Abstract
The effects of different processes for heat treatment on microstructures and mechanical properties of a Ti-Al-V-Cr-Fe-based alloy (TLC002) were investigated based on the Ti-6411 alloy designed by Northwest Institute for Nonferrous Metals Research. The results show that the TLC002 alloy treated with solid solution and aging has high strength and low impact toughness. For the annealed specimens, both strength and impact toughness are high. With the rising annealing temperature from 800 °C to 880 °C, the tensile strength (UTS), yield strength (YS), and impact toughness (αu2) increase, especially for the αu2 from 48.7 J/cm2 to 86.0 J/cm2. The tensile and impact specimens treated with both solid solution and aging and annealing are all typical ductile fractures. Both the size dimension and depth of the dimples for the equiaxed structures are greater than those of the bimodal structures, indicating that the plasticity of the equiaxed structures is superior to that of the bimodal structures. The heat treatment that annealing at 880 °C for 1.5 h and then air cooling leads to qualified mechanical properties and a good match of the strength and plasticity of the TLC002 alloy.
1. Introduction
Titanium alloys have become one of the most ideal main structural materials for ocean engineering due to their comprehensive excellent properties such as low density, high specific strength, high corrosion resistance, and non-magnetism [1,2,3,4]. The service environment of marine equipment made of titanium alloys is influenced by factors such as hydrostatic pressure, flow velocity, seawater corrosion, temperature, etc. [1,5]. Thus, this equipment requires materials with high reliability and durability. Mature systems of titanium alloys for marine engineering have formed and been successfully applied to ships, deep submersibles, and other fields [6,7,8,9,10]. A series of titanium alloys with high specific strength and high corrosion resistance have been developed and widely used in the fields of ocean engineering. For example, the most used alloys in the market are TC4 (Ti-6Al-4V) [11,12], TA15 (Ti-6Al-2Zr-1Mo-1V) [13,14,15], VST-5553 (Ti-5Al-5Mo-5V-3Cr) [16,17], Ti5111 (Ti-5Al-1V-1Sn-1Zr-0.8Mo) [18,19], and Ti80 (Ti-6Al-3Nb-2Zr-1Mo) [20,21,22]. Recently, a large number of major projects have been implemented in various fields such as underwater manned submersibles and deep-sea equipment [1]. Higher requirements have been put forward for promoting standardization, scale, and efficiency, and lowering the cost of the production of titanium alloys.
Worldwide teams have designed and developed a variety of new alloys to meet the demand for titanium alloys for marine engineering such as high strength, impact resistance, corrosion resistance, and weldability. Leading nations in titanium alloy research and development, particularly the United States and Japan, have successfully engineered and commercialized a comprehensive range of cost-effective titanium alloy systems. Based on the Ti-6Al-4V alloy system, Liang et al. [23] have successfully developed the Ti-6Al-5Fe-0.05B-0.05C alloy through phase diagram calculations (CALPHAD) and element substitution strategies, using inexpensive element Fe to replace V, which achieves a tensile strength of 1136 MPa and an elongation of 3.7%. Bodunrin et al. [24] also used cheap alloying element Fe to optimize the composition, producing the Ti-6Al-1V-3Fe alloy with a good strength-ductility balance while reducing raw material costs by 10%. Chirico et al. [25] developed a Ti-5Fe-25Nb powder metallurgy titanium alloy that not only exhibits high strength and low modulus but also demonstrates excellent wear resistance. Gunawarman et al. [26] utilized inexpensive Cr-Fe intermediate alloys to develop Ti-4.3Fe-7.1Cr and Ti-4.3Fe-7.1Cr-3.0Al alloys suitable for medical devices, with strengths exceeding 1100 MPa and good fatigue performance. Li et al. [27] prepared the Ti-2Al-9.2Mo-2Fe alloy using Mo-Fe intermediate alloys, achieving strengths of 1200-1400 MPa while maintaining elongations of 7.5–12%. In order to reduce the cost and maintain the mechanical properties in the meantime, expensive elements can be replaced by inexpensive elements. Additionally, a series of studies have been conducted in China. The Harbin Institute of Technology has designed a corrosion-resistant titanium alloy, Ti-5.5Al-4Zr-1Sn-0.3Mo-1Nb, with a compressive strength of up to 952.49 MPa, ultimate strain of 20.82%, and KIC 70.84 MPa·m1/2 [28]. The Nanjing University of Technology has developed a low-cost Ti-3Al-5Mo-4Cr-2Zr-1Fe alloy [29] with yield strength up to 1240 MPa, elongation 8.62%, and KIC 75.8 MPa·m1/2 [30]. The Ti-5322 alloy (Ti-5Al-3V-2Cr-2Fe) was designed based on the TC4 alloy by adding Cr and Fe rather than Mo, V, and Nb [31]. Compared to the TC4 alloy [12], the Ti-5322 alloy has better strength and toughness and has been widely used [32,33].
In order to make major breakthroughs in low-cost titanium alloy composition design and high-efficiency short-process manufacturing, the Northwest Institute for Nonferrous Metals Research has successfully designed a new low-cost Ti-Al-V-Cr-Fe-based titanium alloy (TLC002) on the basis of d-electron theory and ideal cluster structure. The alloy designation is Ti-6411, and the chemical composition is patent pending. The TLC002 alloy is designed based on TC4 alloy by adding a Cr-Fe intermediate. The addition of Fe and Cr is to further improve the mechanical properties. It is bound to increase the Mo equivalent of the TLC002 alloy since both Fe and Cr are strong β-stable elements. Thus, there will be more β phase retained at room temperature. That is to say, the TLC002 alloy with Fe and Cr added will be a two-phase alloy. In this work, the content of oxygen was increased according to the research of the TLC002 alloy. The microstructures and mechanical properties of titanium alloys depend on the processing to a great extent. As for the plates made of titanium alloys in the fields of ocean engineering, high demands are required for the performance indicator. Therefore, it is extremely important to thoroughly study the influence of different processes of heat treatment on the properties of the TLC002 alloy. The laboratory research was carried out to investigate the microstructures and mechanical properties of the TLC002 alloy with different heat treatments. According to the experimental results, the best heat treatment to make the performance qualified was obtained, contributing to providing guidance for the engineering application of the TLC002 alloy.
2. Experiment
The TLC002 alloy was smelted by a vacuum arc remelting (VAR) furnace ZHT001 according to the nominal composition (patent pending). The chemical composition of the ingot is displayed in Table 1. The measured α/β phase transition temperature (Tβ) of the TLC002 alloy is at 960 ± 10 °C by means of metallographic analysis. The three-heat forging process is shown in Figure 1. The forging deformation was about 70% by upsetting and stretching three times for each heat. To avoid the oxidation of the surface during heating for forging and the forging process, the ingot was ground to remove the surface oxide layer after each time of forging.

Table 1.
Chemical composition of the TLC002 alloy ingot.
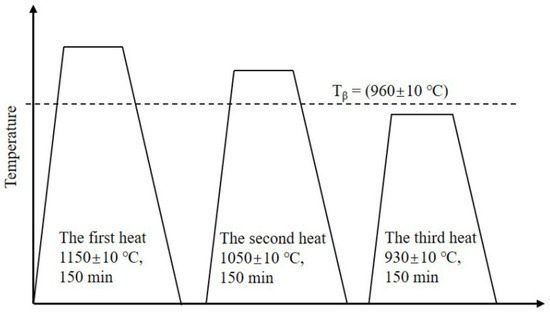
Figure 1.
Diagram of forging for the TLC002 alloy.
The specimens were treated using a chamber electric resistance furnace KSL-1200. Two heat treatment methods were developed: (1) specimens were firstly solution treated at (Tβ—60, 40, 20) °C for 1 h followed by air cooling (AC), and then aged at 500 °C for 4 h followed by air cooling (AC); (2) specimens were annealed at (Tβ—160, 120, 80) °C for 1.5 h followed by air cooling. There are six independent samples for each variant subjected to identical testing conditions in both the tensile and the impact tests. Each presented value is the average of the six results. The mechanical properties were tested by Instron 1195 electronic universal testing machine according to the National Standard [34]. Impact toughness tests were conducted by using the NI300C instrumented Charpy impact testing machine under the National Standard [35]. The schematic diagrams of the dimensions of the tensile specimen and impact specimen, together with the major equipment used during the research, are shown in the Supplementary Material. The microstructure was observed by Olympus MG3 optical microscopy (OM) and JSM-IT510-JEOL (SEM). The fracture morphology was observed by SEM and grain deviation was measured by Image-Pro-Plus analysis software (Version 6.0.0.260). As Figure 2 shows, the microstructure after forging is a uniformly distributed equiaxed structure, including equiaxed primary α phase (αp) and β transition matrix (βt). Both the equiaxed and the bimodal structures are distributed αp and βt. There are secondary α phase (αs) distributed on the βt. The amount of αp is about 56.48%, with an average grain size of 15.30 μm.

Figure 2.
The forged microstructure of the TLC002 alloy.
3. Results and Discussion
3.1. Microstructures
The microstructures after solid solution and aging treatment for the TLC002 alloy are exhibited in Figure 3a–c. The microstructures are bimodal consisting of spherical and rodlike primary α phase (αp) and β transition matrix (βt). When the solution temperature is at 900, 920, and 940 °C, the constitute of αp is 43.93, 30.32, and 18.44%, with the average grain size of 17.12, 9.36, and 5.93 μm, respectively. Consistent with the reported results [36,37], the amount and grain size of αp decreased gradually with increasing solution temperature, especially for the rodlike αp. As Figure 3d–f display, annealed samples show equiaxed microstructures with a spherical and rodlike α phase. The volume fraction of αp is 57.16% when the annealing temperature is at 800 °C. As the annealing temperature increases, the volume fraction of αp decreases from 51.75% to 36.27%. The content of the spherical α phase increases, while the percentage composition of the rod α phase decreases. Figure 4 exhibits the microstructure observed by SEM treated with solid solution and aging (920 °C/1 h, AC + 500 °C/4 h, AC) and annealing (880 °C/1.5 h, AC). It is obvious that the αs of the equiaxed structures is much finer than that of the bimodal structures. The average thickness of αs in Figure 4a,b are 0.34 μm and 0.18 μm, respectively. The possible reason for the coarsening αs is the aging process [16].
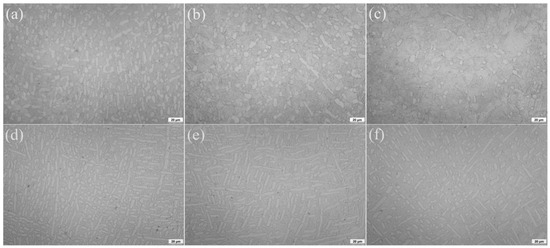
Figure 3.
Optical metallographs of the TLC002 alloy under solid solution and aging (a) 900 °C/1 h, AC + 500 °C/4 h, AC; (b) 920 °C/1 h, AC + 500 °C/4 h, AC; and (c) 940 °C/1 h, AC + 500 °C/4 h, AC; together with specimens under annealing; (d) 800 °C/1.5 h, AC; (e) 840 °C/1.5 h, AC; and (f) 880 °C/1.5 h, AC.
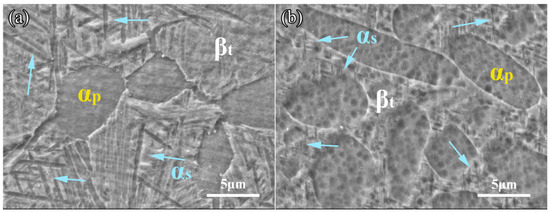
Figure 4.
Microstructures of the TLC002 alloy under (a) solid solution and aging (920 °C/1 h, AC + 500 °C/4 h, AC), and (b) annealing (840 °C/1.5 h, AC). The blue arrows stand for the αs.
Figure 5 exhibits the microstructures and concentration distributions of alloying elements of the TLC002 alloy treated with solid solution and aging (900 °C/1 h, AC + 500 °C/4 h, AC) and annealing (840 °C/1.5 h, AC). The microstructure shown in Figure 4a (900 °C/1 h, AC + 500 °C/4 h, AC) is a bimodal structure with αp phases distributed on βt. The TLC002 alloy was treated with solid solution in the two-phase region. Due to the element partition effect, Al is enriched in the αp phase (Figure 5c). The enrichment of Al can enhance the effect of solid solution strengthening [36]. The concentration of V, Cr, and Fe in the α phase is lower than that in the βt phase (Figure 5b,d,e). According to Figure 5f, the O element is uniformly distributed within αp and βt phases. The concentration distribution of O shows rarely significant differences. As Figure 5g displays, the annealed TLC002 alloy (840 °C/1.5 h, AC) shows equiaxed α phases distributed on βt. Similarly, the enrichment of Al can be observed in the α grains (Figure 5i). The distributions of V, Cr, and Fe in the βt phase are relatively more than that in the α phase (Figure 5h,j,k), while the O element shows no evident concentration gradient between the α and βt phases (Figure 5l). Generally, in the alloy with different heat treatments, Al is enriched in the αp phase. The β-stable elements V, Cr, and Fe appear more in the βt phase than the α phase, whereas the interstitial O atoms are evenly distributed in both α and βt phases.
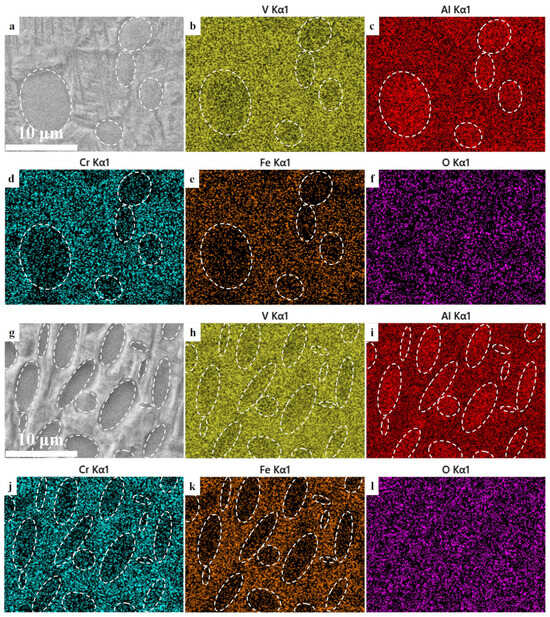
Figure 5.
The (a) bimodal microstructures and concentration distributions of alloying elements (b) V, (c) Al, (d) Cr, (e) Fe, and (f) O of the TLC002 alloy under solid solution and aging (900 °C/1 h, AC + 500 °C/4 h, AC) and (g) equiaxed microstructures and concentration distributions of alloying elements (h) V, (i) Al, (j) Cr, (k) Fe, and (l) O of the TLC002 alloy under annealing (840 °C/1.5 h, AC).
3.2. Mechanical Properties
Representative stress–strain curves in Figure 6a,b display the tensile properties under different heat treatments. Figure 6c,d plot the tensile strength (UTS), yield strength (YS), and impact toughness (αu2) of the solid-soluted and annealed TLC002 alloy. For comparison, the reported UTS, YS, and αu2 of TC4 [38,39] and the previous work of authors are plotted in Figure 6c,d. For specimens treated with solid solution and aging, it can be seen that both the UTS and YS of the TLC002 and TC4 (previous work) maintain a high value and have no obvious change with the increasing solid solution temperature, in line with the reported results of the Ti-55531 alloy [16] but different from that of the TC16 alloy [37]. The possible reason for the difference is that the solution temperature is below (above) the Tβ during experiments of the TLC002 alloy (the TC16 alloy [37]). For annealed specimens, the UTS and YS of the TLC002 are a bit higher than those of TC4. It is obvious that under different heat treatments, both the UTS and YS of the TLC002 are higher than those of TC4 [39], indicating that the addition of new elements can advance the strength. According to the microstructure in Figure 3a–c, with the solid solution temperature at 900 °C, the primary α (αp) and secondary α (αs) phases account for about 43.93 and 38.47%, respectively. As the temperature rises to 920 (940) °C, the content of αp becomes 30.32 (18.44), and the volume fraction of αs is about 46.15 (52.31). The decrease in αp and the increase in αs lead to the difficulty for the grain boundaries to slip during the deformation. In addition, the compatible deformation can barely occur for the αs. Thus, the strength of the TLC002 alloy treated with solid solution and aging is relatively high. The αu2 is lower than the reported results if TC4 [38] and maintains a low value due to the relatively high content of the O element (0.20 wt./%).
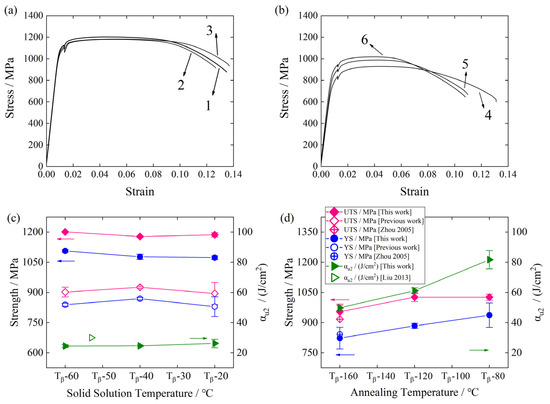
Figure 6.
The representative stress–strain curves of the TLC002 alloy under (a) solid solution and aging (1) 900 °C/1 h, AC + 500 °C/4 h, AC; (2) 920 °C/1 h, AC + 500 °C/4 h, AC; (3) 940 °C/1 h, AC + 500 °C/4 h, AC, as well as (b) annealing (4) 800 °C/1.5 h, AC; (5) 840 °C/1.5 h, AC; (6) 880 °C/1.5 h, AC. The tensile strength (UTS), yield strength (YS), and impact toughness (αu2) of the TLC002 alloy treated with (c) solid solution and aging, and (d) annealing. For comparison, the previous data and available experimental results [38,39] are also displayed.
However, for the annealed alloy, both the UTS and YS increase with the increasing annealing temperature, in line with the reported results [14,40]. Especially, the αu2 increases from 48.7 J/cm2 to 86.0 J/cm2. On the basis of the microstructure analysis of Figure 3d–f, when the annealing temperatures are at 800, 840, and 880 °C, the volume fractions of αp are 57.67, 52.23, and 37.32%, respectively. The volume fractions of αp decline with the increasing annealing temperature. Moreover, the grain size of αp reduces from 25.75 to 9.41 μm with the increase in annealing temperature, and the shape changes from rod-like grains to spherical grains. The variation in the content, size, and shape for the αp phase can reduce the amount of the slip systems of grain boundaries during the deformation, resulting in an increase in the strength. According to the results, the mechanical properties of specimens annealed at 880 °C meet the criteria, and the strength and plasticity are well-matched.
The average UTS, YS, and Young’s Modulus (E) of the TLC002 alloy treated with solid solution, aging, and annealing are listed in Table 2. The UTS, YS, and E of the solid-soluted TLC002 alloy show no significant alteration with the increasing solid solution temperature. However, for the annealed alloy, the UTS, YS, and E present a direct proportionality to the annealing temperature. That is to say, the temperature dependence of both elastic modulus and strength demonstrates remarkable consistency throughout the experimental temperature range.

Table 2.
The average tensile strength (UTS), yield strength (YS), and Young’s Modulus (E) of the TLC002 alloy.
As shown in Figure 7a–f, the fractographies of the heat-treated TLC002 alloy display characteristics of multiple dimples and micropores, corresponding to the reported fractographs [40]. A microcrack can be also observed in Figure 7c. The specimens are typical ductile fractures. As for equiaxed microstructures (Figure 7d–f), many dense and uniform dimples and some micro-voids are distributed on the fracture surfaces. For the bimodal structures (Figure 7a–c), the average diameters of the dimples are about 6.76, 9.28, and 10.83 μm, respectively, and the average diameters of the dimples are about 9.84, 10.72, and 12.65 μm, respectively. The size and depth of the dimples for the equiaxed structures (Figure 7d–f) were larger than those of the bimodal structures (Figure 7a–c), implying that the plasticity of the equiaxed structures is better than that of the bimodal structures.
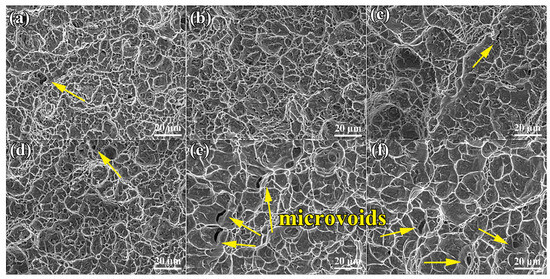
Figure 7.
Tensile fracture morphologies of the TLC002 alloy with different solid solution and aging temperatures: (a) 900 °C/1 h, AC + 500 °C/4 h, AC; (b) 920 °C/1 h, AC + 500 °C/4 h, AC; (c) 940 °C/1 h, AC + 500 °C/4 h, AC, as well as annealing temperatures: (d) 800 °C/1.5 h, AC; (e) 840 °C/1.5 h, AC; (f) 880 °C/1.5 h, AC.
The crack initiation, propagation, and final fracture zones of the impact fracture appearances of the specimens for the TLC002 alloy are characterized. According to Figure 8a2–f2, dimples can be observed in the crack propagation zones for both the bimodal and equiaxed structures, indicating that the large plastic deformation occurred, and the ductile fracture developed. In line with the reported results [18], the final fracture zones for both the solid-soluted specimens (Figure 8a3,b3,c3) and the annealed specimens (Figure 8d3,e3,f3) are relatively flat. However, different from the research [18], cracks (Figure 8a1,c1) and step-faceting (Figure 8b1) can be observed in the crack initiation zones for solid-soluted specimens. Also, there are several cracks observed in the crack initiation zones of the annealed specimens (Figure 8d1,e1).
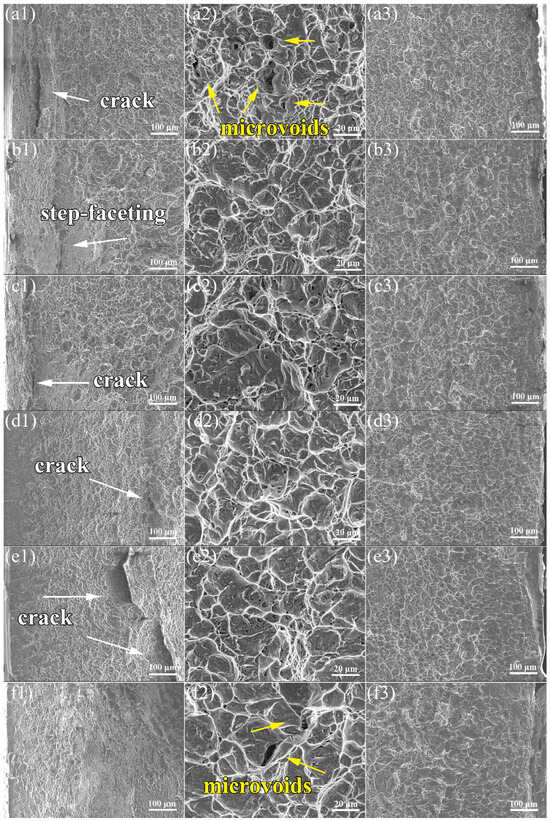
Figure 8.
Impact fracture morphologies of the TLC002 alloy with different solid solution and aging temperatures: (a1–a3) 900 °C/1 h, AC + 500 °C/4 h, AC; (b1–b3) 920 °C/1 h, AC + 500 °C/4 h, AC; (c1–c3), 940 °C/1 h, AC + 500 °C/4 h, AC, as well as annealing temperatures: (d1–d3) 800 °C/1.5 h, AC; (e1–e3) 840 °C/1.5 h, AC; (f1–f3) 880 °C/1.5 h, AC.
The microstructures of the sections for the impact fractures were analyzed. As is exhibited in Figure 9a, for the bimodal structures, voids tend to originate at the αp/βt interface then expand along the αp/βt interface, in line with the reported results [18]. The crack is located at the grain boundary and cut through the βt, resulting in the formation of small dimples on the fracture surface. The region of βt is softer compared to the α phase, thus the plastic deformation is precedent to occur at the βt phase. Due to the Burger’s vector between the lamellar α and β in the βt phase, the dislocations can easily go through the β/α interface. However, the large orientation difference of the αp/βt interface increases the difficulty for dislocations to slip, thus leading to the dislocation pile-up and the concentration of stress at the αp/βt interface. The coordinated deformation of αp and βt can alleviate the concentration of stress to some extent, resulting in the characteristics of microstructure deformation. During the crack propagation, the soft region of βt tends to have plastic deformations and is constrained by surrounding αp grains, leading to torsional deformation of βt. Some of the cracked αp and deformation bands generated in the αp phase can also consume part of the impact energy. The synergistic effect of the torsional deformation for βt and the plastic deformation for αp can effectively release the stress at the crack tip, thus the crack initiation and propagation require more energy. Though the amount of the αp phase in the equiaxed structures is higher than that in the bimodal structures, the length/width ratio of the βt phase in the equiaxed structures is rather large. Different from the reported research [22], the αs of the equiaxed structures is finer than that of the bimodal structures, therefore the crack in the bimodal structures propagates more easily than that in the equiaxed structures.
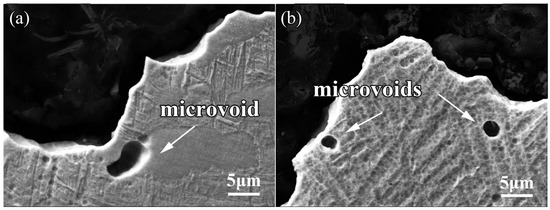
Figure 9.
Impact fracture morphologies of the TLC002 alloy under different heat treatments: (a) 900 °C/1 h, AC + 500 °C/4 h, AC; (b) 880 °C/1.5 h, AC.
4. Conclusions
The effects of different processes for heat treatment on microstructures and mechanical properties of the TLC002 alloy were investigated.
(1) To obtain certified mechanical properties and a good match of the strength and plasticity, the best heat treatment for the TLC002 alloy is 880 °C/1.5 h, AC.
(2) The solid-soluted TLC002 specimens show bimodal microstructures. The annealed samples show equiaxed microstructures.
(3) The samples treated with solid solution and aging have high strength and low impact toughness. For the annealed specimens, the UTS and YS increase with the rising annealing temperature. Additionally, the αu2 increases from 48.7 J/cm2 to 86.0 J/cm2 with the annealing temperature from 800 °C to 880 °C.
(4) Both the solid-soluted and annealed specimens are typical ductile fractures. The plasticity of equiaxed structures is better than that of bimodal structures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15030250/s1, Figure S1: The schematic diagrams of dimensions of tensile specimen and impact specimen; Figure S2: The major equipment used during the research.
Author Contributions
Conceptualization, H.F. and P.G.; methodology, H.F. and P.G.; software, X.T. and F.Q.; validation, H.F. and Z.L.; formal analysis, H.F. and H.W.; investigation, F.Q. and Z.L.; resources, P.G. and H.W.; data curation, H.F.; writing—original draft preparation, H.F.; writing—review and editing, H.F. and P.G.; visualization, X.T.; supervision, S.X. and P.G.; project administration, P.G.; funding acquisition, P.G. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFB3705601), the China Postdoctoral Science Foundation (2024M762659), the Postdoctoral Fellowship of Shaanxi Province (2023BSHGZZHQYXMZZ30), and the National Advanced Rare Metal Materials Technology Innovation Center Project (2024ZG-GCZX-01(1)-06). And The APC was funded by the National Key Research and Development Program of China (2022YFB3705601).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this article and its Supplementary Information Files. The datasets generated and/or analyzed during the current study are not publicly available because the alloy composition is currently under patent application, and the related research project has not yet been concluded. However, the datasets are available from the corresponding author on reasonable request.
Acknowledgments
The authors gratefully acknowledge the National Key Research and Development Program of China (2022YFB3705601), the China Postdoctoral Science Foundation (2024M762659) the Postdoctoral Fellowship of Shaanxi Province (2023BSHGZZHQYXMZZ30), and the National Advanced Rare Metal Materials Technology Innovation Center Project (2024ZG-GCZX-01(1)-06).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chang, H.; Dong, Y.C.; Dan, Z.H.; Li, F.; Guo, Y.H.; Zhou, L. Current Status and Development Trend of Titanium Alloy for Marine Engineering in China. Mater. China 2020, 39, 585–590. [Google Scholar]
- Williams, W.L. Development of structural titanium alloys for marine applications. Ocean Eng. 1969, 1, 375–383. [Google Scholar] [CrossRef]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Charact. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Kang, L.M.; Yang, C. A Review on High-Strength Titanium Alloys: Microstructure, Strengthening, and Properties. Adv. Eng. Mater. 2019, 21, 1801359. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.Q. Current Application and Prospect of Titanium Alloys in Marine Engineering. Dev. Appl. Mater. 2018, 3, 111–116. [Google Scholar]
- Geographic, N. Deepsea Challenger Pilot Sphere. Available online: http://www.deepseachallenge.com/the-sub/pilot-sphere (accessed on 10 August 2024).
- WHOI. Sixty Years of Deep Ocean Research, Exploration, and Discovery with Human-Occupied Vehicle Alvin. 2024. Available online: https://www.whoi.edu/press-room/news-release/alvin-60 (accessed on 10 January 2025).
- Global Dynamics. Alcin a Manned Deep-Ocean Research Project. 2025. Available online: http://gdynx.com/res_dev3.php (accessed on 12 January 2025).
- Busby, R.F. Manned Submersibles. 2025. Available online: http://mannedsubmersibles.org (accessed on 12 January 2025).
- Nanba, N.; Morihana, H.; Nakamura, E. Development of Deep Submergence Research Vehicle SHINKAI 6500. Technol. Rev. Mitsubishi Heavy Ind. Ltd. 1990, 27, 157–168. [Google Scholar]
- Qiang, F.; Xin, S.W.; Tu, X.Y.; Wang, H.; Guo, P.; Hou, H.; Lian, Z.; Zhang, L.; Hou, W. Low-temperature superplastic deformation mechanism of ultra-fine grain Ti-6Al-4V alloy by friction stir processing. J. Mater. Res. Technol. 2024, 30, 7413–7419. [Google Scholar] [CrossRef]
- Zhang, W.J.; Ding, H.; Yang, W.J.; Li, J.Z. Effect of Initial Microstructure on Grain Refinement and Enhanced Low Temperature Superplaticity in Friction Stir Processed Ti-6Al-4V Alloy. Defect Diffus. Forum 2018, 385, 189–194. [Google Scholar] [CrossRef]
- Sha, A.X.; Li, X.W.; Chu, J.P. Common Annealing of TA15 Alloy. Chin. J. Rare Met. 2003, 27, 213–215. [Google Scholar]
- Zhang, J.Y.; Yang, Y.Q.; Chen, Y.; Wen, L.; Zhou, Y.G. Effect of Annealing on the Structure and Properties of TA15 Titanium Alloy. Heat Treat. Met. 2003, 28, 46–48. [Google Scholar]
- Zhao, Y.; Guo, H.Z.; Shi, Z.F.; Zhang, Y.Q.; Yao, Z.K.; Tan, L.J.; Wang, T. Effect of Annealing on Microstructure and Microhardnesws of TA15 Titanium Alloy Processed by Equal Channel Angular Pressing. Adv. Mater. Sci. Technol. 2011, 675–677, 735–738. [Google Scholar]
- Wu, C.; Zhan, M. Effect of solution plus aging heat treatment on microstructural evolution and mechanical properties of near-β titanium alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 997–1006. [Google Scholar] [CrossRef]
- Oryshchenko, A.S.; Gorynin, I.V.; Leonov, V.P.; Kudryavtsev, A.S.; Mikhailov, V.I.; Chudakov, E.V. Marine Titanium Alloys: Present and Future. Inorg. Mater. Appl. Res. 2015, 6, 571–579. [Google Scholar] [CrossRef]
- An, F.P.; Zhang, L.J.; Ning, J.; Zhang, B.; Sun, Z.; Na, S.J. Influence of annealing on the microstructure and Charpy impact toughness of wire arc additive manufactured Ti5111 alloy. Mater. Sci. Eng. A 2022, 860, 144255. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, J.Q.; Zhang, B.B.; Xin, C.; Wu, Y.K.; Zhang, L. Simultaneously improved strength and elongation at cryogenic temperature in Ti-5Al-1V-1Sn-1Zr-0.8Mo alloy with a bimodal structure. Mater. Sci. Eng. A 2021, 824, 141792. [Google Scholar] [CrossRef]
- Mao, P.L.; Cao, Z.X. A Study of Effect of Compositions on Mechanical Properties of Titanium Alloy STi80. J. Shanghai Iron Steel Res. 2001, 4, 10–13. [Google Scholar]
- Yang, J.; Ren, X.L.; Wang, T.; Sun, F.; Zhang, S.; Lei, J.; Li, S.; Chen, H.; Luo, J. Research on preparation of oversized forging billet for Ti80 titanium alloy in ocean engineering. Forg. Stamp. Technol. 2021, 46, 19–22. [Google Scholar]
- Yang, S.L.; Sun, E.J.; Liu, X.Q.; Song, D.; Tao, H.; Zhang, N.; Li, B.; Yu, Y. Effect of Heat Treatment on Microstructure and Properties of Ti6321 Alloy Slab with Different Microstructures. Rare Met. Mater. Eng. 2020, 49, 1002–1008. [Google Scholar]
- Liang, Z.; Miao, J.; Brown, T.; Sachdev, A.K.; Williams, J.C.; Luo, A.A.A. Low-cost and high-strength Ti-Al-Fe-based cast titanium alloy for structural applications. Scr. Mater. 2018, 157, 124–128. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; van der Merwe, J.W.; Alaneme, K.K. On the substitution of vanadium with iron in Ti–6Al–4V: Thermo-Calc simulation and processing map considerations for design of low-cost alloys. Mater. Sci. Eng. A 2020, 791, 139622. [Google Scholar] [CrossRef]
- Chirico, C.; Romero, A.V.; Gordo, E.; Tsipas, S.A. Improvement of wear resistance of low-cost powder metallurgy β-titanium alloys for biomedical applications. Surf. Coat. Technol. 2022, 434, 128207. [Google Scholar] [CrossRef]
- Gunawarman, B.; Niinomi, M.; Akahori, T.; Souma, T.; Ikeda, M.; Toda, H. Mechanical properties and microstructures of low cost β titanium alloys for healthcare applications. Mater. Sci. Eng. C 2005, 25, 304–311. [Google Scholar] [CrossRef]
- Li, C.-L.; Narayana, P.; Reddy, N.; Choi, S.-W.; Yeom, J.-T.; Hong, J.-K.; Park, C.H. Modeling hot deformation behavior of low-cost Ti-2Al-9.2Mo-2Fe beta titanium alloy using a deep neural network. J. Mater. Sci. Technol. 2019, 35, 907–916. [Google Scholar] [CrossRef]
- Luo, L.S.; Su, B.X.; Wang, Y.; Wang, L.; Su, Y.Q.; Guo, J.J. A High-Strength and Corrosion-Resistant Ti-Al-Zr-Sn-Mo-Nb Alloy and Its Preparation Metho. CN110218908A, 10 September 2019. [Google Scholar]
- Chang, H.; Li, J.J.; Gao, H.; Dong, Y.C.; Li, D.; Ni, H.J.; Zhou, L. A Low-Cost Near-β High-Strength Titanium Alloy Adding Fe and Its Preparation Method. CN106521236A, 22 March 2017. [Google Scholar]
- Chen, F.W.; Xu, G.L.; Cui, Y.W.; Chang, H. Optimization of low-cost Ti35421 titanium alloy: Phase transformation, bimodal microstructure, and combinatorial mechanical properties. Materials 2019, 12, 2791. [Google Scholar] [CrossRef]
- Ge, P.; Zhou, W.; Mao, X.N. Effect of rolling deformation on microstructure and properties of Ti-5322 sheet. J. Mater. Eng. 2009, 37 (Suppl. S1), 154–157+162. [Google Scholar]
- Luo, X.; Xu, J.J.; Wang, Y.Q.; Yang, Y.Q.; Umer, M.A.; Zhang, S.Q. Effect of solution and aging treatment on microstructure and tensile properties of Ti-5322 alloy. Heat Treat. Met. 2020, 45, 24–28. [Google Scholar]
- Song, P.; Li, W.B.; Zheng, Y.; Guan, Z.W.; Wang, X.M.; Xu, W.X.; Ge, P. The constitutive behavior of Ti-5Al-3V-2Cr-2Fe under high-velocity impact: Experimental, modeling, and validation. J. Alloys Compd. 2019, 811, 151946. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. China Standard Press: Beijing, China, 2021.
- GB/T 229-2020; Metallic Materials—Charpy Pendulum Impact Test Method. China Standard Press: Beijing, China, 2020.
- An, Y.; Kou, W.J.; Gao, T.; Sun, Q. Effect of solid solution temperature on aging precipitation behavior and properties of Ti-1300 alloy. Heat Treat. Met. 2020, 9, 29–36. [Google Scholar]
- Liu, Q.M.; Zhang, Z.H.; Yang, H.Y.; Liu, S.F. Effects of Solid Solution Temperature on the Structure and Properties of TC16 Titanium Alloy Bars. Adv. Mater. Res. 2014, 881–883, 1588–1591. [Google Scholar] [CrossRef]
- Zhou, W.; Qu, H.L.; Zhao, Y.Q. Effect of Heat Treatment on Microstructure and Mechanical Properties of TC4 Alloy. Hot Work. Technol. 2005, 8, 26–27. [Google Scholar]
- Liu, J.Q. Microstructure and Impact Toughness Resistance of TC4 Titanium Alloy. Hot Work. Technol. 2013, 42, 63–66. [Google Scholar]
- Li, Z.; Su, B.; Chen, C.; Luo, L.; Wang, L.; Su, Y.; Guo, J. Composition Optimization, Microstructure and Mechanical Properties of Ti-Al-Nb-Zr-Mo Alloy with High Strength and Corrosion Resistance. Spec. Cast. Nonferrous Alloys 2020, 40, 591–595. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).