Cotton Swab-Based Surface-Enhanced Raman Spectroscopy Substrate for Ultrasensitive Detection with Year-Long Stability and Multiple Recyclability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for SERS Substrate Fabrication
2.2. Characterization
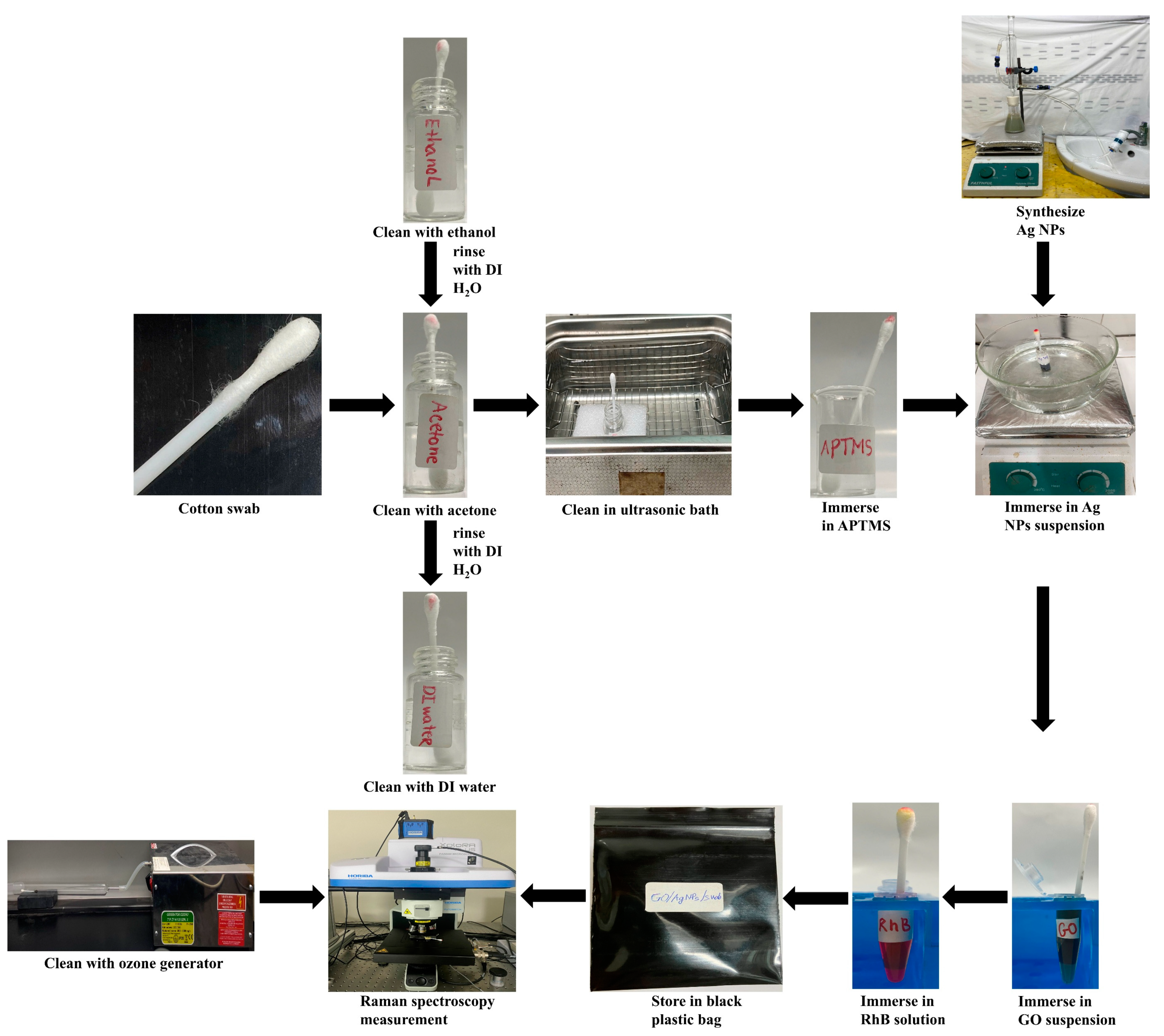
2.3. Methods of SERS Substrate Fabrication
2.4. Performance Tests of SERS Substrates
3. Results and Discussion
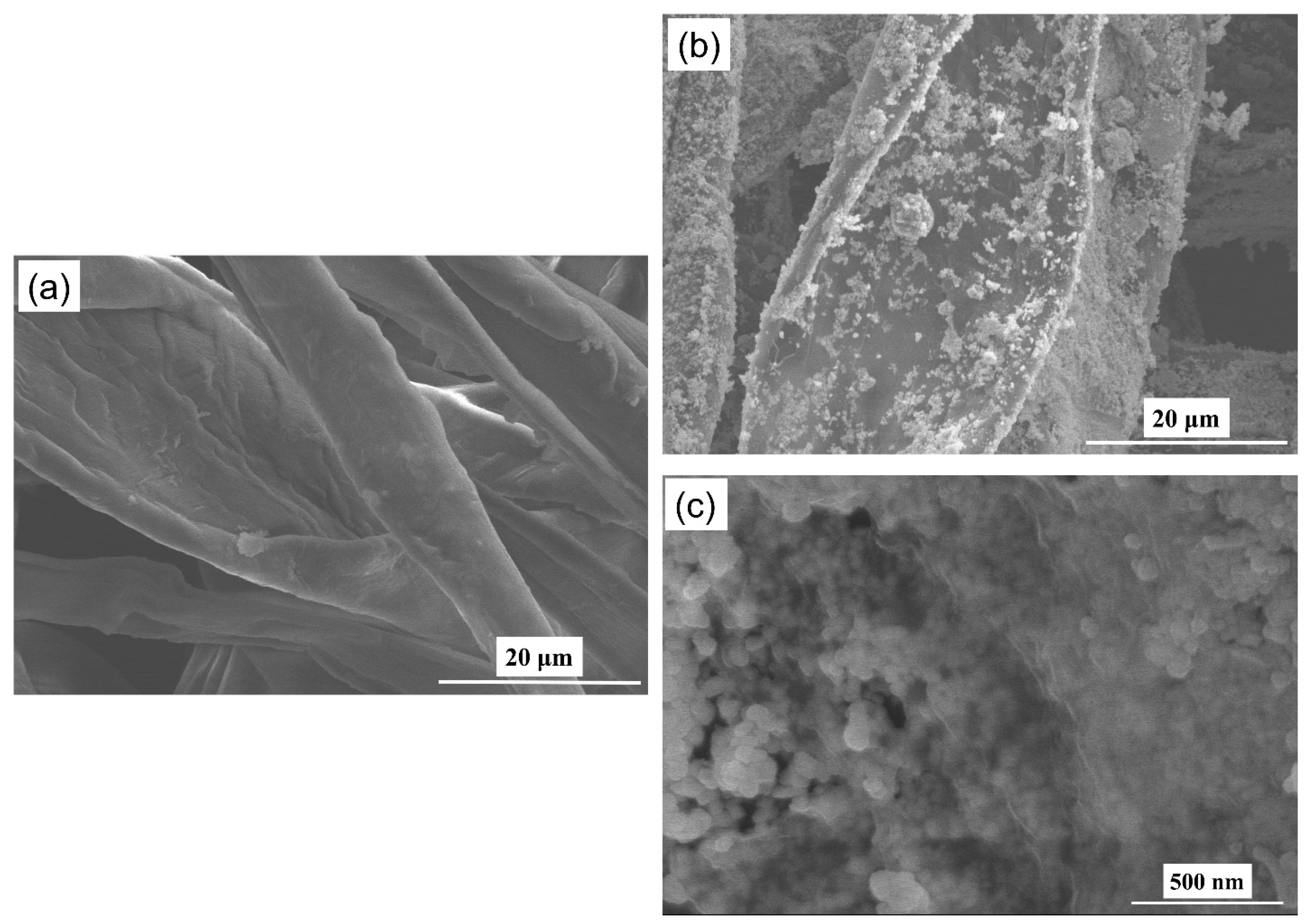
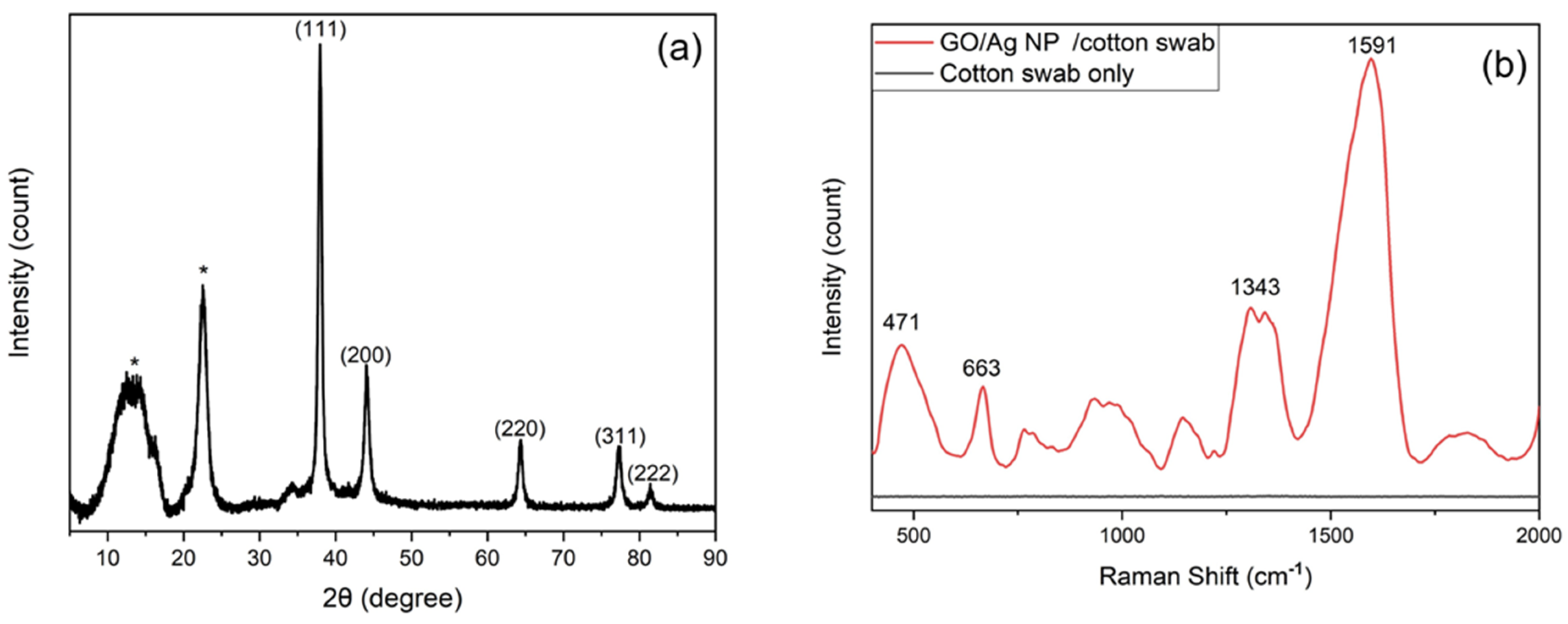
3.1. Characterization of Cotton Swab-Based SERS Substrates
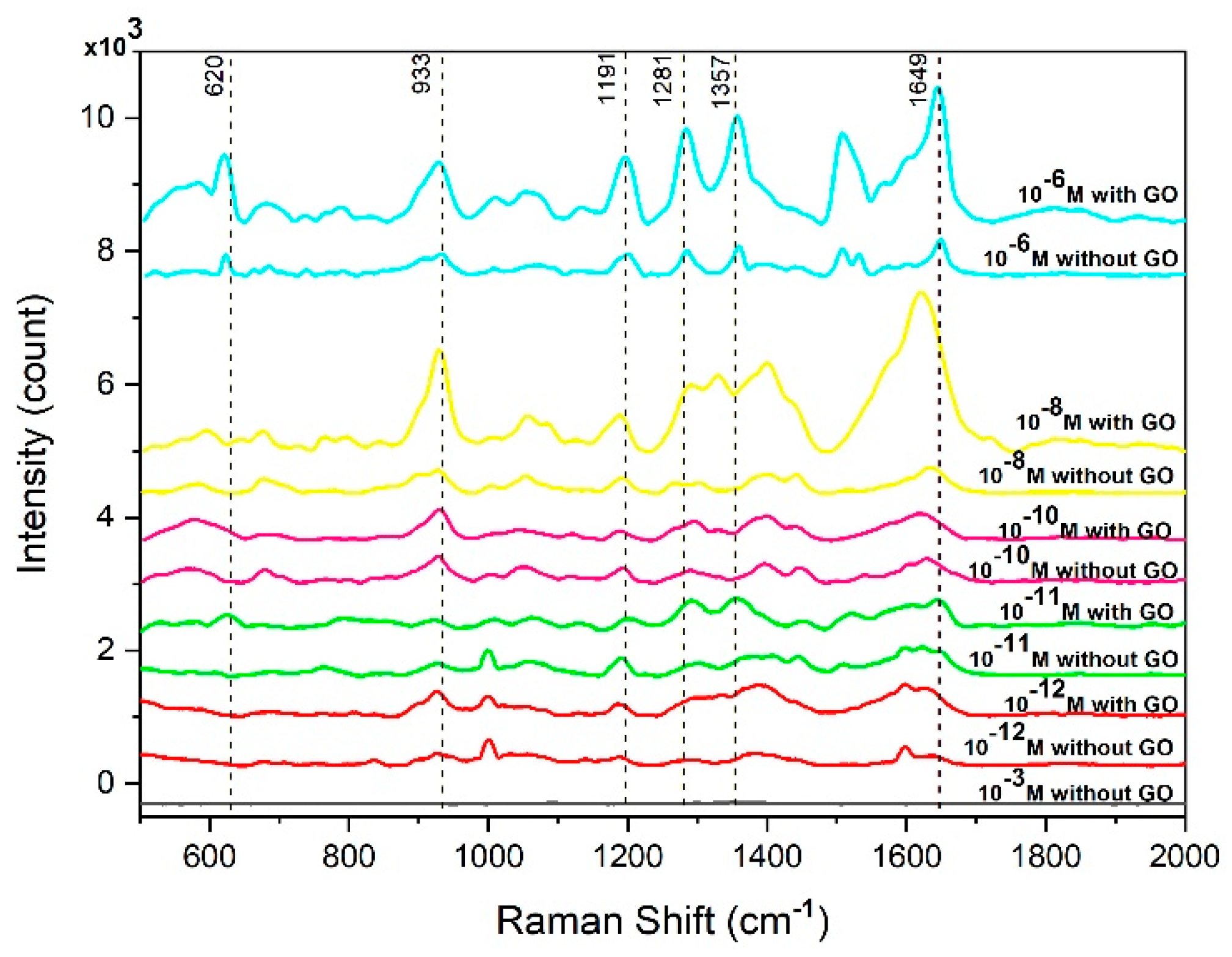
3.2. Performance of Cotton Swab-Based SERS Substrates in Detecting RhB and Thiophanate Methyl
3.3. Investigation of the Stability and Recyclability of Cotton Swab-Based SERS Substrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, L.; Yang, Y.; Wu, D.; Zhang, Y.; Cheng, W.; Tang, X. Fabrication of a metal organic framework (MOF)-modified Au nanoparticle array for sensitive and stable SERS sensing of paraquat in cereals. J. Food Sci. 2023, 88, 1769–1780. [Google Scholar] [CrossRef]
- Xie, W.; Xu, A.; Yeung, E.S. Determination of NAD+ and NADH in a Single Cell under Hydrogen Peroxide Stress by Capillary Electrophoresis. Anal. Chem. 2009, 81, 1280–1284. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, Q. New advances in signal amplification strategies for DNA methylation detection in vitro. Talanta 2024, 273, 125895. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Liu, Y.; Fales, A.M.; Ngo, H.; Wang, H.; Register, J.K.; Yuan, H.; Norton, S.J.; Griffin, G.D. SERS Nanosensors and Nanoreporters: Golden Opportunities in Biomedical Applications. WIREs Nanomed. Nanobiotechnology 2014, 7, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef]
- Hoppmann, E.P.; Yu, W.W.; White, I.M. Highly sensitive and flexible inkjet printed SERS sensors on paper. Methods 2013, 63, 219–224. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Young, M.A.; Lyandres, O.; van Duyne, R.P. Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2005, 127, 4484–4489. [Google Scholar] [CrossRef]
- Qiu, Y.; Kuang, C.; Liu, X.; Tang, L. Single-Molecule Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 4889. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Brolo, A.G.; Lindquist, N.C. Single-Molecule Surface-Enhanced Raman Spectroscopy: Challenges, Opportunities, and Future Directions. ACS Nano 2024, 18, 25930–25938. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Tran, M.; Roy, S.; Kmiec, S.; Whale, A.; Martin, S.; Sundararajan, S.; Padalkar, S. Formation of Size and Density Controlled Nanostructures by Galvanic Displacement. Nanomaterials 2020, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Dao, C.T.; Luong, N.T.Q.; Cao, A.T.; Kieu, M.N. Detection of a Sudan dye at low concentrations by surface-enhanced Raman spectroscopy using silver nanoparticles. Commun. Phys. 2019, 29, 521–526. [Google Scholar] [CrossRef]
- Tran, M.; Whale, A.; Padalkar, S. Exploring the efficacy of platinum and palladium nanostructures for organic molecule detection via Raman spectroscopy. Sensors 2018, 18, 147. [Google Scholar] [CrossRef]
- Kundu, S.; Yi, S.-I.; Ma, L.; Chen, Y.; Dai, W.; Sinyukov, A.M.; Liang, H. Morphology dependent catalysis and surface enhanced Raman scattering (SERS) studies using Pd nanostructures in DNA, CTAB and PVA scaffolds. Dalton Trans. 2017, 46, 9678–9691. [Google Scholar] [CrossRef]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can graphene be used as a substrate for Raman enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, S.X.; Hu, C.F.; Zhang, W.; Zhong, H.Q.; Guo, Z.Y. pH-dependent surface-enhanced Raman scattering of aromatic molecules on graphene oxide. J. Raman Spectrosc. 2013, 44, 75–80. [Google Scholar] [CrossRef]
- He, R.; Lai, H.; Wang, S.; Chen, T.; Xie, F.; Chen, Q.; Liu, P.; Chen, J.; Xie, W. Few-layered vdW MoO3 for sensitive, uniform and stable SERS applications. Appl. Surf. Sci. 2020, 507, 145116. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.J.; Kim, H.; Yi, G.C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in non-plasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef]
- Sun, L.; Hu, H.; Zhan, D.; Yan, J.; Liu, L.; Teguh, J.S.; Yeow, E.K.L.; Lee, P.S.; Shen, Z. Plasma Modified MoS2 Nanoflakes for Surface Enhanced Raman Scattering. Small 2014, 10, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.Y.F.; Rusin, C.T.; McDermott, M.T. Gold nanostars as a colloidal substrate for in-solution SERS measurements using a handheld Raman spectrometer. Analyst 2020, 145, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Bantz, K.; Lindquist, N.; Haynes, C.; Oh, S. Vertically oriented sub-10-nm plasmonic nanogap arrays. Nano Lett. 2010, 10, 2231–2236. [Google Scholar] [CrossRef]
- Wu, Y.; Hang, T.; Komadina, J.; Ling, H.; Li, M. High-adhesive superhydrophobic 3D nanostructured silver films applied as sensitive, long-lived, reproducible and recyclable SERS substrates. Nanoscale 2014, 6, 9720–9726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qin, X.; Yin, D.; Gong, M.; Yang, L.; Zhao, B.; Ruan, W. Rapid monitoring of benzylpenicillin sodium using Raman and surface enhanced Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 474–478. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Y.; Han, S.; Yan, Y.; Zhou, L.; Chen, W.; Zhou, P.; Chen, X.; Roy, V. Controlled assembly of silver nanoparticles monolayer on 3D polymer nanotubes and their applications. Small 2014, 10, 4645–4650. [Google Scholar] [CrossRef]
- Tran, M.; Fallatah, A.; Whale, A.; Padalkar, S. Utilization of inexpensive carbon-based substrates as platforms for sensing. Sensors 2018, 18, 2444. [Google Scholar] [CrossRef]
- Gong, Z.; Du, H.; Cheng, F.; Wang, C.; Wang, C.; Fan, M. Fabrication of SERS swab for direct detection of trace explosives in fingerprints. Appl. Mater. Interfaces 2014, 6, 21931–21937. [Google Scholar] [CrossRef]
- Hoang, M.D.; Nguyen, T.D.; Nguyen, C.T.L.; Luong, A.D.; Le, L.N.; Tran, K.V.; Huynh, K.C.; Nguyen, K.D.; Tran, M.H. Hybrid SERS substrate based on cotton swab for sensitive detection of organic molecules. Mater. Res. Express 2024, 11, 025002. [Google Scholar]
- Kavitha, C.; Bramhaiah, K.; John, N.S.; Ramachandran, B.E. Low cost, ultra-thin films of reduced graphene oxide–Ag nanoparticle hybrids as SERS based excellent dye sensors. Chem. Phys. Lett. 2015, 629, 81–86. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Competitive surface-enhanced Raman scattering effects in noble metal nanoparticle-decorated graphene sheets. Phys. Chem. Chem. Phys. 2011, 13, 21116–21120. [Google Scholar] [CrossRef]
- Ngo, D.X.; Tran, H.Q.; Le, V.V.; Le, T.T.; Le, T.A. Multiwalled carbon nanotubes/silver nanocomposite as effective SERS platform for detection of methylene blue dye in water. J. Sci. Adv. Mater. Devices 2016, 1, 84–89. [Google Scholar]
- Tran, H.T.; Nguyen, H.M.; Mai, H.H.; Pham, T.V.; Sai, D.C.; Nguyen, B.T.; Pham, H.N.; Nguyen, T.T.; Ho, H.K.; Nguyen, T.V. Toxic Gases on β12 Borophene: The Selective Adsorption. VNU J. Sci. Math. Phys. 2020, 36, 1–6. [Google Scholar]
- Fang, H.; Zhang, C.X.; Liu, L.; Zhao, Y.M.; Xu, H.J. Recyclable three-dimensional Ag nanoparticle-decorated TiO2 nanorod arrays for surface-enhanced Raman scattering. Biosens. Bioelectron. 2015, 64, 434–441. [Google Scholar] [CrossRef]
- Kumar, S.; Lodhi, D.K.; Singh, J.P. Highly sensitive multifunctional recyclable Ag–TiO2 nanorod SERS substrates for photocatalytic degradation and detection of dye molecules. RSC Adv. 2016, 6, 45120–45126. [Google Scholar] [CrossRef]
- Xu, S.C.; Zhang, Y.X.; Luo, Y.Y.; Wang, S.; Ding, H.L.; Xu, J.M.; Li, G.H. Ag-decorated TiO2 nanograss for 3D SERS-active substrate with visible light self-cleaning and reactivation. Analyst 2013, 138, 4519–4525. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Le, H.X.; Dao, T.N.; Nguyen, L.T.; Binard, G.; de Marcillac, W.D.; Maitre, A.; Nguyen, L.Q.; Coolen, L.; Pham, N.T. Surface-enhanced Raman scattering from semiconductor and graphene quantum dots coupled to metallic-film-on-nanosphere substrates. Appl. Phys. A 2019, 125, 337. [Google Scholar]
- Gong, T.; Zhu, Y.; Zhang, J.; Ren, W.; Quan, J.; Wang, N. Surface-enhanced Raman scattering from semiconductor and graphene quantum dots coupled to metallic-film-on-nanosphere substrates. Carbon 2015, 87, 385–394. [Google Scholar] [CrossRef]
- Xu, S.; Man, B.; Jiang, S.; Wang, J.; Wei, J.; Xu, S.; Liu, H.; Gao, S.; Liu, H.; Li, Z.; et al. Graphene/Cu nanoparticle hybrids fabricated by chemical vapor deposition as surface-enhanced Raman scattering substrate for label-free detection of adenosine. ACS Appl. Mater. Interfaces 2015, 7, 10977–10987. [Google Scholar] [CrossRef]
- Sun, L.; He, J.; An, S.; Zhang, J.; Ren, D.J. Facile one-step synthesis of Ag@ Fe3O4 core–shell nanospheres for reproducible SERS substrates. Mol. Struct. 2013, 1046, 74–81. [Google Scholar] [CrossRef]
- He, X.; Wang, H.; Zhang, Q.; Li, Z.; Wang, X. Exotic 3D Hierarchical ZnO–Ag Hybrids as Recyclable Surface-Enhanced Raman Scattering Substrates for Multifold Organic Pollutant Detection. Eur. J. Inorg. Chem. 2014, 2014, 2432–2439. [Google Scholar] [CrossRef]
- Weng, X.; Feng, Z.; Guo, Y.; Feng, J.J.; Hudson, S.P.; Zheng, J.; Ruan, Y.; Laffir, F.; Pita, I. Recyclable SERS substrates based on Fe 2 O 3–Ag hybrid hollow microspheres with crumpled surfaces. New J. Chem. 2016, 40, 5238–5244. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Khai, T.V.; Long, L.N.; Khoi, N.H.T.; Thang, N.H. Effects of Hydrothermal Reaction Time on the Structure and Optical Properties of ZnO/Graphene Oxide Nanocomposites. Crystals 2023, 12, 1825. [Google Scholar] [CrossRef]
- Priya, P.S.; Nandhini, P.P.; Vaishnavi, S.; Pavithra, V.; Almutairi, M.H.; Almutairi, B.O.; Arokiyaraj, S.; Pachaiappan, R.; Arockiaraj, J. Rhodamine B, an organic environmental pollutant induces reproductive toxicity in parental and teratogenicity in F1 generation in vivo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 280, 109898. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, W.; Sui, H.; Kitahama, Y.; Ruan, W.; Ozaki, Y.; Zhao, B.J. Exploring the Effect of Intermolecular H-Bonding: A Study on Charge-Transfer Contribution to Surface-Enhanced Raman Scattering of p-Mercaptobenzoic Acid. Phys. Chem. C 2014, 118, 10191–10197. [Google Scholar] [CrossRef]
- Visbal, C.A.; Cervantes, W.R.; Marín, L.; Betancourt, J.; Pérez, A.; Diosa, J.E.; Rodríguez, L.A.; Mosquera-Vargas, E. The Fabrication of Gold Nanostructures as SERS Substrates for the Detection of Contaminants in Water. Nanomaterials 2024, 14, 1525. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Codex Pesticides Residues in Food Online Database. In Codex Alimentarius—International Food Standards; FAO: Roma, Italy, 2023; Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/en/ (accessed on 12 December 2024).
- Wu, Z.; Zhang, C.; Stair, P.C. Influence of absorption on quantitative analysis in Raman spectroscopy. Catal. Today 2006, 113, 40–47. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Lombardi, J.R.; Leona, M.J. Sample self-absorption in surface-enhanced Raman spectroscopy (SERS): Influence of the resonance, dilution and depth of the measurements. Raman. Spectrosc. 2017, 48, 647–652. [Google Scholar] [CrossRef]
- Nguyen, H.T.N.; Le, T.N.T.; Nguyen, P.T.P.; Nguyen, H.D.; Nguyen, T.L.M. Potential application of gold nanospheres as a surface plasmon resonance based sensor for in-situ detection of residual fungicides. Sensors 2020, 20, 2229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Sun, D.W.; Pu, H. Determination of trace thiophanate-methyl and its metabolite carbendazim with teratogenic risk in red bell pepper (Capsicumannuum L.) by surface-enhanced Raman imaging technique. Food Chem. 2017, 218, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef] [PubMed]
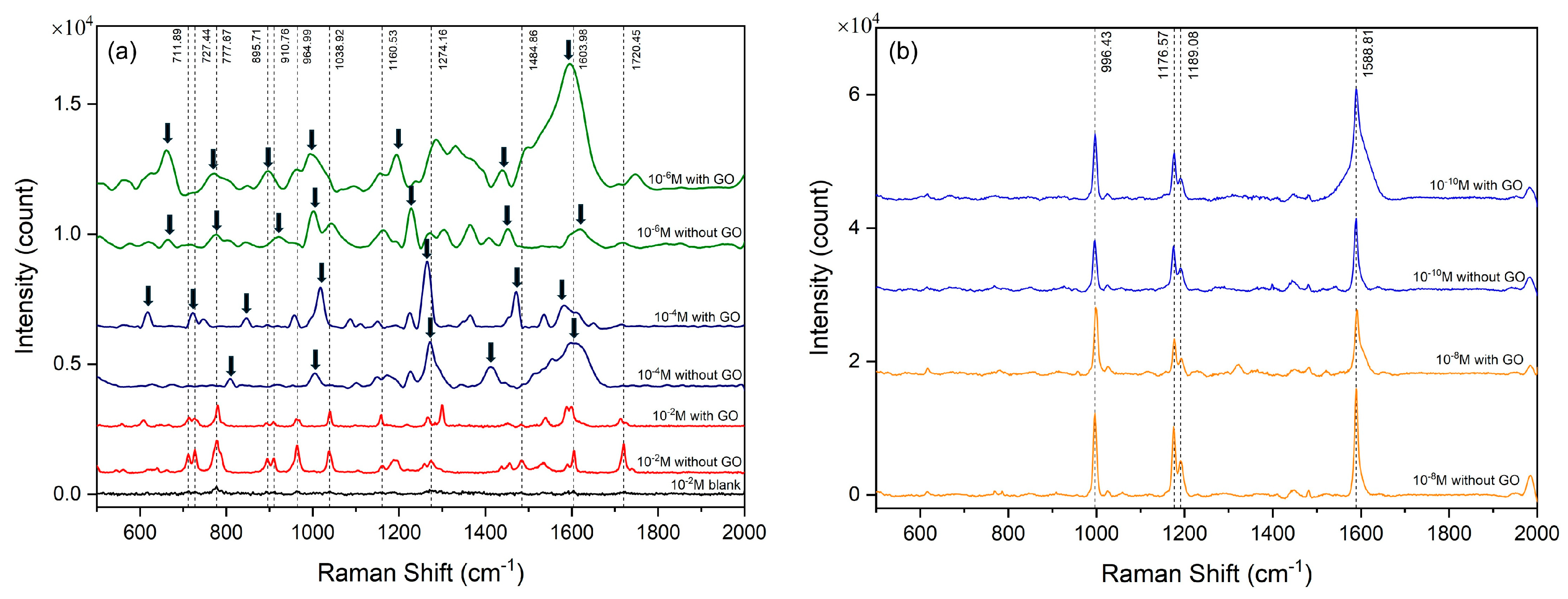
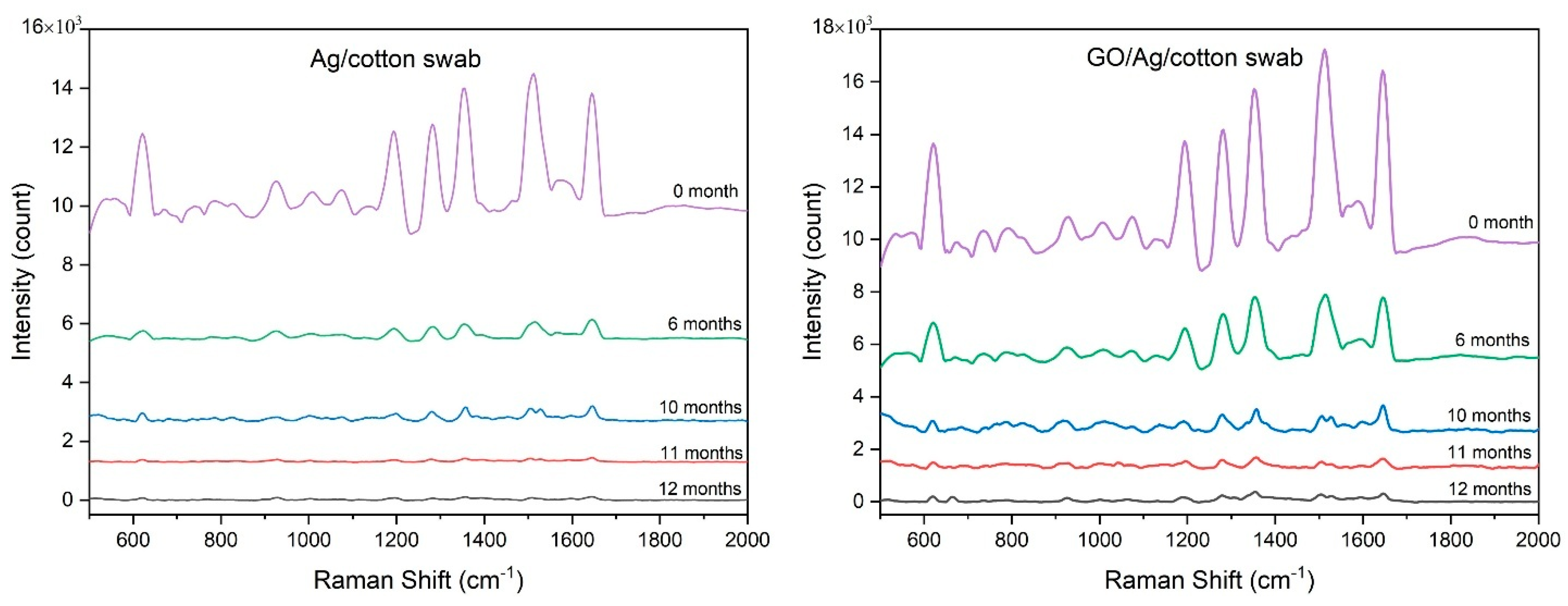
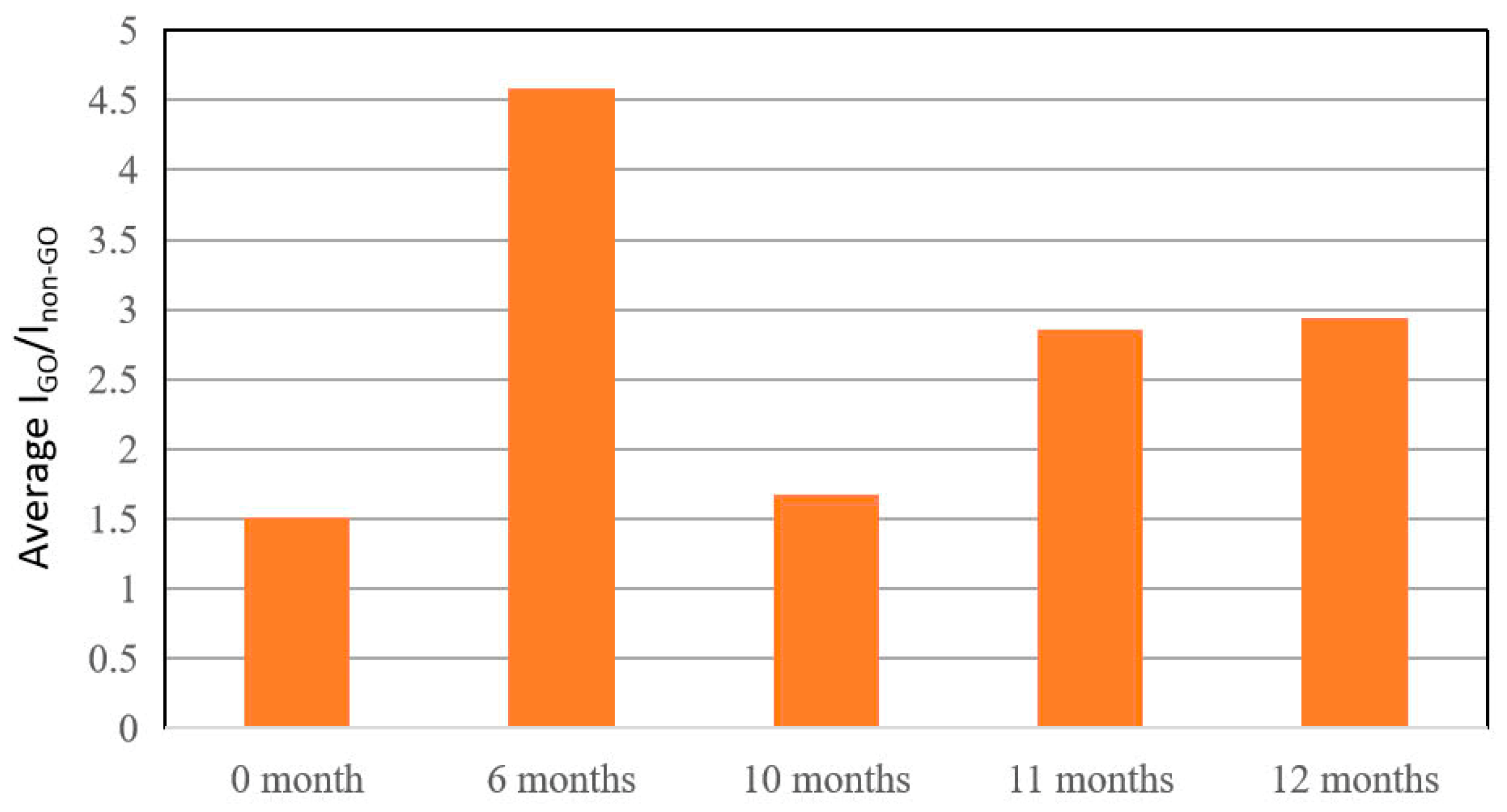
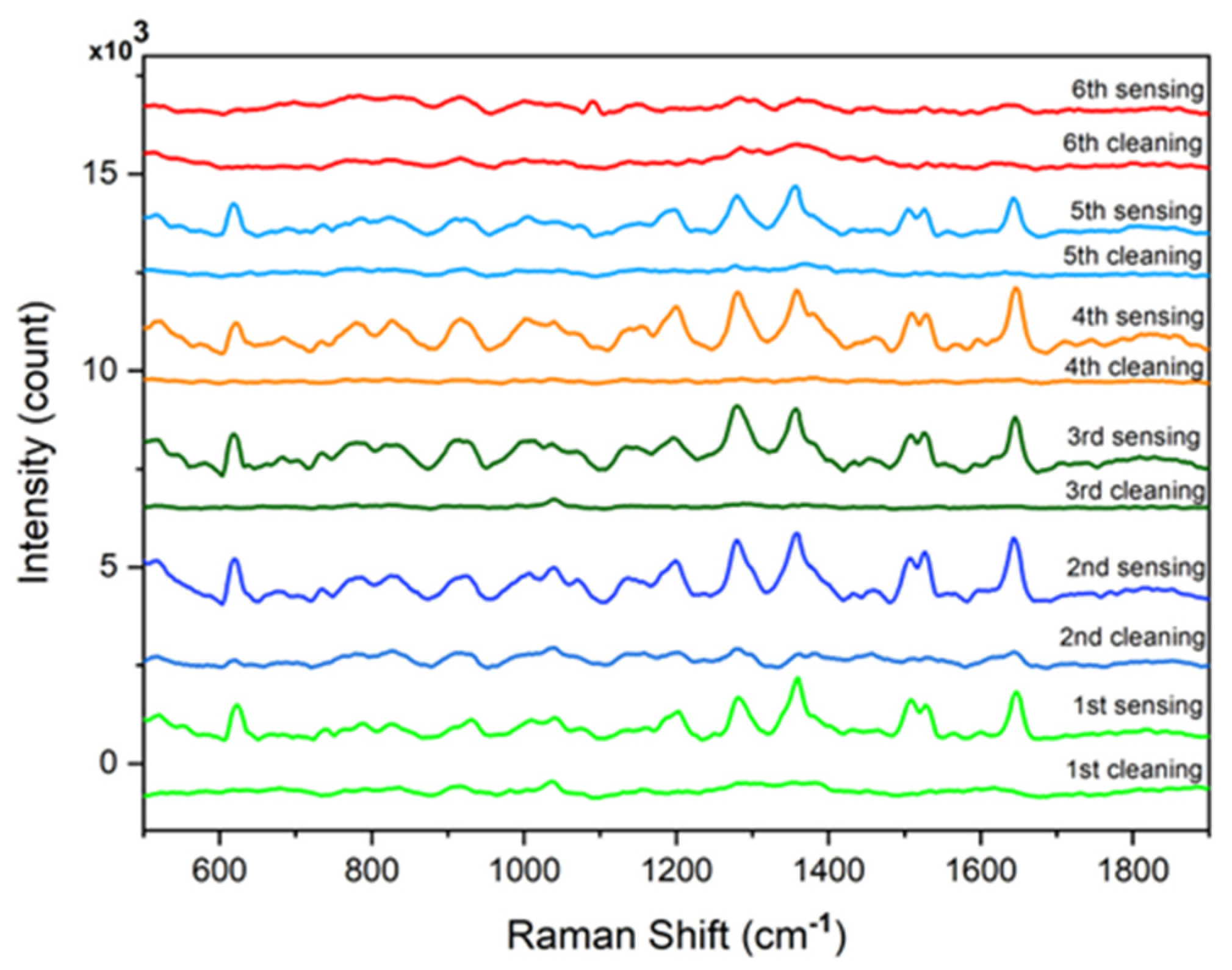
| Raman Shift (cm−1) | Assignment |
|---|---|
| 1644 | Aromatic C–C stretch |
| 1591 | C–H stretch |
| 1551 | Aromatic C–C stretch |
| 1528 | C–H stretch |
| 1508 | Aromatic C–C stretch |
| 1426 | C–H stretch |
| 1360 | Aromatic C–C stretch |
| 1284 | Aromatic C–C stretch |
| 1199 | C–H in-plane bend |
| 1130 | C–H stretch |
| 932 | C–H stretch |
| 773 | C–H stretch |
| 622 | C–C–C stretch |
| 355 | |
| 278 | |
| 240 | Ag–N stretch |
| 213 |
| Raman Shift (cm−1) | Assignment |
|---|---|
| 1708 | C=O stretching, N–H stretching, N–H deformation, –CH3 deformation |
| 1601 | C=C stretching, N–H deformation, C–H deformation |
| 1538 | C–N stretching, N–H deformation |
| 1298 | C–O–C stretching, N–H deformation, C–H deformation |
| 1267 | C–O stretching, N–H deformation, C–H deformation |
| 1154 | N–C–N asymmetric stretching, –CH3 deformation, C–C stretching |
| 1039 | C–H deformation, C–O stretching |
| 959 | C=S stretching, C–H deformation |
| 898 | C–S stretching, C–H deformation |
| 779 | C=S stretching, N–H deformation, C–H deformation |
| 726 | N–H wagging |
| 717 | C–H deformation, N-H bending |
| 613 | –N–C=S deformation |
| 465 | C–O–C deformation |
| 325 | C–C stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.H.; Luong, A.D.; Nguyen, C.L.T.; Hoang, M.D.; Tran, K.V. Cotton Swab-Based Surface-Enhanced Raman Spectroscopy Substrate for Ultrasensitive Detection with Year-Long Stability and Multiple Recyclability. Crystals 2025, 15, 240. https://doi.org/10.3390/cryst15030240
Tran MH, Luong AD, Nguyen CLT, Hoang MD, Tran KV. Cotton Swab-Based Surface-Enhanced Raman Spectroscopy Substrate for Ultrasensitive Detection with Year-Long Stability and Multiple Recyclability. Crystals. 2025; 15(3):240. https://doi.org/10.3390/cryst15030240
Chicago/Turabian StyleTran, Minh H., Anh D. Luong, Chi L. T. Nguyen, Minh D. Hoang, and Khai V. Tran. 2025. "Cotton Swab-Based Surface-Enhanced Raman Spectroscopy Substrate for Ultrasensitive Detection with Year-Long Stability and Multiple Recyclability" Crystals 15, no. 3: 240. https://doi.org/10.3390/cryst15030240
APA StyleTran, M. H., Luong, A. D., Nguyen, C. L. T., Hoang, M. D., & Tran, K. V. (2025). Cotton Swab-Based Surface-Enhanced Raman Spectroscopy Substrate for Ultrasensitive Detection with Year-Long Stability and Multiple Recyclability. Crystals, 15(3), 240. https://doi.org/10.3390/cryst15030240




