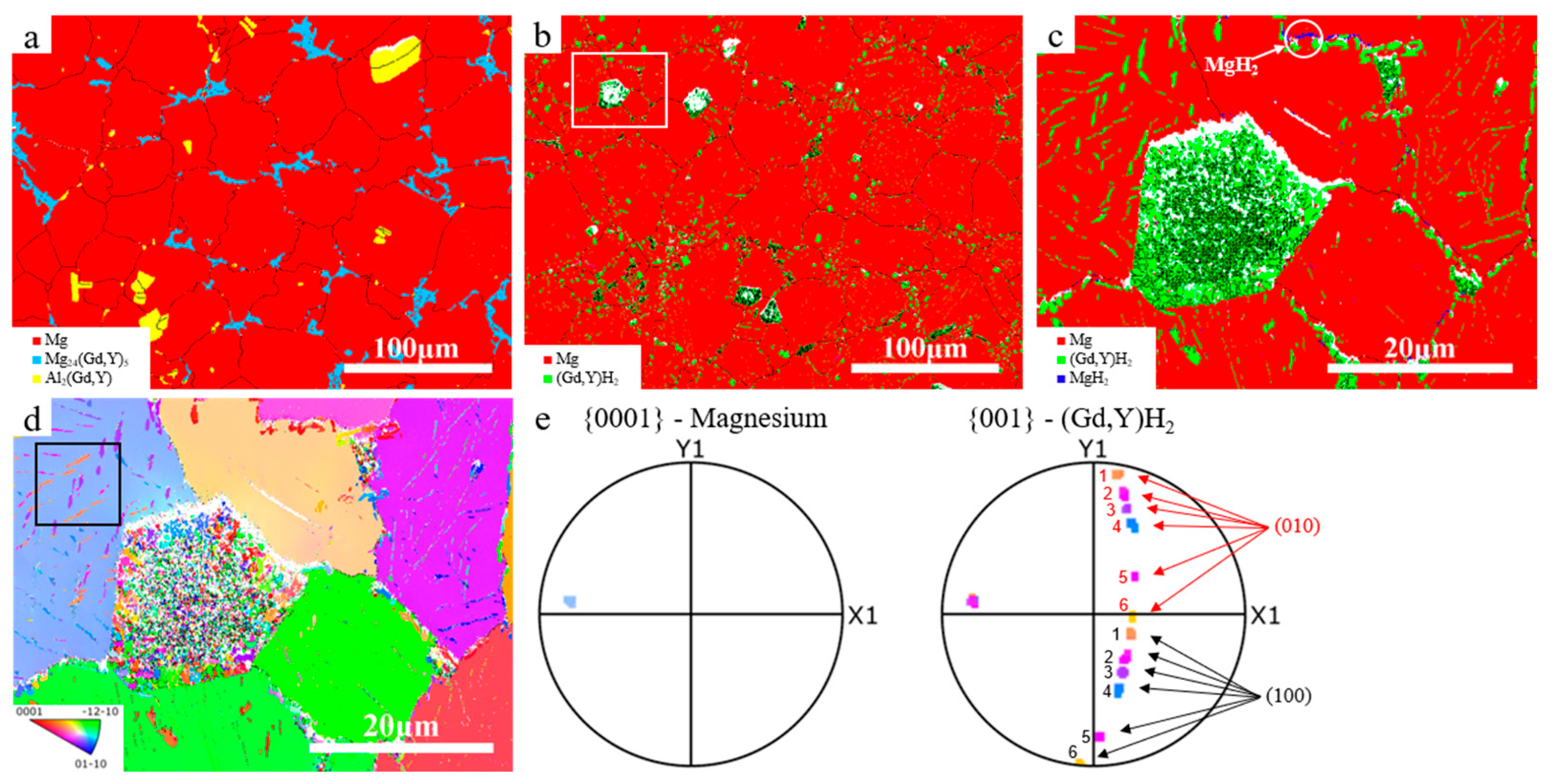
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Li, Y.H.; Zhang, N.; Li, Z.; Lin, X.; Zhu, W.; Lu, C.; Ding, W.J.; Zou, J.X. Nanostructuring of Mg-Based Hydrogen Storage Materials: Recent Advances for Promoting Key Applications. Nano-Micro Lett. 2023, 15, 36–62. [Google Scholar]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen trapping and embrittlement in metals—A review. Int. J. Hydrog. Energy 2024, in press. [Google Scholar] [CrossRef]
- Behvar, A.; Haghshenas, M.; Djukic, M.B. Hydrogen embrittlement and hydrogen-induced crack initiation in additively manufactured metals: A critical review on mechanical and cyclic loading. Int. J. Hydrog. Energy 2024, 58, 1214–1239. [Google Scholar] [CrossRef]
- Lindblom, D.; Halilović, A.E.; Woracek, R.; Tengattini, A.; Helfen, L.; Dahlberg, C.F.O. In-situ neutron imaging of delayed crack propagation of high strength martensitic steel under hydrogen embrittlement conditions. Mater. Sci. Eng. A 2024, 895, 146215. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Q.Z.; Yan, Y. Processing, microstructure, mechanical properties, and hydrogen embrittlement of medium-Mn steels: A review. J. Mater. Sci. Technol. 2024, 201, 44–57. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, J.Y.; Wang, Z.R.; Du, F.S. Effect of pre-strain on hydrogen embrittlement of 7075 aluminum alloy and molecular dynamics simulation. Int. J. Hydrog. Energy 2024, 88, 626–637. [Google Scholar] [CrossRef]
- Safyari, M.; Schnall, M.; Haunreiter, F.; Moshtaghi, M. Design of hydrogen embrittlement resistant 7xxx-T6 aluminum alloys based on wire arc additive manufacturing: Changing nanochemistry of strengthening precipitates. Mater. Des. 2024, 243, 113030. [Google Scholar] [CrossRef]
- Cheng, H.X.; Xu, J.J.; Luo, H.; Duan, G.P.; Zhao, Q.C.; Guo, Y.L. Enhanced hydrogen embrittlement resistance of additive manufacturing Ti-6Al-4V alloy with basket weave structure. Corros. Sci. 2024, 236, 112231. [Google Scholar] [CrossRef]
- Feng, X.D.; Xu, Y.W.; Shi, Y.; Gu, Y.F.; Korzhyk, V. Effects of microstructure and morphological distribution on hydrogen-embrittlement sensitivity of Ti–6Al–4V alloy welded joint. Int. J. Hydrog. Energy 2024, 50, 361–371. [Google Scholar] [CrossRef]
- Sun, B.Z.; Ren, J.X.; Wang, J.; Lv, X.L.; Zeng, Y.Z.; Chen, J.S. On Shapes and Orientations of Precipitation Strengthening Phases in an Aged Mg–La Alloy. Cryst. Growth Des. 2023, 23, 997–1013. [Google Scholar] [CrossRef]
- Ni, R.; Boehlert, C.J.; Zeng, Y.; Chen, B.; Huang, S.J.; Zheng, J.; Zhou, H.; Wang, Q.D.; Yin, D.D. Automated analysis framework of strain partitioning and deformation mechanisms via multimodal fusion and computer vision. Int. J. Plast. 2024, 182, 104119. [Google Scholar] [CrossRef]
- Kamilyan, M.; Silverstein, R.; Eliezer, D. Hydrogen trapping and hydrogen embrittlement of Mg alloys. J. Mater. Sci. 2017, 52, 11091–11100. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.K.; Wang, B.J.; Zhang, Z.Q.; Xu, X.B.; Wang, D.L.; Lv, X. Effect of I-phase formation on hydrogen embrittlement behaviour of as-cast Mg-8 wt%Li based alloys. Corros. Sci. 2024, 240, 112467. [Google Scholar] [CrossRef]
- He, B.; Li, Y.X.; Xiong, Z.H.; Chu, S.F.; Chen, K.; Li, M.W.; Zhou, L.P.; Zeng, X.Q. Effect of high-temperature hydrogenation on mechanical properties of a Mg-B4C composite. J. Alloys Compd. 2024, 975, 172949. [Google Scholar] [CrossRef]
- Chiu, C.; Su, C.-J.; Yu, W.-H.; Rabkin, E. Microstructure and mechanical properties of Mg–GdH2 composite prepared by internal hydrogenation. J. Mater. Sci. 2022, 57, 11649–11662. [Google Scholar] [CrossRef]
- Lapovok, R.; Zolotoyabko, E.; Berner, A.; Skripnyuk, V.; Lakin, E.; Larianovsky, N.; Xu, C.J.; Rabkin, E. Hydrogenation effect on microstructure and mechanical properties of Mg-Gd-Y-Zn-Zr alloys. Mater. Sci. Eng. A 2018, 719, 171–177. [Google Scholar] [CrossRef]
- Vajda, P. Hydrogen in rare-earth metals, including RH2+x phases. Handb. Phys. Chem. Rare Earths 1995, 20, 207–291. [Google Scholar]
- Vajda, P.; Andre, G. Commensurate and incommensurate magnetic structures in rare-earth. J. Alloys Compd. 2001, 326, 151–156. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Kelly, P.M. Crystallographic features of phase transformations in solids. Prog. Mater. Sci. 2009, 54, 1101–1170. [Google Scholar] [CrossRef]
- Huang, Y.D.; Yang, L.; You, S.H.; Gan, W.M.; Kainer, K.U.; Hort, N. Unexpected formation of hydrides in heavy rare earth containing magnesium alloys. J. Magnesium Alloys 2016, 4, 173–180. [Google Scholar] [CrossRef]
- Peng, Q.M.; Huang, Y.D.; Meng, J.; Li, Y.D.; Kainer, K.U. Strain induced GdH2 precipitate in Mg–Gd based alloys. Intermetallics 2011, 19, 382–389. [Google Scholar] [CrossRef]
- Wu, L.Y.; Li, Y.a.; Cheng, Y.L.; Linghu, F.; Jiang, F.L.; Chen, G.; Teng, J.; Fu, D.F.; Zhang, H. Microstructure evolution and corrosion resistance improvement of Mg–Gd–Y–Zn–Zr alloys via surface hydrogen treatment. Corros. Sci. 2021, 191, 109746. [Google Scholar] [CrossRef]
- Wei, Q.H.; Yuan, L.; Ma, X.; Zheng, M.Y.; Shan, D.B.; Guo, B. Strengthening of low-cost rare earth magnesium alloy Mg-7Gd-2Y–1Zn-0.5Zr through multi-directional forging. Mater. Sci. Eng. A 2022, 831, 142144. [Google Scholar] [CrossRef]
- Xu, W.L.; Su, C.; Chen, X.H.; Tan, J.; Feng, L.; Wen, C.; Bai, J.Y.; Pan, F.S. Achieving superior elevated-temperature strength of Mg-12Gd-3Y alloys by Nd addition. Mater. Sci. Eng. A 2023, 867, 144730. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Li, Y.X.; Cao, F.Y.; Qiu, D.; Yang, Y.; Wang, J.Y.; Zhang, H.; Ying, T.; Ding, W.J.; Zeng, X.Q. Towards development of a high-strength stainless Mg alloy with Al-assisted growth of passive film. Nat. Commun. 2022, 13, 5838. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Shang, X.Q.; Zhang, H.; Qi, X.X.; Li, Y.X.; Zeng, X.Q. Influence of Al2Y particles on mechanical properties of Mg-11Y-1Al alloy with different grain sizes. Mater. Sci. Eng. A 2022, 831, 142166. [Google Scholar] [CrossRef]
- Wang, Z.P.; Shen, Z.; Liu, Y.; Zhao, Y.H.; Zhu, Q.C.; Chen, Y.W.; Wang, J.Y.; Li, Y.X.; Lozano-Perez, S.; Zeng, X.Q. The effect of LPSO phase on the high-temperature oxidation of a stainless Mg-Y-Al alloy. J. Magnesium Alloys 2024, 12, 4045–4052. [Google Scholar] [CrossRef]
- Easton, M.A.; Gibson, M.A.; Qiu, D.; Zhu, S.M.; Gröbner, J.; Schmid-Fetzer, R.; Nie, J.F.; Zhang, M.X. The role of crystallography and thermodynamics on phase selection in binary magnesium–rare earth (Ce or Nd) alloys. Acta Mater. 2012, 60, 4420–4430. [Google Scholar] [CrossRef]
- Kelly, P.M.; Zhang, M.X. Edge-to-Edge Matching—The Fundamentals. Metall. Mater. Trans. A 2006, 37, 833–839. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Fu, K.; Li, G.L.; Li, J.G.; Liu, Y.; Tian, W.H.; Zheng, J.; Li, X.G. Study on the thermodynamics of the gadolinium-hydrogen binary system (H/Gd = 0.0–2.0) and implications to metallic gadolinium purification. J. Alloys Compd. 2016, 673, 131–137. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Weatherly, G.C. On the crystallography of precipitation. Prog. Mater. Sci. 2005, 50, 181–292. [Google Scholar] [CrossRef]
- Hwangsun, K.; Howook, C.; Juhyun, O.; Sangmin, L.; Ho, K.; Eun, S.P.; Lee, S.w.; Lee, G.D.; Miyoung, K.; Heung, N.H. Elucidating the role of a unique step-like interfacialstructure of η4 precipitates in Al-Zn-Mg alloy. Sci. Adv. 2023, 9, 7426. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Li, Y.; Su, Y.; Chu, S.; Xiong, Z.; Qiu, D.; Zeng, X. Crystallography and Morphology of (Gd,Y)H2 Hydride in a Mg-Gd-Y-Al Alloy. Crystals 2025, 15, 249. https://doi.org/10.3390/cryst15030249
Chen K, Li Y, Su Y, Chu S, Xiong Z, Qiu D, Zeng X. Crystallography and Morphology of (Gd,Y)H2 Hydride in a Mg-Gd-Y-Al Alloy. Crystals. 2025; 15(3):249. https://doi.org/10.3390/cryst15030249
Chicago/Turabian StyleChen, Kun, Yangxin Li, Yang Su, Shufen Chu, Zhihao Xiong, Dong Qiu, and Xiaoqin Zeng. 2025. "Crystallography and Morphology of (Gd,Y)H2 Hydride in a Mg-Gd-Y-Al Alloy" Crystals 15, no. 3: 249. https://doi.org/10.3390/cryst15030249
APA StyleChen, K., Li, Y., Su, Y., Chu, S., Xiong, Z., Qiu, D., & Zeng, X. (2025). Crystallography and Morphology of (Gd,Y)H2 Hydride in a Mg-Gd-Y-Al Alloy. Crystals, 15(3), 249. https://doi.org/10.3390/cryst15030249






