Synthesis and Characterization of ONO Pincer Ligand Precursors and Metal Complexes with Ethyl, Isopropyl and Tert-Butyl Wingtip Groups
Abstract
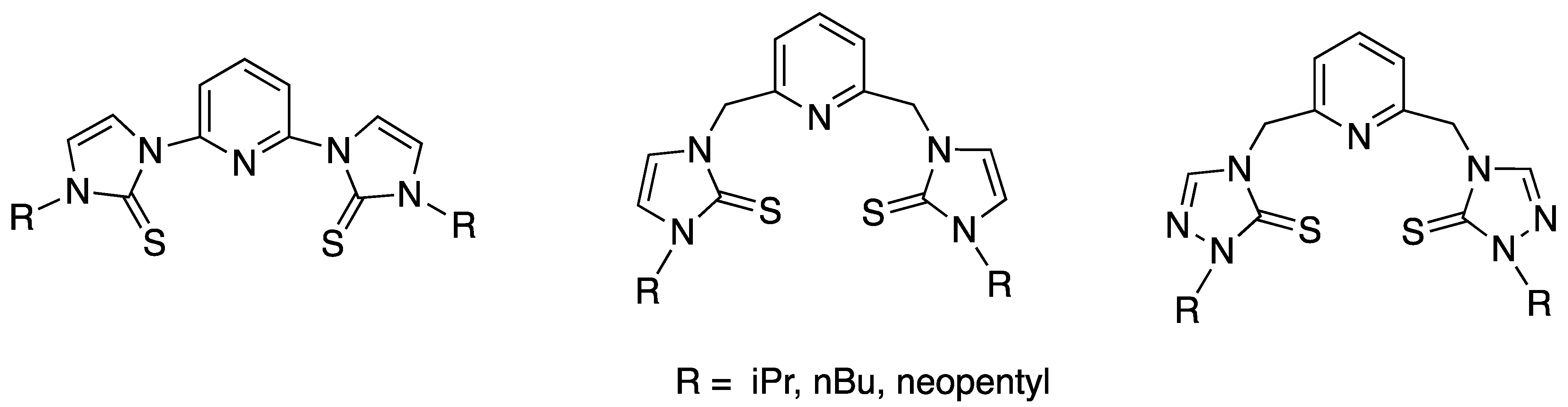
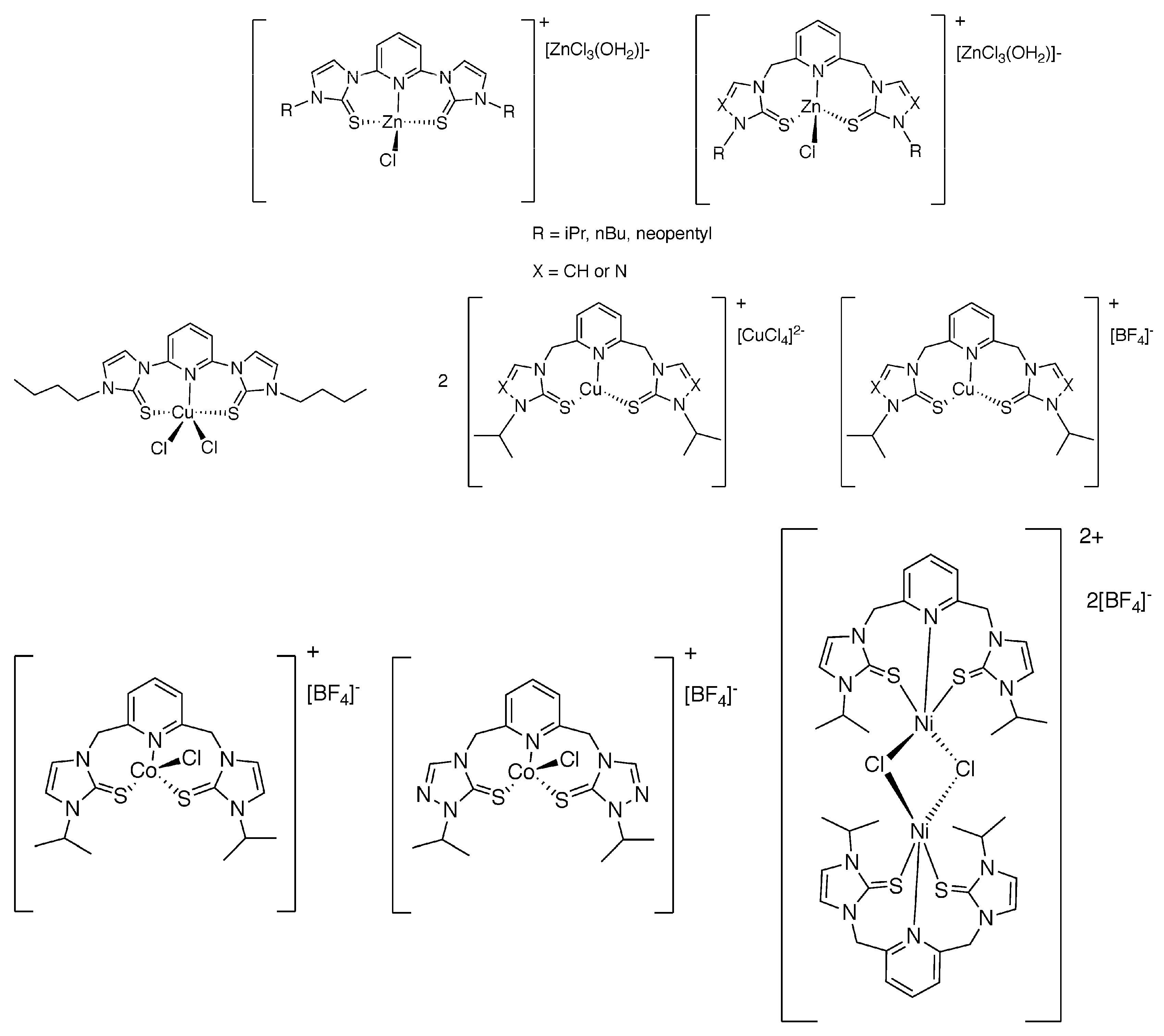
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. General Procedures for Ligand Syntheses and Metal Complexes
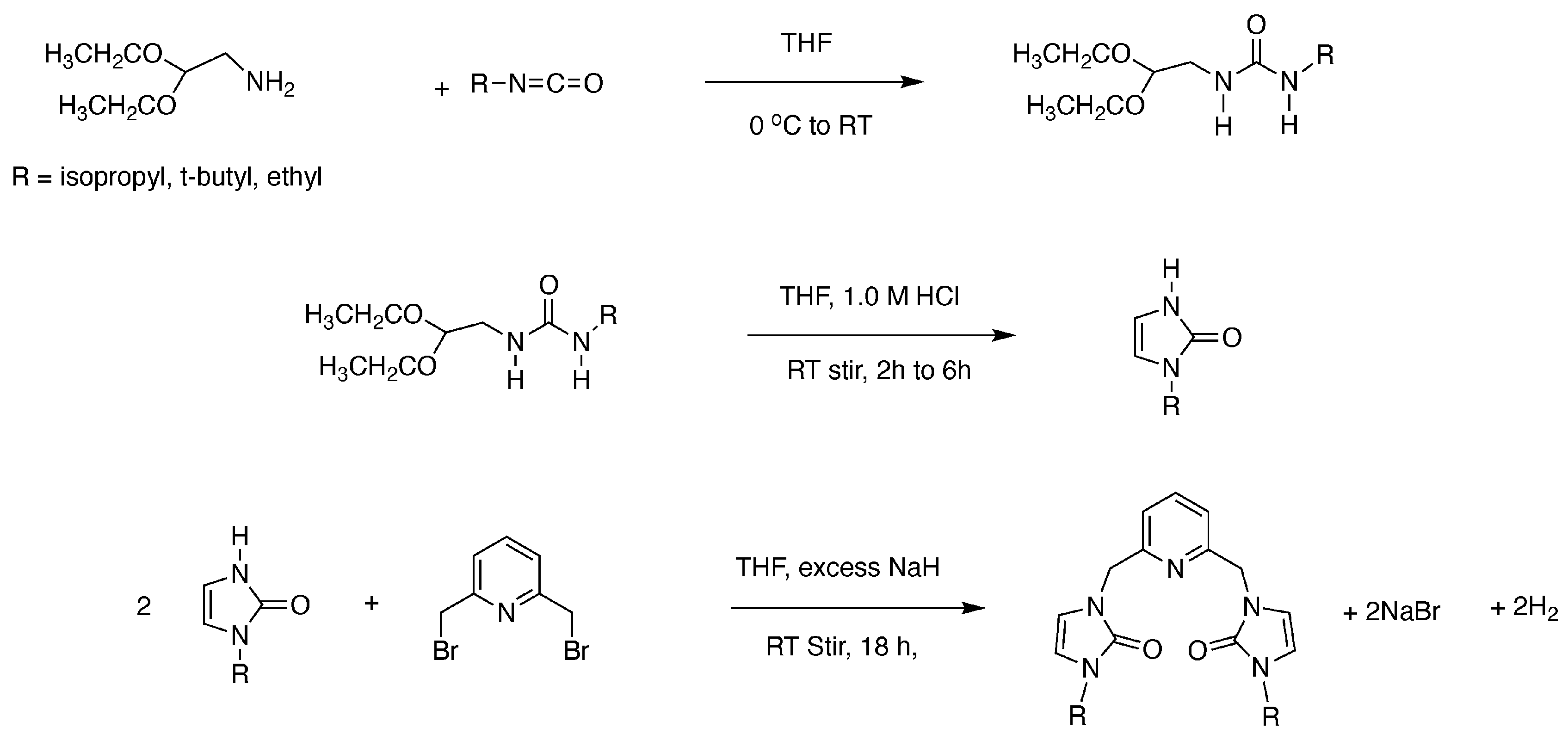
2.3. Synthesis of Ligand Precursors
2.3.1. Preparation of 1-(2,2-Diethoxyethyl)-3-isopropylurea
ESI-MS (Positive Ion Mode) m/z: 241.15 (M + Na)
2.3.2. Preparation of 1-(2,2-Diethoxyethyl)-3-t-butylurea
ESI-MS (Positive Ion Mode) m/z: 255.1684 (M + Na)
2.3.3. Preparation of 1-(2,2-Diethoxyethyl)-3-ethylurea
ESI-MS (Positive Ion Mode) m/z: 227.1385 (M + Na)
2.3.4. Preparation of 1-Isopropyl-1,3-dihydro-2H-imidazol-2-one
ESI-MS (Positive Ion Mode) m/z: 127.0858 (M + H)
2.3.5. Preparation of 1-t-Butyl-1,3-dihydro-2H-imidazol-2-one
ESI-MS (Positive Ion Mode) m/z: 141.1024 (M + H)
2.3.6. Preparation of 1-Ethyl-1,3-dihydro-2H-imidazol-2-one
ESI-MS (Positive Ion Mode) m/z: 113.0720 (M + H)
2.3.7. Preparation of 3,3′-(Pyridine-2,6-diylbis(methylene))bis(1-isopropyl-1,3-dihydro-2H-imidazole-2-one
ESI-MS (Positive Ion Mode) m/z: 378.1870 (M + Na)
2.3.8. Preparation of 3,3′-(Pyridine-2,6-diylbis(methylene))bis(1-t-butyl-1,3-dihydro-2H-immidazol-2-one Product
ESI-MS (Positive Ion Mode) m/z: 384.2423 (M + H)
2.3.9. Preparation of 3,3′-(Pyridine-2,6-diylbis(methylene))bis(1-ethyl-1,3-dihydro-2H-immidazol-2-one Product
ESI-MS (Positive Ion Mode) m/z: 328.1797 (M + H)
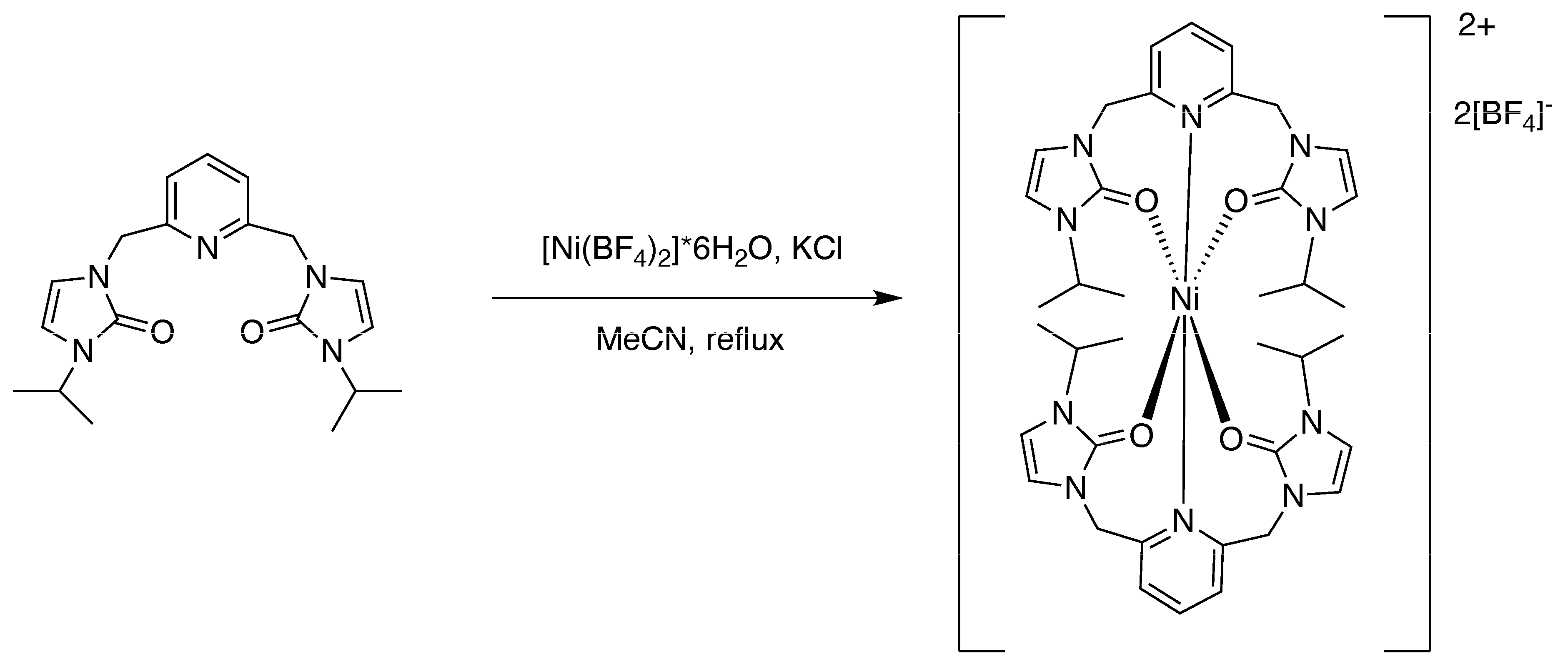
2.4. Preparation of nickel(II) and cobalt(II) Complexes
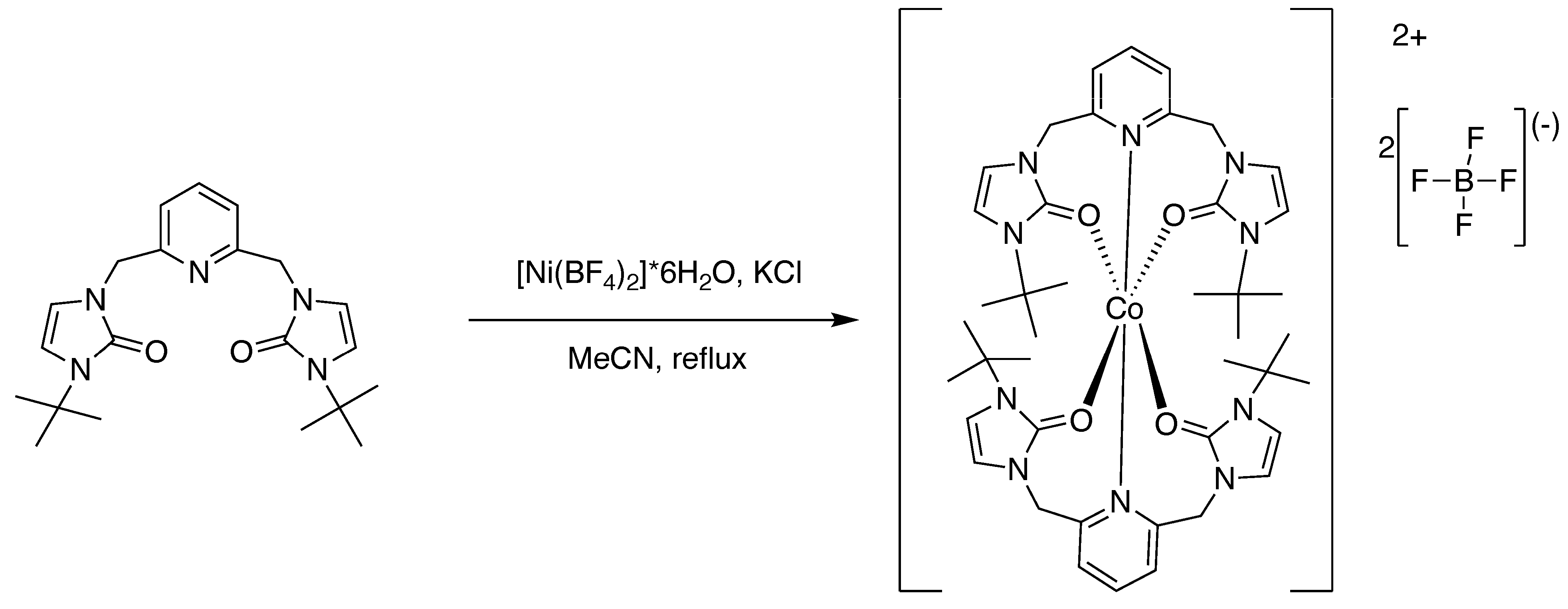
2.4.1. Preparation of Bis-(n3-O,O,N)-[2,6-bis(N-isopropyl-N’-methyleneimidazole-2-one)pyridine]nickel(II) Tetrafluoroborate [1]
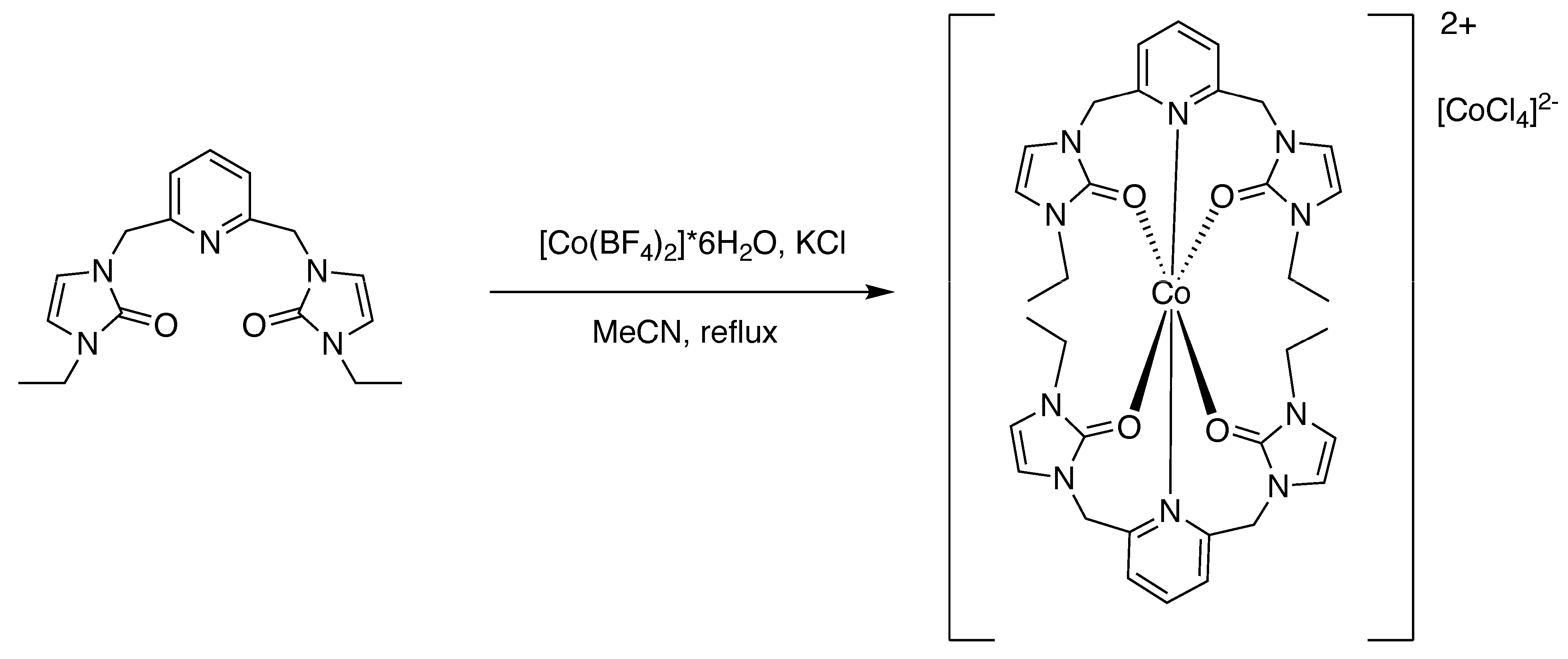
2.4.2. Preparation of Bis-(n3-O,O,N)-[2,6-bis(N-ethyl-N’-methyleneimidazole-2-one)pyridine]cobalt(II) Tetrachlorocobaltate [2]
2.4.3. Preparation of Bis-(n3-O,O,N)-[2,6-bis(nt-butyl-N’-methyleneimidazole-2-one)pyridine]cobalt(II) Tetrafloroborate [3]
- Characterization:
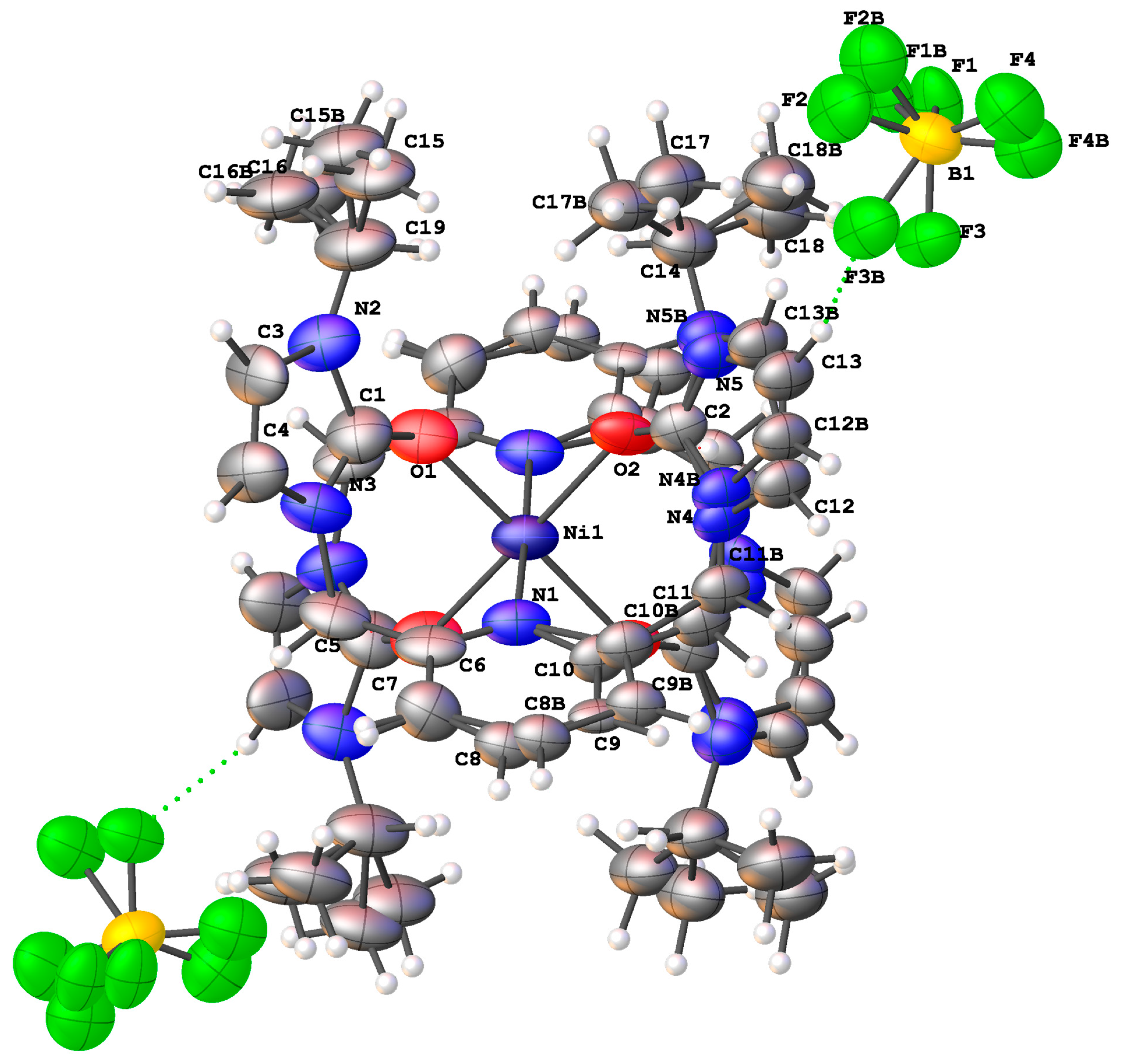
- Single Crystal X-Ray Crystallography
- Nickel(II) complex [1], cobalt(II) complex [2], cobalt(II) complex [3]
- Experimental
3. Results and Discussion
3.1. Preparation and Characterization of the Ligand Precursors
3.2. Preparation of the Metal Complexes
3.3. Magnetic Susceptibility of the Metal Complexes
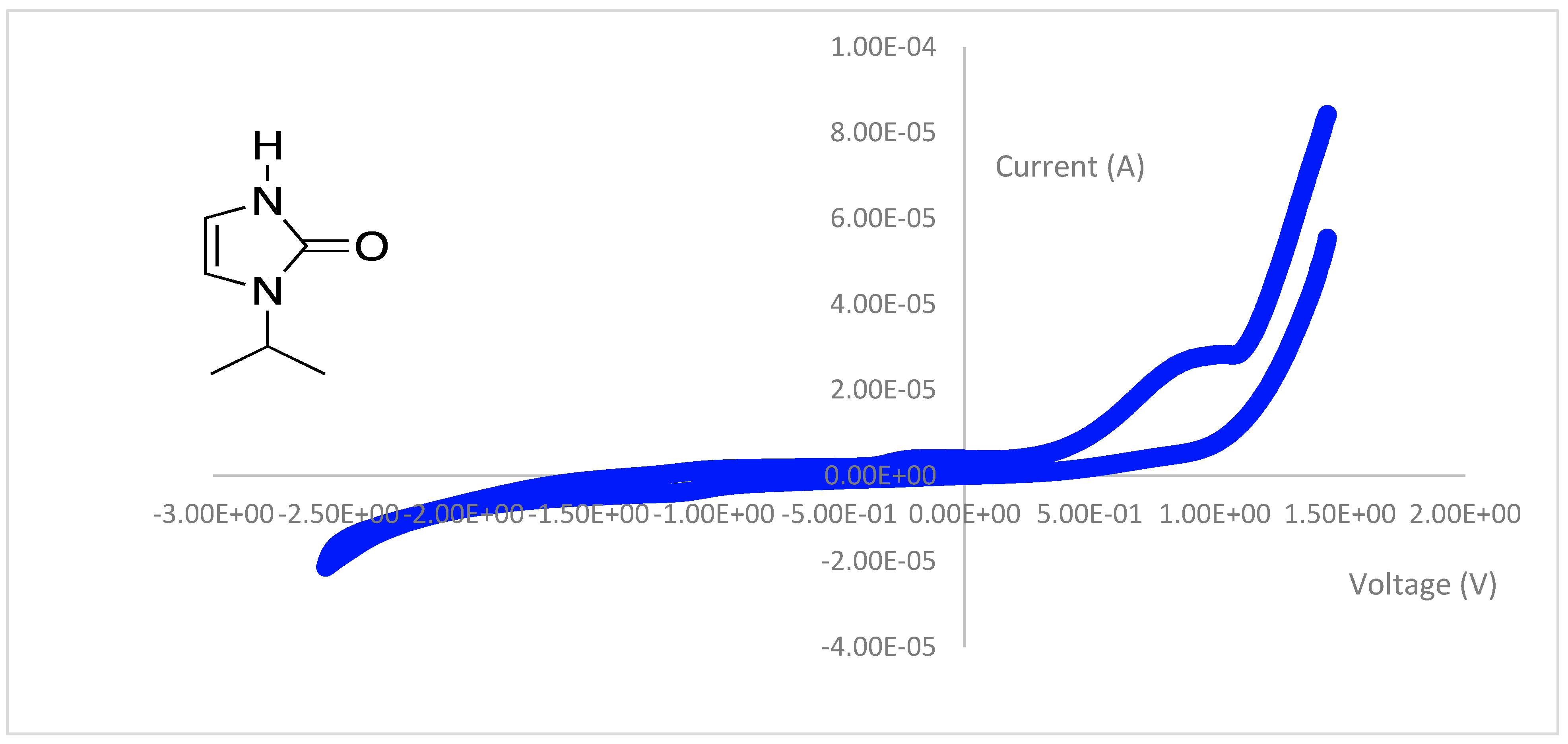
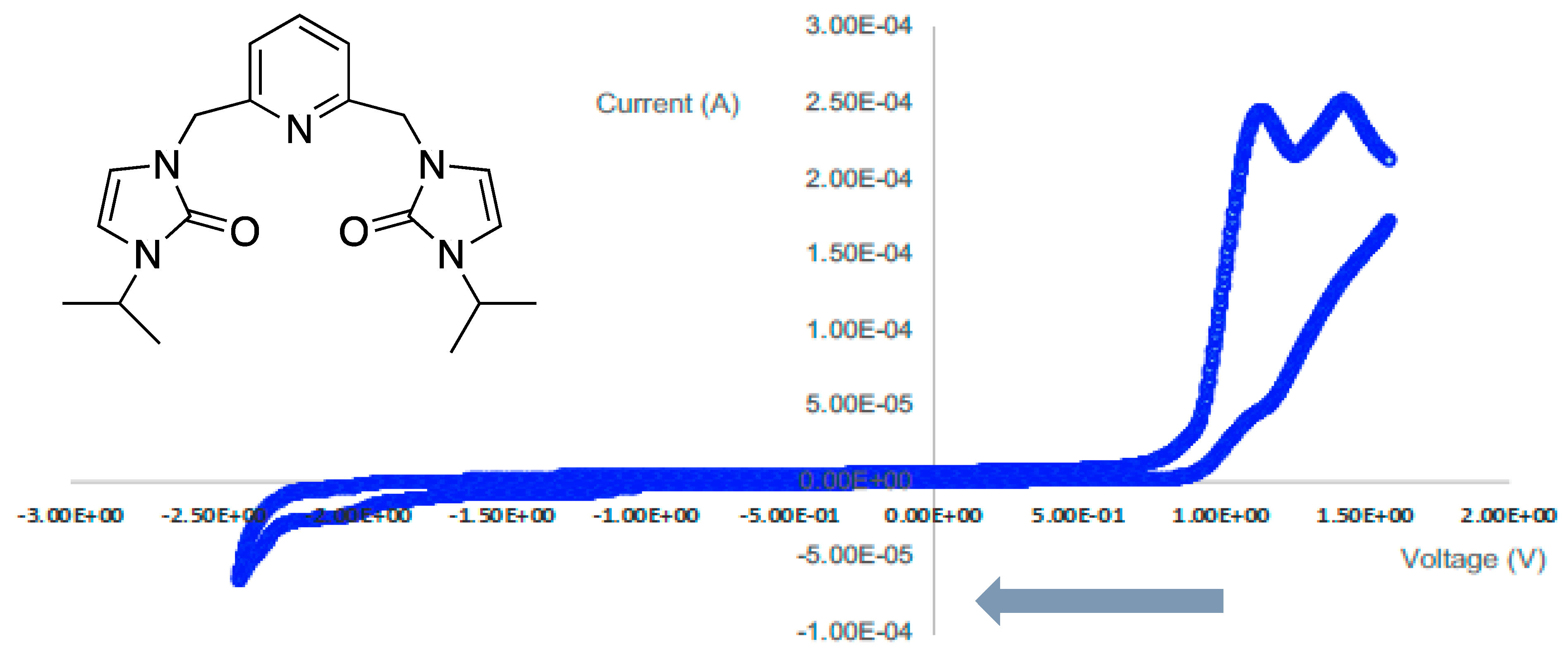
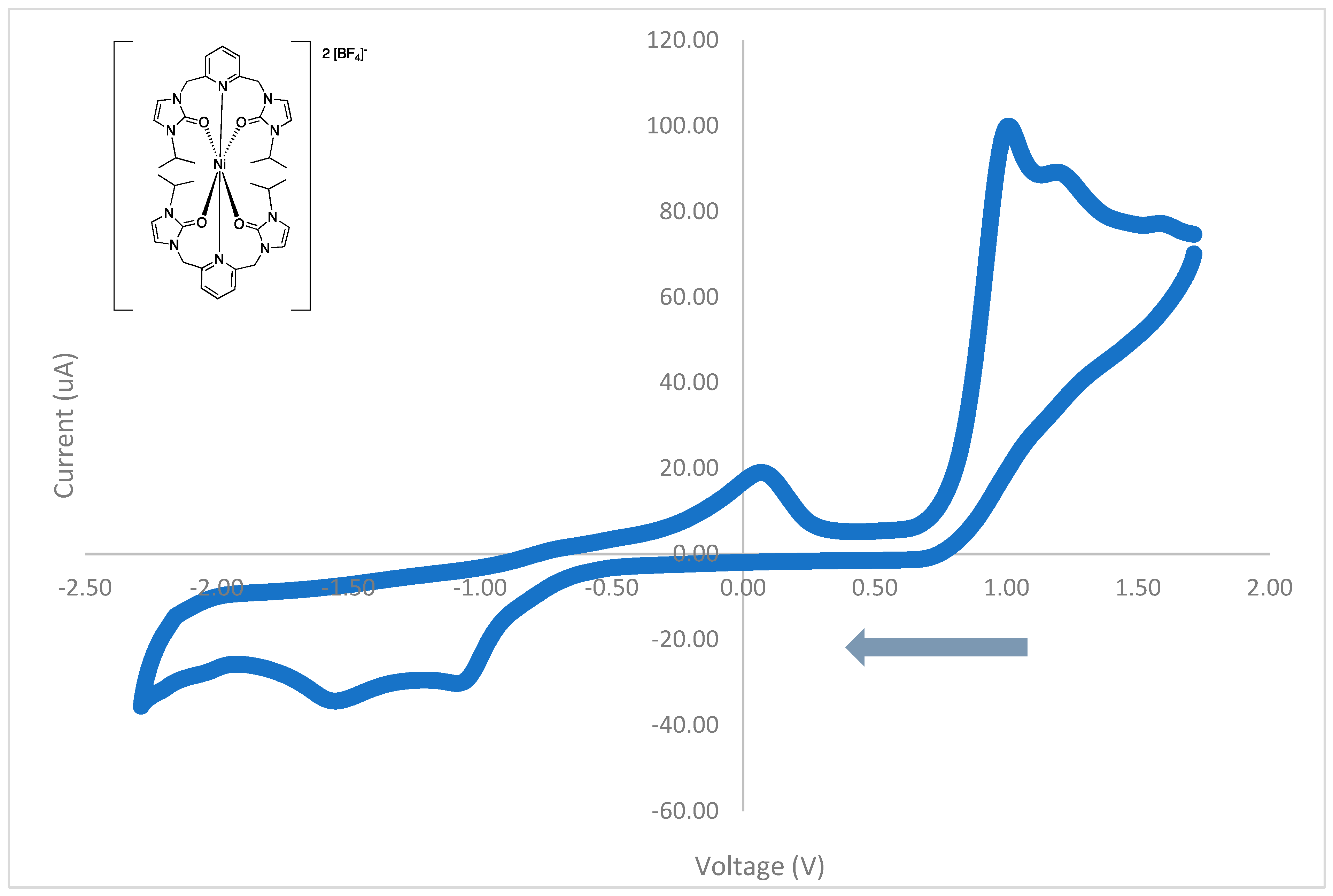
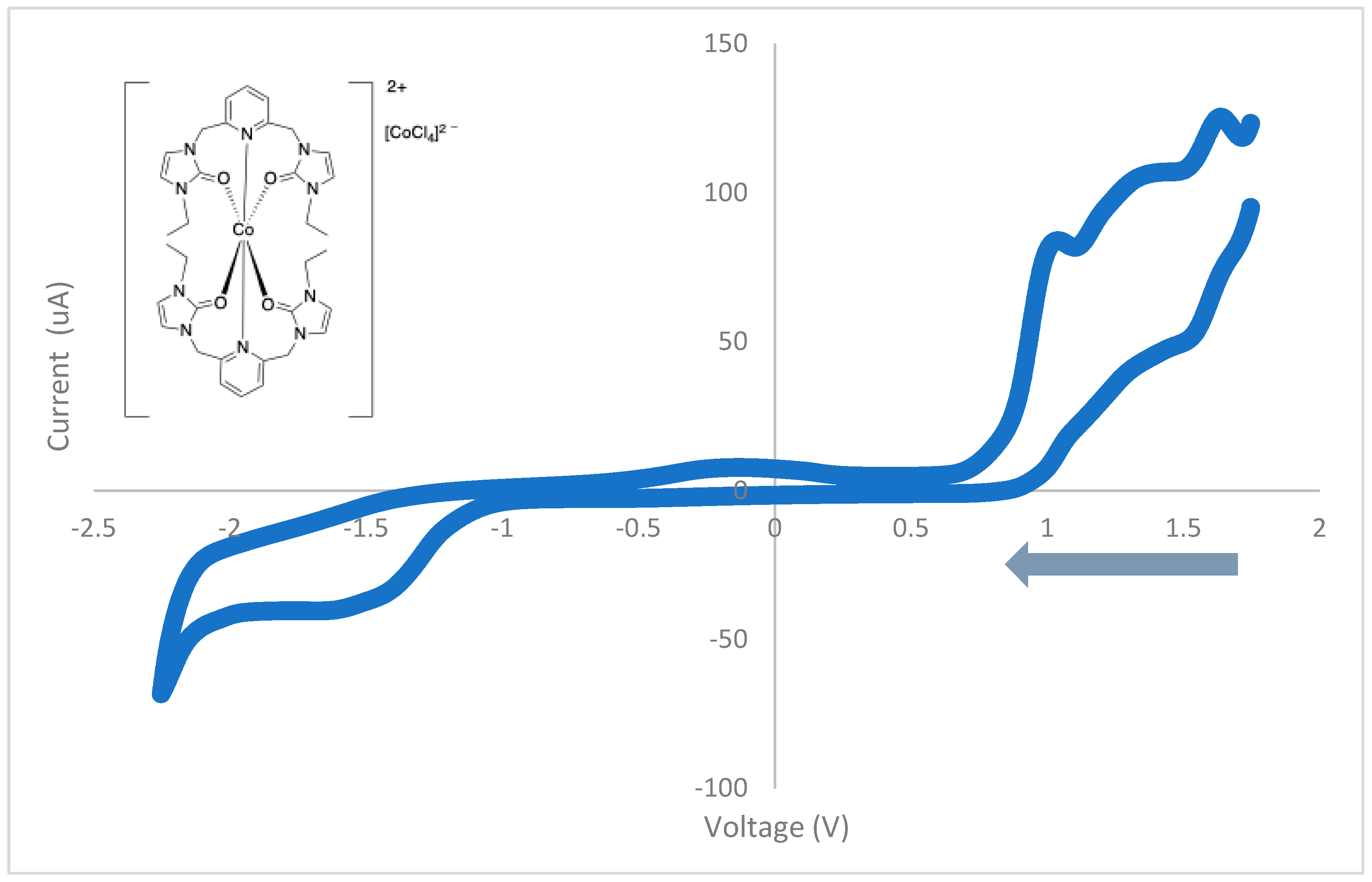
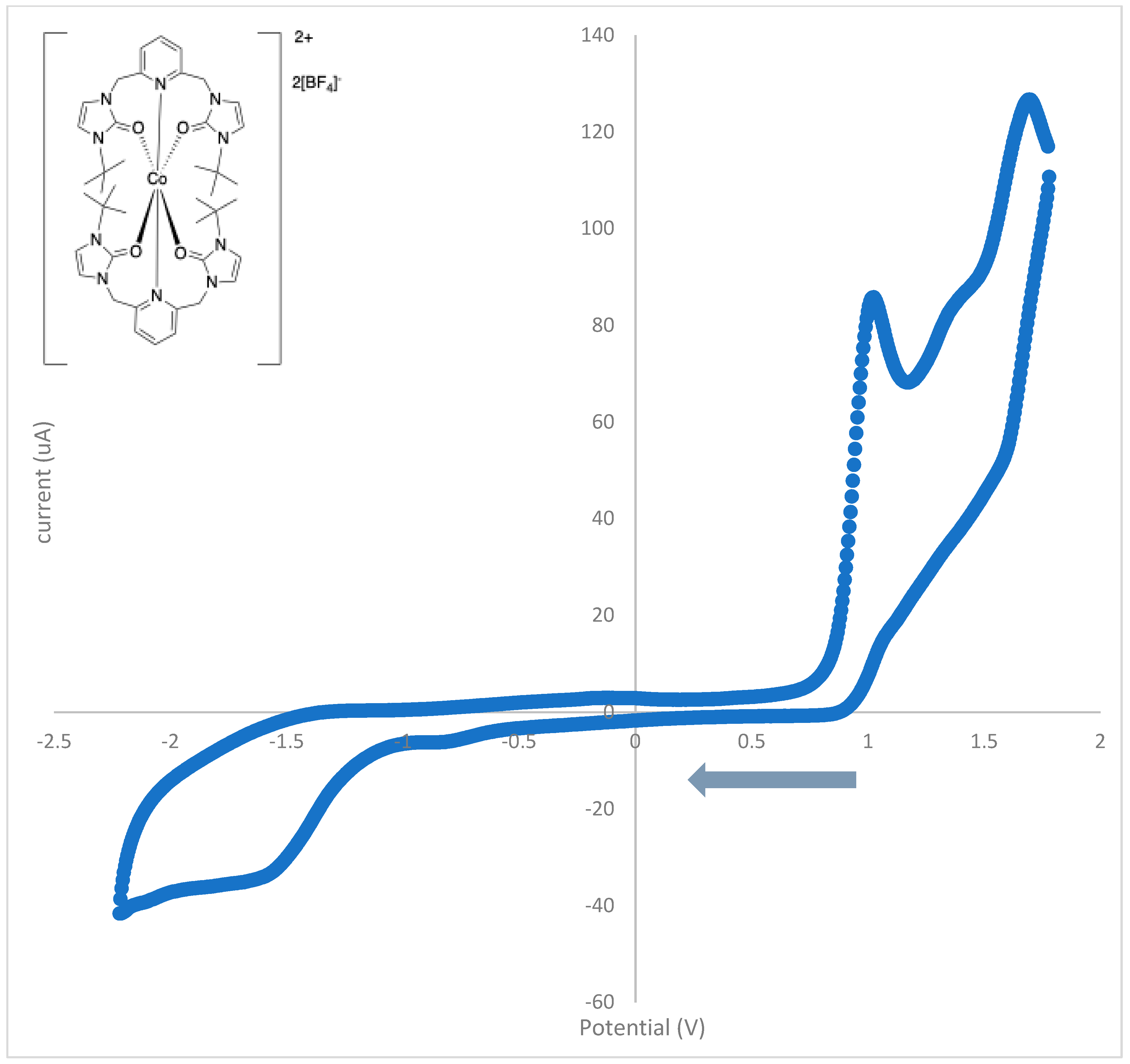
3.4. Cyclic Voltammetry
3.5. UV-Visible Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peris, E.; Crabtree, R.H. Key Factors in Ligand Pincer Design. Chem. Soc. Rev. 2018, 47, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Gunanathan, C.; Milstein, D. Bond Activation and Catalysis by Ruthenium Pincer Complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.J.; Wendt, O.F. (Eds.) Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis; Wiley VCH: Weinheim, Germany, 2014. [Google Scholar]
- Van Koten, G.; Milstein, D. (Eds.) Organometallic Pincer Complexes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Van Koten, G.; Gossage, R.A. The Privileged Pincer-Metal Platform: Coordination Chemistry & Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Miecznikowski, J.R.; Lo, W.; Lynn, M.A.; O’Loughlin, B.E.; DiMarzio, A.P.; Martinez, A.M.; Lampe, L.; Foley, K.M.; Keilich, L.C.; Lisi, G.P.; et al. Syntheses, Characterization, Density Functional Theory Calculations, and Activity of Tridentate SNS Zinc Pincer Complexes. Inorg. Chim. Acta 2011, 376, 515–524. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lo, W.; Lynn, M.A.; Jain, S.; Keilich, L.C.; Kloczko, N.F.; O’Loughlin, B.E.; DiMarzio, A.P.; Foley, K.M.; Lisi, G.P.; et al. Syntheses, Characterization, Density Functional Theory Calculations, and Activity of Tridentate SNS Zinc Pincer Complexes Based on Bis-Imidazole or Bis-Triazole Precursors. Inorg. Chim. Acta 2012, 387, 25–36. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef]
- Sunderland, J.R.; Tao, X.; Butrick, E.E.; Keilich, L.C.; Villa, C.E.; Miecznikowski, J.R.; Jain, S. Investigation of liver alcohol dehydrogenase catalysis using an NADH biomimetic and comparison with a synthetic zinc model complex. Polyhedron 2016, 114, 145–151. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lynn, M.A.; Jasinski, J.P.; Lo, W.; Bak, D.; Pati, M.; Butrick, E.E.; Drozdoski, A.E.R.; Archer, K.A.; Villa, C.E.; et al. Synthesis and characterization of three-and five-coordinate copper(II) complexes based on SNS pincer ligand precursors. Polyhedron 2014, 80, 157–165. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Lynn, M.A.; Jasinski, J.P.; Reinheimer, E.; Bak, D.; Pati, M.; Butrick, E.E.; Drozdoski, A.E.R.; Archer, K.A.; Villa, C.E.; et al. Synthesis, Characterization, and Computational Study of Three-Coordinate SNS Copper(I) Complexes Based on Bis-Thione Ligand Precursors. J. Coord. Chem. 2014, 67, 29–44. [Google Scholar] [CrossRef]
- Lynn, M.A.; Miecznikowski, J.R.; Jasinski, J.P.; Kaur, M.; Mercado, B.Q.; Reinheimer, E.; Almanza, E.; Kharbouch, R.M.; Smith, M.R.; Zygmont, S.E.; et al. Copper(I) SNS Pincer Complexes: Impact of Ligand Design and Solvent Coordination on Conformer Interconversion from Spectroscopic and Computational Studies. Inorg. Chim. Acta 2019, 495, 118996. [Google Scholar] [CrossRef]
- Mast, Z.; Huntzinger, C.G.; Stinson, T.A.; Myren, T.H.T.; Kharbouch, R.M.; Almanza, E.M.; Zygmont, S.E.; Miecznikowski, J.R.; Luca, O.R. Cu(I) SNS triazole and imidazole pincers as electrocatalyst precursors for solar fuel production. Inorg. Chem. Front. 2020, 7, 1012–1015. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Zygmont, S.E.; Jasinski, J.P.; Kaur, M.; Almanza, E.; Kharbouch, R.M.; Bonitatibus, S.C.; Mircovich, E.E.; Le Magueres, P.; Reinheimer, E.; et al. Synthesis, Characterization, and Electrochemistry of SNS Cobalt(II) Tridentate Complexes. Transit. Met. Chem. 2022, 47, 127–137. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Mircovich, E.E.; Bertolotti, N.R.; Corbett, M.J.; Jasinski, J.P.; Reinheimer, E. Synthesis, single crystal structure and spectroscopic characterization of a nickel(II) complex that contains a SNS tridentate ligand. J. Chem. Crystallogr. 2022, 52, 287–296. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Jasinski, J.P.; Bonitatibus, S.C.; Almanza, E.; Kharbouch, R.M.; Zygmont, S.E.; Landy, K.R. Preparation of SNS cobalt(II) pincer model complexes of liver alcohol dehydrogenase. J. Vis. Exp. 2020, 157, e60668. [Google Scholar] [CrossRef]
- O’Reilly, M.E.; Veige, A.S. Trianionic pincer and pincer-type metal complexes and catalysts. Chem. Soc. Rev. 2014, 43, 6325–6369. [Google Scholar] [CrossRef]
- Elamathi, C.; Butcher, R.; Prabhakaran, R. Preparation, characterizations and biological evaluations of new copper(II) complexes containing ONO pincer type ligands. Appl. Organomet. Chem. 2018, 32, e4364. [Google Scholar] [CrossRef]
- Masood, S.; Jamshaid, M.; Zafar, M.N.; Mughal, E.U.; Ashfaq, M.; Tahir, M.N. ONO-pincer Zn(II) & Cd(II) complexes: Synthesis, structural characterization, Hirshfeld surface analysis and CT-DNA interactions. J. Mol. Struct. 2024, 1295, 136571. [Google Scholar]
- Szigethy, G.; Heyduk, A.F. Aluminum complexes of the redox active [ONO] pincer ligand. Dalton Trans. 2012, 41, 8144–8152. [Google Scholar] [CrossRef]
- Wong, J.L.; Higgins, R.F.; Bhowmick, I.; Cao, D.X.; Szigethy, G.; Ziller, J.W.; Shores, M.P.; Heyduk, A.F. Bimetallic iron-iron and iron-zinc complexes of the redox active ONO pincer ligand. Chem. Sci. 2016, 7, 1594–1599. [Google Scholar] [CrossRef]
- Hananouchi, S.; Krull, B.T.; Ziller, J.W.; Furche, F.; Heyduk, A.F. Metal effects on ligand non-innocence in Group 5 complexes of the redox-active ONO pincer ligand. Dalton Trans. 2014, 43, 17991–18000. [Google Scholar] [CrossRef]
- O’Reilly, M.E.; Ghiviriga, I.; Abboud, K.A.; Veige, A.S. A New ONO3− Trianionic Pincer-Type Ligand for Generating Highly Nucleophilic Metal–Carbon Multiple Bonds. J. Am. Chem. Soc. 2012, 134, 11185–11195. [Google Scholar] [CrossRef]
- Pascaulini, M.E.; DiRusso, N.V.; Quintero, P.A.; Thuijs, A.E.; Pinkowicz, D.; Abboud, K.A.; Dunbar, K.R.; Christou, G.; Meisel, M.W.; Veige, A.S. Synthesis, Characterization, and Reactivity of Iron(III) Complexes Supported by a Trianionic ONO3− Pincer Ligand. Inorg. Chem. 2014, 53, 13078–13088. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Greger, I.; Perez-Torrente, J.J.; Jimenez, M.V.; Modrego, F.J.; Lahoz, F.J.; Oro, L.A. ONO Dianionic Pincer-Type Ligand Precursors for the Synthesis of σ,π-Cyclooctenyl Iridium(III) Complexes: Formation Mechanism and Coordination Chemistry. Organometallics 2013, 32, 6903–6917. [Google Scholar] [CrossRef]
- Delgado-Rangel, L.H.; Reyes-Marquez, V.; Moreno-Narvaez, M.E.; Aragon-Muriel, A.; Parra-Unda, J.R.; Cruz-Navarro, J.A.; Martinez-Torres, M.A.; Valdes, H.; Morales-Morales, D. Biological Activity of Vanadium Pincer Complexes. New J. Chem. 2025. Advance Article. [Google Scholar] [CrossRef]
- Dhibar, P.; Chandra, A.; Paul, P.; Samaresh, B. Ancillary ligand induced variation in electronic spectral and catalytic properties of heteroleptic ONO-pincer complexes of ruthenium. New J. Chem. 2025, 49, 2674–2684. [Google Scholar] [CrossRef]
- Pennamuthiriyan, A.; Rengan, R.; Malecki, J.G. Sustainable Synthesis of Substituted 1,3,5-Triazines by [ONO]-Pincer-Supported Nickel(II) Complexes via an Acceptorless Dehydrogenative Coupling Strategy. J. Org. Chem. 2025, 90, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Garcia-Bosch, I. Metal Complexes Bearing Redox-Active Supporting Ligands that promote Chemical Transformations involving protons and electrons. In Redox-Active Ligands: Concepts and Catalysis; Desage-El Murr, M., Ed.; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Awe, B.; Swart, G.; Erasmus, E.; Malan, F.P. Synthesis, characterization, and catalytic evaluation of Ru-ONO complexes featuring N- and P-based ligands. Eur. J. Inorg. Chem. 2024, 28, e202400619. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Nicaise, O.J.C.N.; Mercado, B.Q.; Araujo, A.J.; Bertolotti, N.R.; Erickson, S.L.; Trucchio, J.P.; Corbett, M.J.; Padover, C.J.; Coulombe, S.L.; et al. CCDC 2402486: Experimental Crystal Structure Determination. CSD Commun. 2025. [Google Scholar] [CrossRef]
- Wong, O.; Tsuzuki, N.; Richardson, M.; Rytting, H.; Konishi, R.; Higuchi, T. An Improved Synthesis of 1-alykyl-4-imidazoline-2-ones. Heterocycles 1987, 26, 3153–3158. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Gonzalez Nieves, K.; Pinero Cruz, D.M. Crystal structure of a nickel compound comprising two nickel(II) complexes with different ligand environments: [Ni(tren)(H2O)2][Ni(H2O)6](SO4)2. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 314–317. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Krause Bauer, J.A. cis-Bis(ethylenediamine)bis(pyridine)nickel(II) dintrate. Cryst. Struct. Commun. 1999, C55, 1772–1773. [Google Scholar] [CrossRef]
- Glocker, G. Carbon-oxygen bond energies and bond distances. J. Phys. Chem. 1958, 62, 1049–1054. [Google Scholar] [CrossRef]
- Ribeiro, M.A.; Lanznaster, M.; Silva, M.M.P.; Resende, J.A.L.C.; Pinheiro, M.V.B.; Krambrock, K.; Stumpf, H.O.; Pinheiro, C.B. Cobalt lawsone complexes: Searching for new valence tautomers. Dalton Trans. 2013, 42, 5462. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Jasinski, J.P.; Ostrowski, T.J.; Landy, K.R.; Bonitatibus, S.C.; Smolinsky, A.N.; Bertolotti, N.R. Crystal Structure and spectroscopic properties of aqua-dichloro{1,1′-[(pyridine-2,6-diyl-κN)(bis-(methylene)]bis(4-butyl-4,5-dihydro-1H-1,2,4-triazole-5-thione-κN2)}cobalt(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, E76, 1757–1761. [Google Scholar] [CrossRef]
- Fang, Z.-Y.; Zhang, L.; Ma, J.-P.; Zhao, L.; Wang, S.-L.; Xie, N.-H.; Liu, Y.-Q.; Guo, X.-Y.; Qin, J. Dinuclear cobalt and nickel complexes of a mercaptoacetic acid substituted 1,2,4 triazole ligand: Syntheses, structures, and urease inhibitory studies. Acta Crystallogr. Sect. C Struct. Chem. 2019, C75, 1658–1665. [Google Scholar] [CrossRef]


| Nickel(II) Complex [1] with Isopropyl Wingtip Groups | Cobalt(II) Complex [2] with Ethyl Wingtip Groups | Cobalt(II) Complex [3] with t-Butyl Wingtip Groups [35] | |
|---|---|---|---|
| Identification code | 007a-22125 | 007c-22060 | syn-23135 |
| Empirical formula | C41H58.50B2F8N11.50NiO6 | C34H42Cl4Co2N10O4 | C42H58B2CoF8N10O4 |
| Formula weight | 1040.82 | 914.43 | 999.53 |
| Temperature | 93.15 K | 93(2) K | 93(2) K |
| Wavelength | 1.54184 Å | 0.71073 Å | 1.54184 Å |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | C 1 2 1 | P21/n | P-1 |
| Unit cell dimensions | a = = 21.7639(8) Å α = 90°. | a = 17.7907(7) Å α = 90°. | a = 9.7774(4) Å α = 77.657(4)°. |
| b = 11.0649(5) Å β = 90.609(3)°. | b = 21.5278(6) Å β = 95.063(3)°. | b = 12.2396(6) Å β = 80.273(4)°. | |
| c = 10.9225(4) Å γ = 90°. | c = 21.8597(7) Å γ = 90°. | c = 20.7461(10) Å γ = 80.397(4)°. | |
| Volume | 2630.16(18) Å3 | 8339.5(5) Å3 | 2368.3(2) Å3 |
| Z | 2 | 8 | 2 |
| Density (calculated) | 1.314 Mg/m3 | 1.457 Mg/m3 | 1.402 Mg/m3 |
| Absorption coefficient | 1.244 mm−1 | 1.101 mm−1 | 3.566 mm−1 |
| F(000) | 1086 | 3760 | 1042 |
| Crystal size | 0.200 × 0.200 × 0.020 mm3 | 0.200 × 0.200 × 0.050 mm3 | 0.090 × 0.050 × 0.020 mm3 |
| Crystal color and habit | green plate | Blue Plate | light pink block |
| Diffractometer | Rigaku Saturn 944+ CCD | Dectris Pilatus 3R | XtaLAB Synergy, Dualflex, HyPix-Arc 100 |
| Theta range for data collection | 4.048 to 66.595°. | 2.962 to 25.350° | 2.201 to 51.861°. |
| Index ranges | −25 <= h <= 25, −13 <= k <= 13, −13 <= l <= 13 | −21 <= h <= 21, −25 <= k <= 25, −26 <= l <= 26 | −9 <= h <= 9, −12 <= k <= 12, −20 <= l <= 21 |
| Reflections collected | 44657 | 74637 | 11852 |
| Independent reflections | 4622 [R(int) = 0.0778] | 15242 [R(int) = 0.0587] | 5008 [R(int) = 0.0334] |
| Observed reflections (I > 2sigma(I)) | 3645 | 12,395 | 4414 |
| Completeness to theta = 66.595° | 99.60% | 99.80% | 95.10% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 1.00000 and 0.70348 | 1.00000 and 0.56773 | 1.00000 and 0.80167 |
| Solution method | SHELXT-2014/5 [37] | SHELXT-2014/5 [37] | SHELXT-2014/5 [37] |
| Refinement method | SHELXL-2014/7 [37] | SHELXL-2014/7 [37] | SHELXL-2014/7 [37] |
| Data/restraints/parameters | 4622/439/432 | 15242/25/992 | 5008/0/616 |
| Goodness-of-fit on F2 | 1.051 | 1.189 | 1.029 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0749, wR2 = 0.2113 | R1 = 0.0783, wR2 = 0.1650 | R1 = 0.0402, wR2 = 0.1026 |
| R indices (all data) | R1 = 0.0899, wR2 = 0.2271 | R1 = 0.0952, wR2 = 0.1713 | R1 = 0.0473, wR2 = 0.1070 |
| Extinction coefficient | n/a | n/a | n/a |
| Largest diff. peak and hole | 0.789 and −0.618 e.Å−3 | 1.160 and −0.563 e.Å−3 | 0.479 and −0.323 e.Å−3 |
| Bond | Bond Distance (Å) |
|---|---|
| Ni(1)-O(1) | 2.028(7) |
| Ni(1)-O(1)#1 | 2.028(7) |
| Ni(1)-O(2)#1 | 2.062(6) |
| Ni(1)-O(2) | 2.062(6) |
| Ni(1)-N(1) | 2.296(4) |
| Ni(1)-N(1)#1 | 2.296(4) |
| O(1)-C(1) | 1.290(11) |
| O(2)-C(2) | 1.240(10) |
| Bond | Bond Angle (°) |
|---|---|
| O(1)-Ni(1)-O(1)#1 | 97.2(4) |
| O(1)#1-Ni(1)-O(2) | 179.1(4) |
| O(1)#1-Ni(1)-O(2)#1 | 81.83(17) |
| O(1)-Ni(1)-O(2) | 81.83(17) |
| O(1)-Ni(1)-O(2)#1 | 179.1(4) |
| O(1)-Ni(1)-N(1) | 90.3(3) |
| O(1)#1-Ni(1)-N(1)#1 | 90.3(3) |
| O(1)#1-Ni(1)-N(1) | 88.8(3) |
| O(1)-Ni(1)-N(1)#1 | 88.8(3) |
| O(2)-Ni(1)-O(2)#1 | 99.1(4) |
| O(2)#1-Ni(1)-N(1)#1 | 91.3(3) |
| O(2)#1-Ni(1)-N(1) | 89.6(3) |
| O(2)-Ni(1)-N(1) | 91.3(3) |
| O(2)-Ni(1)-N(1)#1 | 89.6(3) |
| N(1)-Ni(1)-N(1)#1 | 178.6(6) |
| Bond | Bond Distance (Å) |
|---|---|
| Co(1)-O(4) | 2.044(5) |
| Co(1)-O(1) | 2.047(4) |
| Co(1)-O(2) | 2.060(5) |
| Co(1)-O(3) | 2.073(5) |
| Co(1)-N(1) | 2.348(5) |
| Co(1)-N(6) | 2.356(5) |
| O(1)-C(8) | 1.227(8) |
| O(2)-C(13) | 1.236(9) |
| O(3)-C(25) | 1.225(9) |
| O(4)-C(31) | 1.219(8) |
| Bond | Bond Angle (°) |
|---|---|
| O(4)-Co(1)-O(1) | 98.13(17) |
| O(4)-Co(1)-O(2) | 85.56(19) |
| O(1)-Co(1)-O(2) | 166.85(18) |
| O(4)-Co(1)-O(3) | 164.77(18) |
| O(1)-Co(1)-O(3) | 87.25(17) |
| O(2)-Co(1)-O(3) | 92.40(18) |
| O(4)-Co(1)-N(1) | 80.15(17) |
| O(1)-Co(1)-N(1) | 95.73(16) |
| O(2)-Co(1)-N(1) | 97.34(17) |
| O(3)-Co(1)-N(1) | 85.16(17) |
| O(4)-Co(1)-N(6) | 95.91(18) |
| O(1)-Co(1)-N(6) | 83.30(17) |
| O(3)-Co(1)-N(6) | 98.87(18) |
| N(1)-Co(1)-N(6) | 175.79(17) |
| Bond | Bond Length (Å) |
|---|---|
| Co-O(1) | 2.034(2) |
| Co-O(2) | 2.040(2) |
| Co-O(3) | 2.059(2) |
| Co-O(4) | 2.075(2) |
| Co-N(6) | 2.379(3) |
| Co-N(1) | 2.385(3) |
| Bond | Bond Angle (°) |
|---|---|
| O(4)-Co(1)-O(1) | 98.08(19) |
| O(4)-Co(1)-O(2) | 85.6(2) |
| O(1)-Co(1)-O(2) | 166.9(2) |
| O(4)-Co(1)-O(3) | 164.8(2) |
| O(1)-Co(1)-O(3) | 87.24(19) |
| O(2)-Co(1)-O(3) | 92.4(2) |
| O(4)-Co(1)-N(1) | 80.22(19) |
| O(1)-Co(1)-N(1) | 95.78(18) |
| O(2)-Co(1)-N(1) | 97.26(19) |
| O(3)-Co(1)-N(1) | 85.13(18) |
| O(4)-Co(1)-N(6) | 95.93(19) |
| O(1)-Co(1)-N(6) | 83.34(19) |
| O(2)-Co(1)-N(6) | 83.8(2) |
| O(3)-Co(1)-N(6) | 98.80(19) |
| N(1)-Co(1)-N(6) | 175.91(19) |
| Peak Potential (V) | Assignment | Type |
|---|---|---|
| 1.63 | oxidation | irreversible |
| 1.26 | oxidation | irreversible |
| 1.17 | reduction | irreversible |
| 1.03 | oxidation | irreversible |
| 0.12 | oxidation | irreversible |
| −1.05 | reduction | irreversible |
| −1.52 | reduction | irreversible |
| −1.89 | oxidation | irreversible |
| Peak Potential (V) | Assignment | Type |
|---|---|---|
| 1.66 | oxidation | irreversible |
| 1.52 | reduction | irreversible |
| 1.36 | oxidation | irreversible |
| 1.06 | oxidation | irreversible |
| −1.34 | reduction | irreversible |
| −1.46 | reduction | irreversible |
| Peak Potential (V) | Assignment | Type |
|---|---|---|
| 1.70 | oxidation | irreversible |
| 1.56 | reduction | irreversible |
| 1.34 | oxidation | irreversible |
| 1.04 | oxidation | irreversible |
| −0.84 | reduction | irreversible |
| −1.57 | reduction | irreversible |
| Wavelength (nm) | Molar Absorptivity (M−1cm−1) |
|---|---|
| 574 | 41 |
| 594 | 59 |
| 639 | 63 |
| Wavelength (nm) | Molar Absorptivity (M−1cm−1) |
|---|---|
| 596 | 1290 |
| 687 | 1690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miecznikowski, J.R.; Nicaise, O.J.C.; Mercado, B.Q.; Araujo, A.J.; Bertolotti, N.R.; Erickson, S.L.; Trucchio, J.P.; Corbett, M.J.; Padover, C.J.; Coulombe, S.L.; et al. Synthesis and Characterization of ONO Pincer Ligand Precursors and Metal Complexes with Ethyl, Isopropyl and Tert-Butyl Wingtip Groups. Crystals 2025, 15, 227. https://doi.org/10.3390/cryst15030227
Miecznikowski JR, Nicaise OJC, Mercado BQ, Araujo AJ, Bertolotti NR, Erickson SL, Trucchio JP, Corbett MJ, Padover CJ, Coulombe SL, et al. Synthesis and Characterization of ONO Pincer Ligand Precursors and Metal Complexes with Ethyl, Isopropyl and Tert-Butyl Wingtip Groups. Crystals. 2025; 15(3):227. https://doi.org/10.3390/cryst15030227
Chicago/Turabian StyleMiecznikowski, John R., Olivier J. C. Nicaise, Brandon Q. Mercado, Abigail J. Araujo, Natalia R. Bertolotti, Samantha L. Erickson, Joseph P. Trucchio, Michael J. Corbett, Connor J. Padover, Stephanie L. Coulombe, and et al. 2025. "Synthesis and Characterization of ONO Pincer Ligand Precursors and Metal Complexes with Ethyl, Isopropyl and Tert-Butyl Wingtip Groups" Crystals 15, no. 3: 227. https://doi.org/10.3390/cryst15030227
APA StyleMiecznikowski, J. R., Nicaise, O. J. C., Mercado, B. Q., Araujo, A. J., Bertolotti, N. R., Erickson, S. L., Trucchio, J. P., Corbett, M. J., Padover, C. J., Coulombe, S. L., Wheeler, A. J., & Ouellette, I. P. (2025). Synthesis and Characterization of ONO Pincer Ligand Precursors and Metal Complexes with Ethyl, Isopropyl and Tert-Butyl Wingtip Groups. Crystals, 15(3), 227. https://doi.org/10.3390/cryst15030227