1. Introduction
Hybrid supercapacitors (HSCs) are a promising class of energy storage devices characterized by their high-power density, extended cycle life, and superior rate capability [
1,
2,
3]. These features make HSCs ideal for applications requiring rapid charge-discharge cycles, such as portable electronics and electric vehicles [
4]. Additionally, their ability to combine the energy storage mechanism of batteries with the high-power delivery of traditional supercapacitors has further fueled interest in their development [
2]. Optimizing HSC performance requires careful selection of electrode materials. The ideal electrode materials should exhibit superior specific capacitance, remarkable conductivity, and robust operational stability to fulfill both high power and energy density demands [
5,
6,
7].
Transition metal-based compounds, particularly cobalt-based materials, have garnered considerable attention as promising electrode candidates for HSCs [
8]. These materials are highly attractive due to their rich redox chemistry, high electrochemical activity, and good electrical conductivity, which collectively contribute to their superior charge-storage capability [
8,
9,
10]. Among these compounds, cobalt selenides (Co
xSe
y) are known for their remarkable electrochemical properties, including high specific capacity, enhanced conductivity, and exceptional stability in alkaline electrolytes [
11,
12,
13]. In recent studies, Co
xSe
y has shown promise for supercapacitor applications. For instance, Zhao et al. [
14] reported mesoporous Co
0.85Se nanowire arrays with an exceptional specific capacitance of 1800 F g⁻¹ at 1 mV s⁻¹. Liu et al. [
15] synthesized porous CoSe
2 nanosheets with a high specific capacitance of 333 F g⁻¹ at 1 A g⁻¹ and remarkable durability (100.97% retention over 25,000 cycles at 5 A g⁻¹). Zhu et al. [
16] developed CoSe nanosheets, achieving a specific capacity of 70.6 mAh g⁻¹ at 1 A g⁻¹ and retaining 52.8% of their capacity at 100 A g⁻¹. Similarly, Dhanasekar et al. [
17] fabricated Co
9Se
8@RGO electrodes with a specific capacitance of 1278 F g⁻¹ at 1 A g⁻¹. These findings highlight the significant potential of Co
xSe
y as an electrode material for HSCs.
Despite their promising performance, the practical application of Co
xSe
y-based materials is often hindered by challenges such as low conductivity and limited cycling stability. Addressing these limitations requires innovative strategies, including the synthesis of nanostructured materials, the formation of composites, and the use of conductive substrates [
12,
13,
14,
15,
16,
17,
18]. Among these approaches, direct synthesis of Co
xSe
y on conductive substrates, such as nickel foam (NF), has demonstrated particular effectiveness [
13,
19,
20,
21]. NF is an ideal conductive substrate for electrode materials because of its large surface area, excellent electrical performance, and lightweight composition. The direct synthesis of Co
xSe
y on NF offers a stable framework that enhances conductivity, supports efficient charge transfer, and provides robust mechanical stability during extended cycling [
13,
19,
20,
21].
Nonstoichiometric Co
0.85Se has gained recent attention as a potential material for energy storage technologies, particularly in capacitive devices [
22,
23,
24,
25,
26,
27]. The direct synthesis of Co
0.85Se in various morphologies, such as nanosheets, nanowires, nanoparticles, hollow spheres, and thin films, has demonstrated improved morphological control and enhanced electrochemical performance [
28,
29,
30,
31]. Among these morphologies, sphere-like structures have demonstrated notable advantages by maximizing the active surface area, improving electrolyte access, and facilitating ion diffusion, thereby achieving superior charge-discharge efficiency [
28]. In our recent study, we fabricated Co
0.85Se microspheres through a two-step hydrothermal process and showcased its effectiveness as an electrocatalyst for the hydrogen evolution reaction [
32].
This study reports the direct fabrication of Co0.85Se microsphere-like structures on NF substrates using a two-step hydrothermal approach. The distinctive morphology of the Co0.85Se/NF electrode significantly enhances electrochemical performance by expanding the contact area and facilitating ion diffusion. These factors are crucial for achieving high capacity and long-term cycling stability. Co0.85Se/NF electrodes demonstrated an impressive specific capacity of 719 C g⁻¹ at 1 A g⁻¹, excellent rate capabilities, and outstanding cycling stability in a 2M KOH electrolyte. An HSC device, constructed with Co0.85Se/NF as the positive electrode and activated carbon (AC) as the negative electrode, demonstrated remarkable performance, achieving an energy density of 66.6 Wh kg⁻¹, a power density of 849.3 W kg⁻¹, and capacitance retention of 81.1% after 10,000 cycles. These results underscore the potential of Co0.85Se microspheres on NF substrates for advanced energy storage applications.
3. Results and Discussion
A two-step hydrothermal synthesis process was employed to prepare microsphere-like Co
0.85Se structures on NF. First, a hydrothermal method was used to grow Co(OH)F microcrystals on the NF substrate. Second, the Co(OH)F microcrystals were converted into microsphere-like Co
0.85Se structures via a selenization process. A schematic representation of this synthesis process for microsphere-like Co
0.85Se structures is shown in
Figure S1. Field-emission scanning electron microscopy (FESEM) was used to comprehensively characterize the morphology of the synthesized electrode materials.
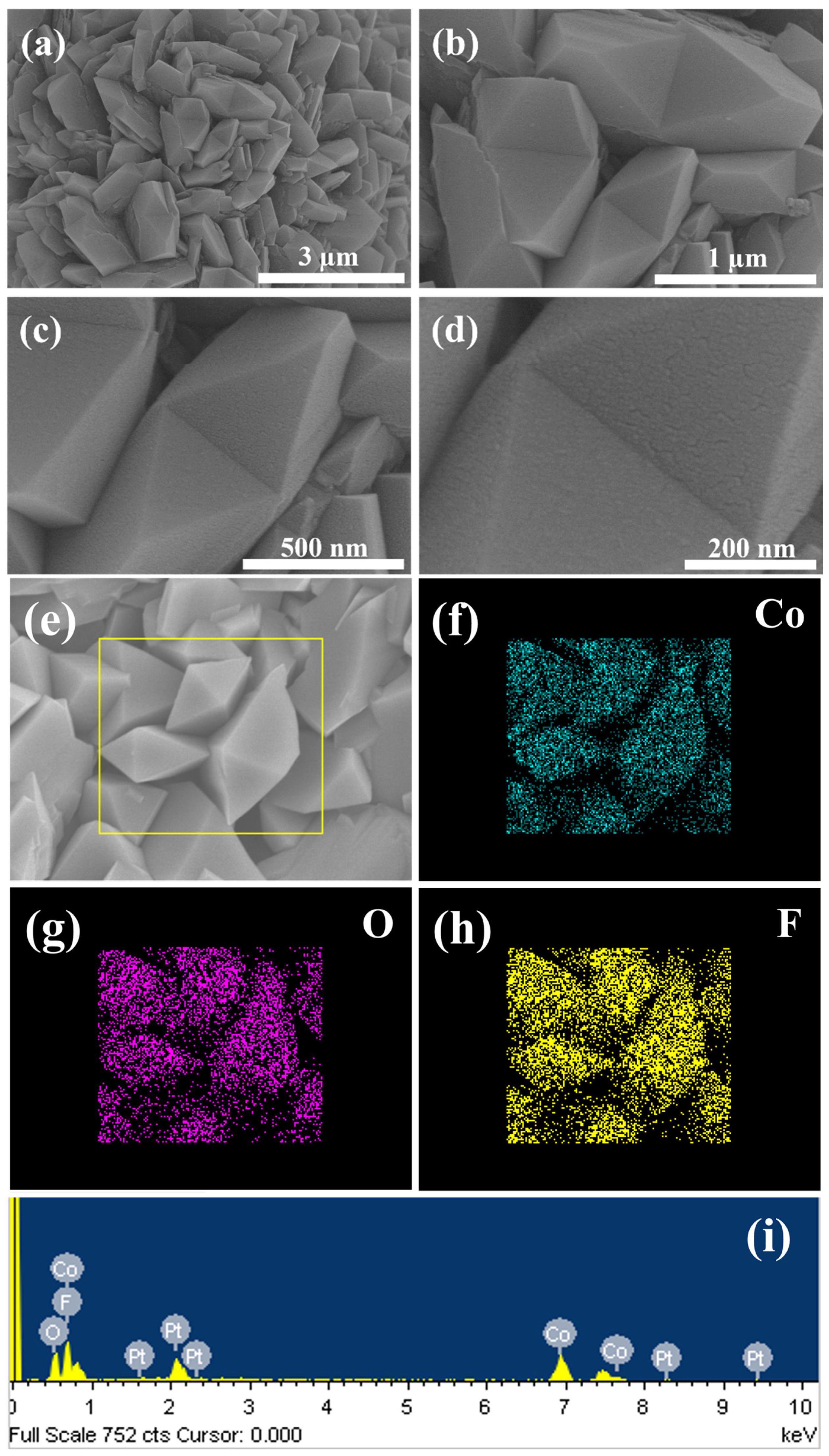
Figure 1 presents FESEM images of Co(OH)F microcrystals obtained from the initial hydrothermal step. The low-magnification images (
Figure 1a,b) reveal that the material comprises interconnected microcrystals arranged in a well-distributed configuration; however, its structure is non-uniform. These microcrystals exhibit distinct geometric shapes, including tetrahedral, octahedral, and dodecahedral forms, with an average size of approximately 1 µm. High-magnification images (
Figure 1c,d) provide a closer view of the microcrystals, highlighting their irregular and robust surfaces, which indicate a hard texture. These structural features are expected to greatly enhance the structural integrity and longevity of the electrode in electrochemical applications. The energy dispersive X-ray spectroscopy (EDS) analysis pattern of the Co(OH)F microcrystals is presented in
Figure 1i, confirming the presence of cobalt (Co), oxygen (O), and fluorine (F). Additionally, the elemental mapping images (
Figure 1f–h) demonstrate the even distribution of these elements within the microcrystals, validating the homogeneous composition of the material.
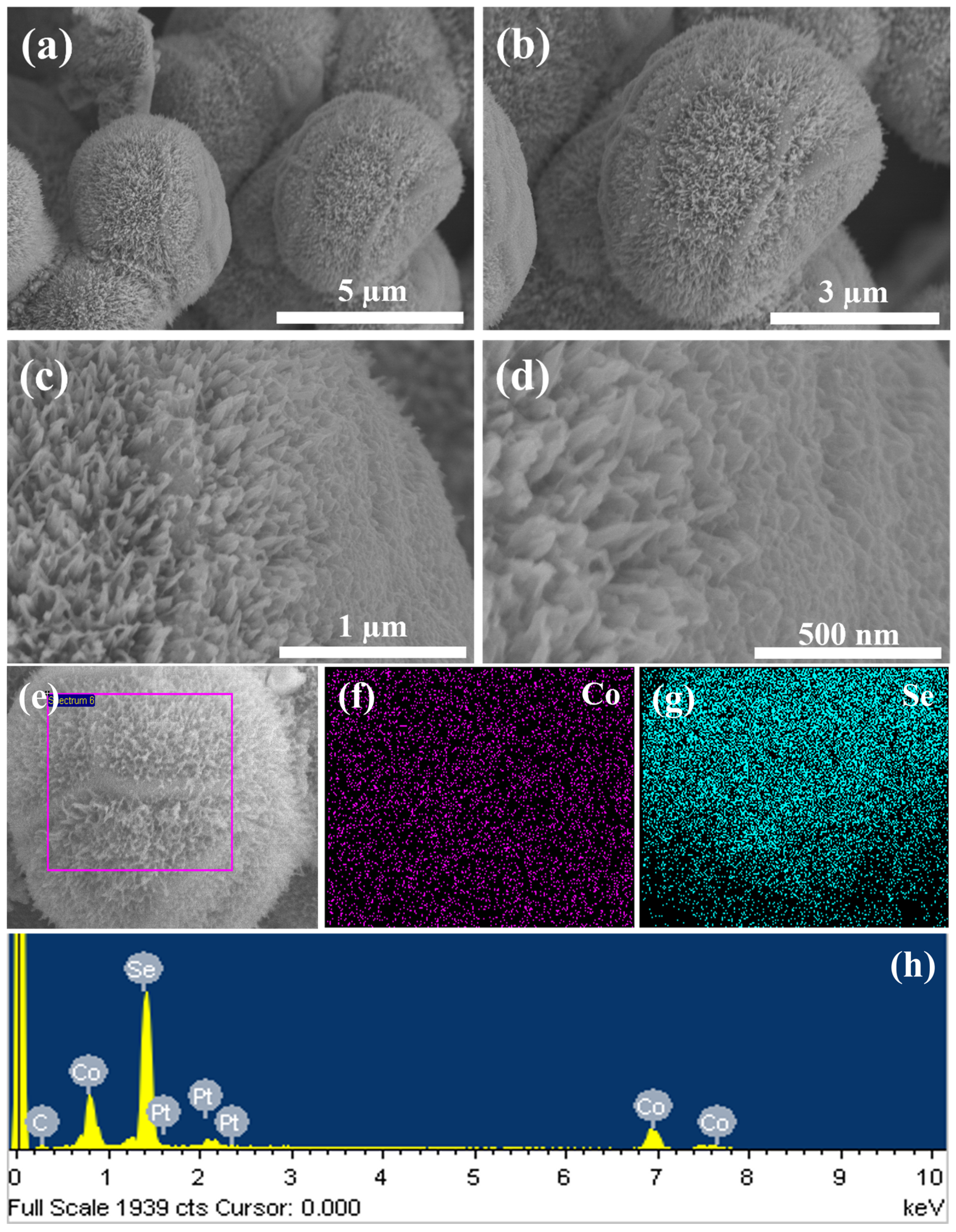
Figure 2 shows FESEM images of the selenized product obtained by the second hydrothermal step. As depicted in
Figure 2a, the morphology of the Co
0.85Se structure resembles the Co(OH)F microcrystals; however, during the selenization process, the interconnected microcrystals transform into a hierarchical microsphere-like structure (
Figure 2b). High-resolution FESEM images reveal the unique surface microstructure of Co
0.85Se microspheres (
Figure 2c). These microspheres comprise numerous spike-like subunits on their surfaces, which aggregate to form the characteristic hierarchical structure—a unique feature attributed to the selenization process. The high magnification image (
Figure 2d) further highlights the rough protuberances on the surface of the Co
0.85Se microspheres. This surface morphology, combined with the hierarchical arrangement, enhances material interconnectivity and increases the available active surface area. These structural features are crucial for optimizing charge storage and transfer, making Co
0.85Se microspheres particularly suitable for supercapacitor applications [
28,
32]. Elemental mappings and the EDX spectrum of Co
0.85Se are shown in
Figure 2e–h, confirming the even dispersion of Co and selenium (Se) throughout the microsphere structure and further supporting the consistent makeup of the material. The small peaks of platinum (Pt) detected in the EDX spectrum of both Co(OH)F microcrystals (
Figure 1i) and Co
0.85Se (
Figure 2h) are attributed to the Pt coating applied during the FESEM analysis.
The microstructural characteristics of the Co
0.85Se microspheres were further analyzed using field-emission transmission electron microscopy (FETEM).
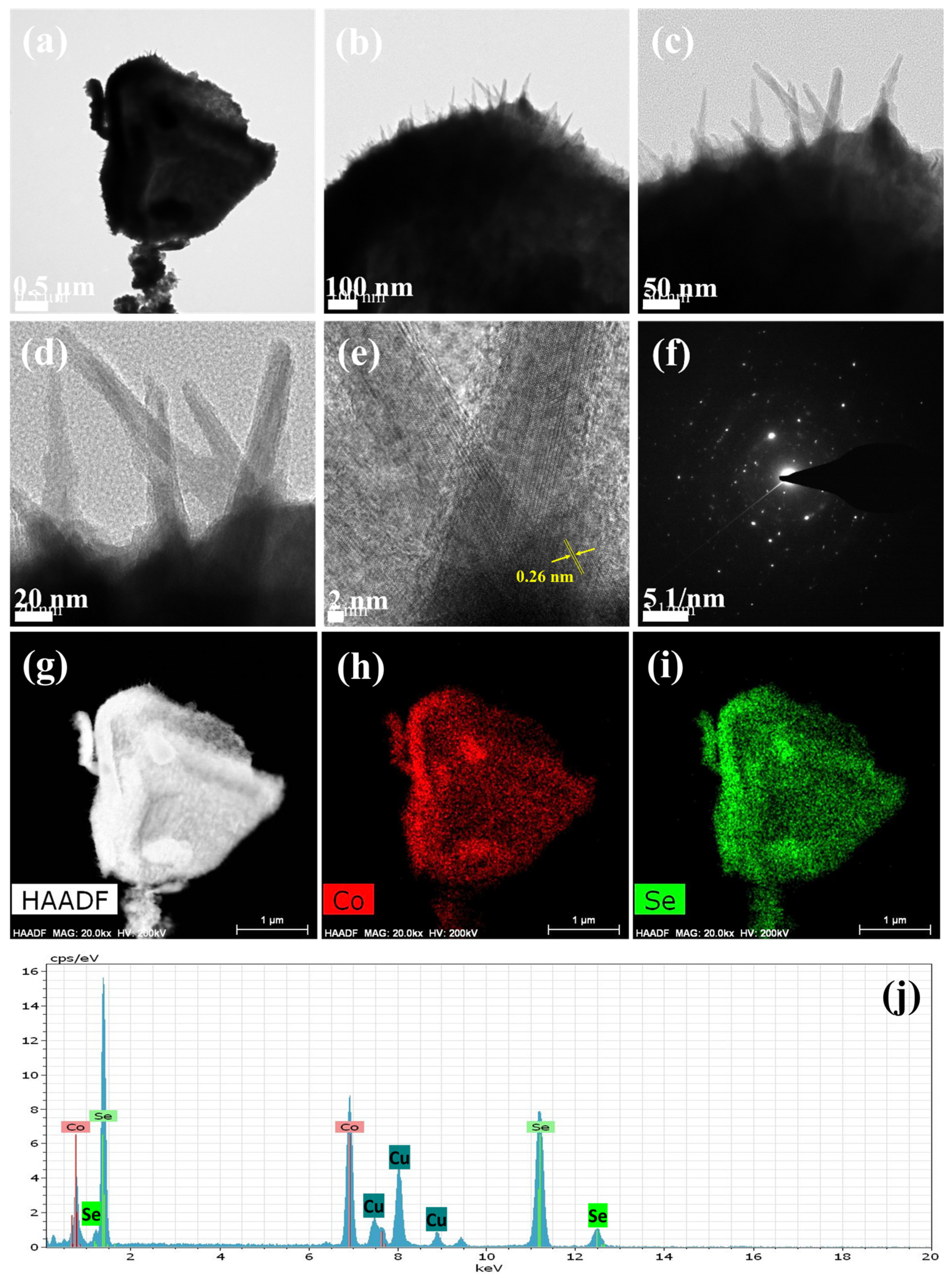
Figure 3a,b present FETEM images at various magnifications, confirming the hierarchical nature of the microspheres, aligning with previous FESEM findings. These microspheres consist of densely packed spike-like subunits, which contribute to their distinctive morphology (
Figure 3c,d). High-resolution TEM (HRTEM) analysis (
Figure 3e) revealed well-defined lattice planes, indicating the crystalline structure of Co
0.85Se. The observed lattice spacing of approximately 0.26 nm corresponds to the (101) plane of Co
0.85Se [
14,
22], verifying the material’s phase composition and crystalline structure. The selected area electron diffraction (SAED) pattern (
Figure 3f) exhibited sharp diffraction spots, further validating the crystallinity and ordered structure of Co
0.85Se. Elemental analysis using EDS in TEM mode revealed an even distribution of Co and Se throughout the microsphere-like structure. The elemental mapping images (
Figure 3h,i) based on the HAADF image (
Figure 3g) confirm the even distribution of these elements, reinforcing the chemical uniformity of the material. Additionally, the EDS spectrum (
Figure 3j) verifies the presence of Co and Se without detectable impurities. This microstructural and compositional homogeneity is critical for the exceptional electrochemical behavior of Co
0.85Se in supercapacitor applications. It enhances charge storage and transfer properties, ensuring consistent and reliable energy storage performance.
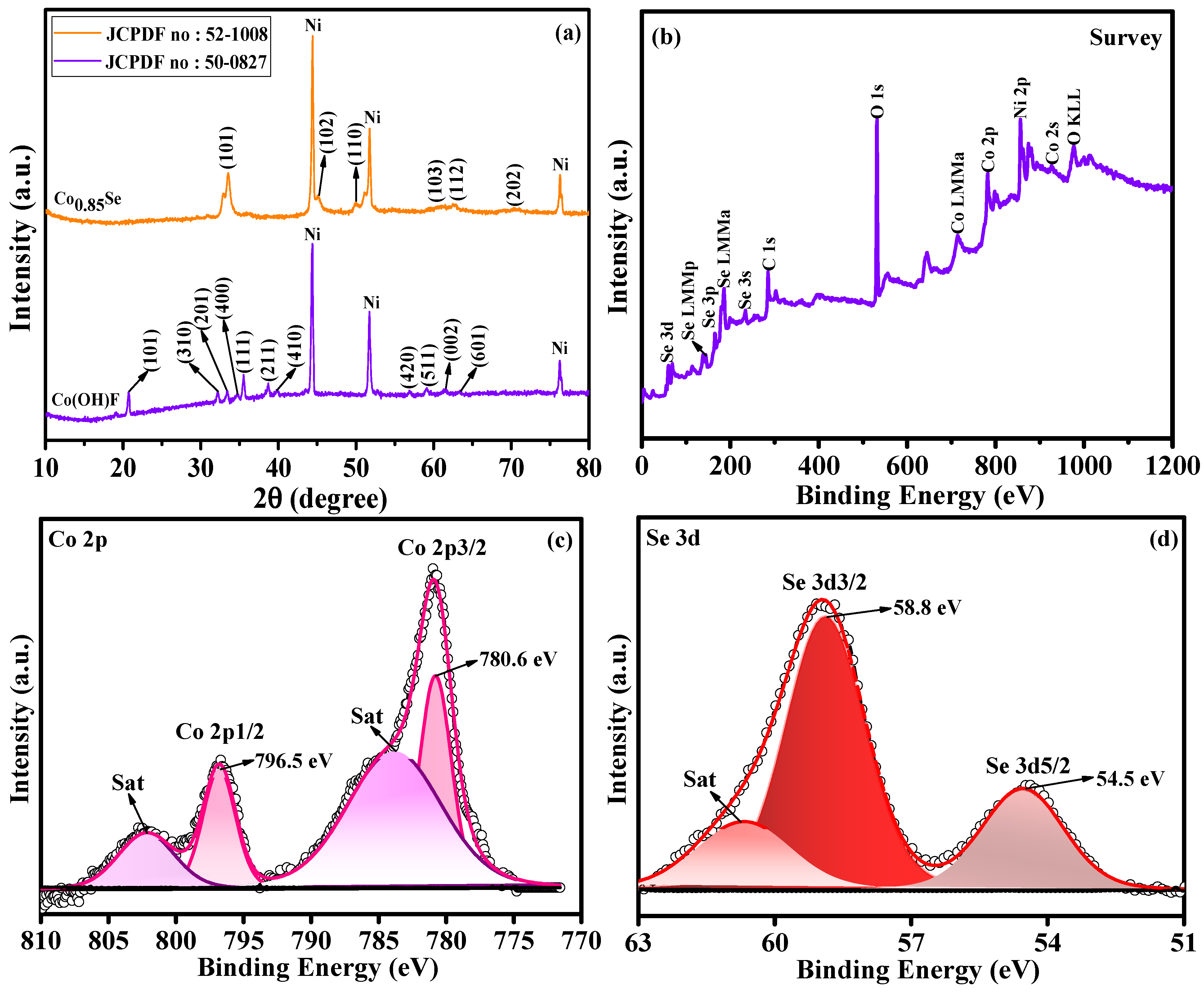
The phase compositions of the synthesized materials were analyzed by X-ray diffraction (XRD).
Figure 4a shows the XRD pattern of the as-synthesized Co(OH)F precursor obtained from the initial hydrothermal step, where all observed peaks are in close agreement with the orthorhombic Co(OH)F phase (JCPDS No. 50–0827) [
33]. After selenization, the XRD pattern (
Figure 4a) of the product closely matches that of hexagonal close-packed Co
0.85Se (JCPDS No. 52–1008) [
23,
26], with peaks at approximately 33.6°, 45.0°, 51.1°, 60.5°, 62.5°, and 70.2° corresponding to Co
0.85Se. These results confirm the successful transformation of Co(OH)F to Co
0.85Se during the selenization process. Furthermore, the Co
0.85Se/NF electrode material derived from Co(OH)F/NF exhibited no discernable diffraction peaks from impurities, indicating that the selenization process produced a high-crystalline product.
The XPS analysis of Co
0.85Se microspheres offers an understanding of Co and Se oxidation states in the material. The survey spectrum (
Figure 4b) shows the detection of C, O, Co, and Se, with carbon and oxygen attributed to the reference material and surface oxidation, respectively. The Co 2p XPS spectrum (
Figure 4c) displayed a doublet with peaks at 780.8 eV (Co 2p3/2) and 796.7 eV (Co 2p1/2), indicating that cobalt is predominantly in the +2 oxidation state (Co
2+). These observations are consistent with reported data for pure Co
0.85Se [
25,
30,
32]. Additionally, satellite peaks at 783.8 and 802.2 eV, characteristic of Co
2+, indicate interactions between the cobalt 2p core level and valence electrons. In the Se core-level spectrum (
Figure 4d), peaks observed at 54.5 and 58.8 eV are assigned to Se 3d5/2 and Se 3d3/2, respectively, which are characteristic of metal–selenide bonds (M–Se). These peaks confirm Co–Se bonding in the cobalt selenide structure [
16,
30,
32]. The satellite peak at approximately 60.6 eV, similar to that observed for Co
2⁺, indicates shake-up interactions. These results indicate that the Co
0.85Se microspheres comprised Co
2+ and Se in a metal–selenide configuration, consistent with the expected chemical structure of cobalt selenide.
The electrochemical performance of Co(OH)F/NF and Co
0.85Se/NF was evaluated using a three-electrode setup to determine their suitability as electrode materials for supercapacitors. The Co(OH)F/NF and Co
0.85Se/NF materials, prepared in advance, were used as working electrodes, with a graphite rod and Hg/HgO electrode functioning as the counter and reference electrodes, respectively. A 2M KOH aqueous solution served as the electrolyte.
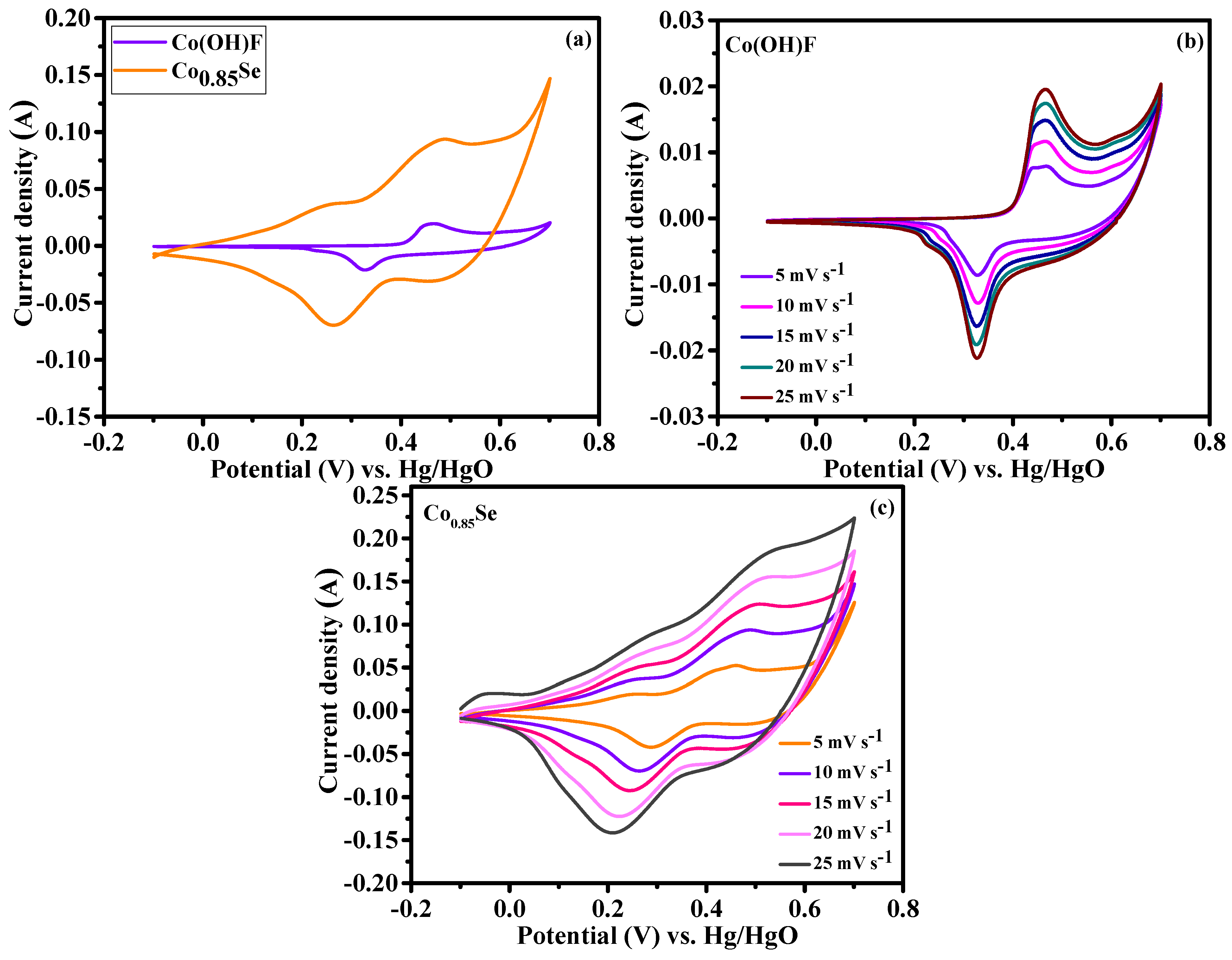
Figure 5a presents the cyclic voltammetry (CV) curves of Co(OH)F/NF and Co
0.85Se/NF electrode materials, recorded at a scan rate of 10 mV s⁻¹. Both materials exhibited CV profiles characteristic of battery-type behavior, featuring quasi-symmetric redox peaks [
31]. These peaks indicate Faradaic processes driven by reversible reactions involving cobalt ions [
33,
34]. Co(OH)F exhibited stable redox activity because of its interconnected microcrystal network, which promotes efficient ion diffusion and consistent electron transfer. By contrast, Co
0.85Se exhibited enhanced redox activity, as evidenced by broader peak currents, indicating superior charge storage capability.
Figure 5b,c show the CV curves of Co(OH)F/NF and Co
0.85Se/NF, respectively, obtained at scan rates ranging from 5 to 25 mV s⁻¹. Both materials retained their characteristic redox peaks even at higher scan rates, indicating excellent electrochemical reversibility. For Co(OH)F/NF, an increase in peak currents with scan rate indicates efficient ion transport. Similarly, Co
0.85Se/NF exhibited broader and more intense redox peaks at higher scan rates, demonstrating superior charge storage capacity and faster charge transfer kinetics. The separation between the anodic and cathodic peaks increased slightly with increasing scan rates for both materials, which can be attributed to polarization effects [
24,
35]. Despite this increase, the well-defined redox peaks confirmed the battery-type behavior of both electrodes, with Co
0.85Se exhibiting a greater current response, highlighting its enhanced activity and superior charge dynamics. Furthermore, the small intensities observed for both oxidation and reduction currents when Co(OH)F and Co
0.85Se are used in 2M KOH (
Figure 5b,c) are related to the electrochemical processes occurring in the electrolyte solution. These currents correspond to the reversible redox reactions involving cobalt ions, particularly the transitions between different oxidation states of cobalt, such as Co
2⁺ and Co
3⁺. In the case of Co(OH)F and Co
0.85Se, these reactions are typically associated with the Co
2⁺/Co
3⁺ redox couple, which occurs in the alkaline medium. The small peak intensities indicate that these redox processes are relatively weak or less pronounced, possibly due to factors such as the limited surface area available for electrochemical reactions or the Co(OH)F and Co
0.85Se structures’ stability under the applied conditions.
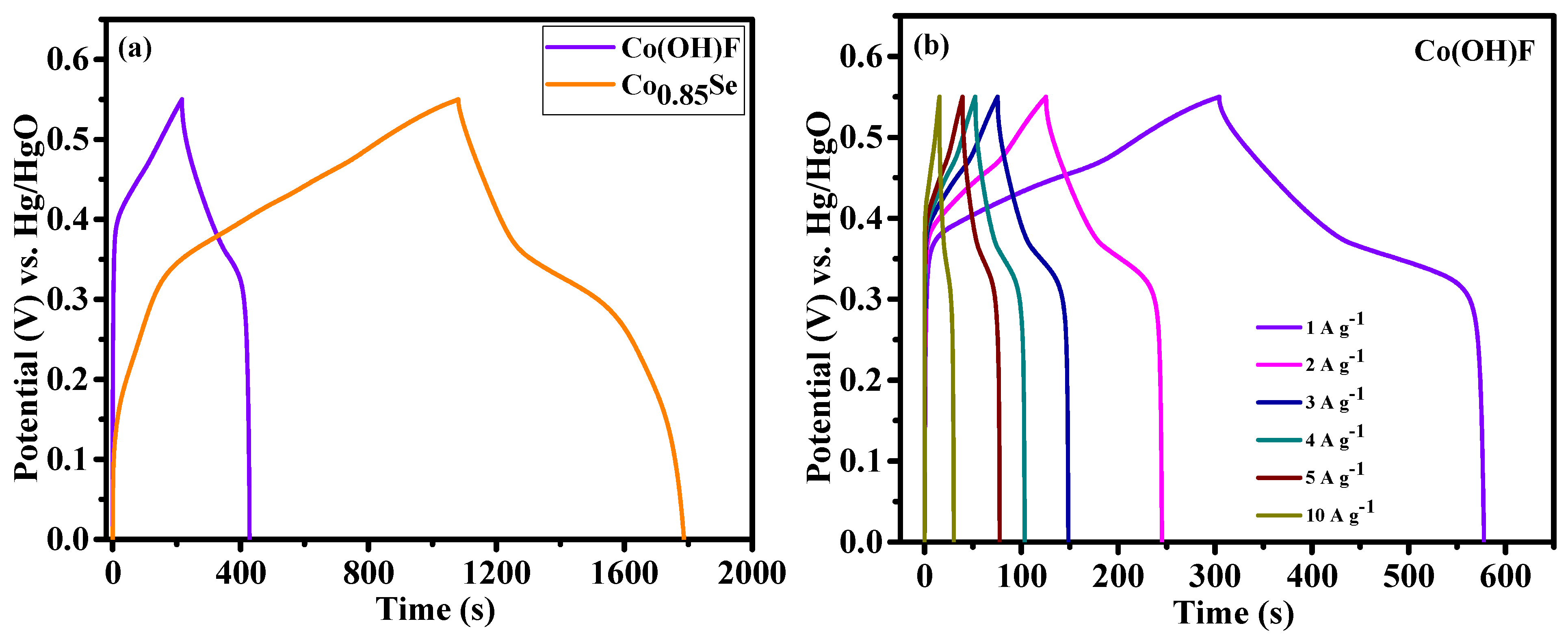
The galvanostatic charge-discharge (GCD) profiles were employed to examine the charge-discharge behavior of Co(OH)F/NF and Co
0.85Se/NF electrodes at varying current densities.
Figure 6a illustrates a comparison of the GCD profiles for the two materials, recorded within a potential range of 0–0.55 V at a current density of 1 A g⁻¹. The voltage characteristics of both Co(OH)F/NF and Co
0.85Se/NF exhibited typical characteristics of battery-type materials, with distinct charge and discharge plateaus. These features correlate with the findings from the CV analysis and suggest a reversible Faradaic process, which is essential to the capacity behavior of these materials. The specific capacities were calculated using
Equation (S1). At a current density of 1 A g
−1, the specific capacities of Co(OH)F/NF and Co
0.85Se/NF were 212.7 and 719 C g
−1, respectively. This significant difference in capacity, with Co
0.85Se/NF achieving over three times the capacity of Co(OH)F/NF, highlights its superior electrochemical performance at this current density. This improved performance is likely due to the spike-like subunits and rough surface morphology of the Co
0.85Se microspheres, which enhanced their conductivity and ion accessibility.
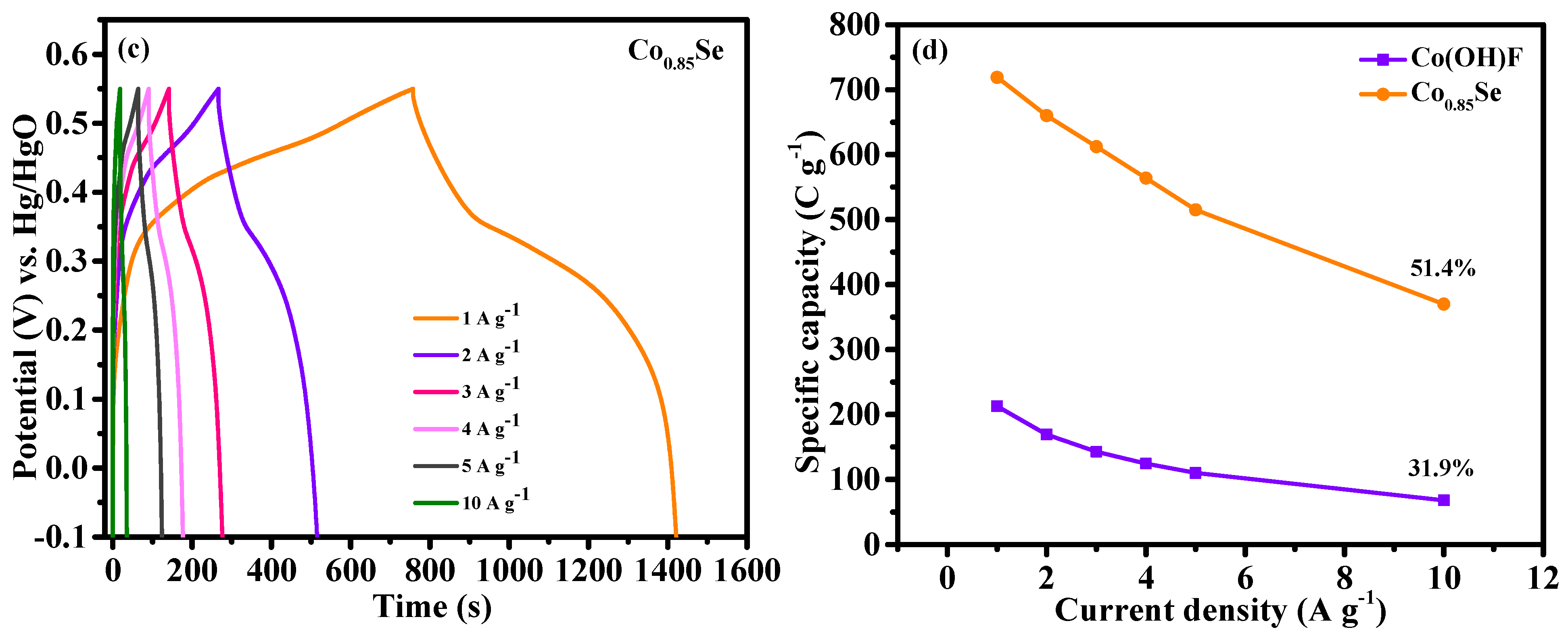
Figure 6b,c show the detailed GCD curves for the Co(OH)F/NF and Co
0.85Se/NF electrodes for a range of current densities from 1 to 10 A g
−1. The Co
0.85Se/NF electrode exhibited remarkable specific capacities of 719, 660, 612, 564, 515, and 370 C g
−1 at current densities of 1, 2, 3, 4, 5, and 10 A g
−1, respectively. By contrast, the Co(OH)F/NF electrode achieved specific capacities of 212.7, 169.2, 142.8, 124.4, 110, and 68 C g
−1 at the same current density. The Co
0.85Se/NF electrode exhibited remarkable electrochemical performance, outperforming previously reported Co-based electrode materials. For instance, it outperforms CoSe nanosheets (254.1 C g
−1 or 70.6 mAh g
−1) [
16], Co
0.85Se-2 nanosheets (111.6 C g
−1 or 31.0 mAh g
−1) [
25], Co
0.85Se thin films (59.2 C g
−1 or 16.47 mAh g
−1) [
31], Co(OH)F-60 (389.9 C g
−1) [
33], V-doped Co
0.85Se nanowire arrays (460.0 C g
−1) [
36], Ni
0.6Co
0.4Se (602.6 C g
−1) [
37], Co(CO
3)
0.5(OH).0.11H
2O (693.0 C g
−1) [
38], and Co(OH)F/Ni(OH)
2 (104.0 C g
−1) [
39]. This exceptional capacity, maintained across a range of current densities, underscores the outstanding electrochemical characteristics of the Co
0.85Se/NF electrode compared with other state-of-the-art materials.
Figure 6d illustrates the specific capacities of Co(OH)F/NF and Co
0.85Se/NF as a function of current density. Co
0.85Se/NF consistently exhibited higher specific capacities than Co(OH)F/NF at all current densities. This exceptional performance can be ascribed to the higher electrical conductivity and enhanced ion diffusion of Co
0.85Se/NF, which facilitates faster electron transfer and greater active material utilization. The capacity retention for both electrodes declined as the current densities were increased from 1 to 10 A g⁻¹. Co
0.85Se/NF achieved a rate performance of 51.4%, surpassing Co(OH)F/NF (31.9%). At lower current densities (1, 2, and 3 A g
−1), both materials exhibited relatively long charge-discharge times, indicating high reversibility and specific capacity. With the rise in current density, the charge-discharge duration is shortened due to the reduced time for ion diffusion and charge transfer [
34,
38]. At higher current densities (5 and 10 A g
−1), Co(OH)F/NF exhibited a notable reduction in specific capacity, reflecting slower ion diffusion and lower rate capability. The observed reduction in performance at higher current densities indicates that Co(OH)F/NF may exhibit limited conductivity or structural stability under high current demands. In contrast, Co
0.85Se/NF maintained a relatively higher specific capacity profile even at high current densities. This result indicates Co
0.85Se’s superior rate capability due to its enhanced electrical conductivity and ion diffusion properties, allowing for efficient charge storage and faster charge-discharge cycles.
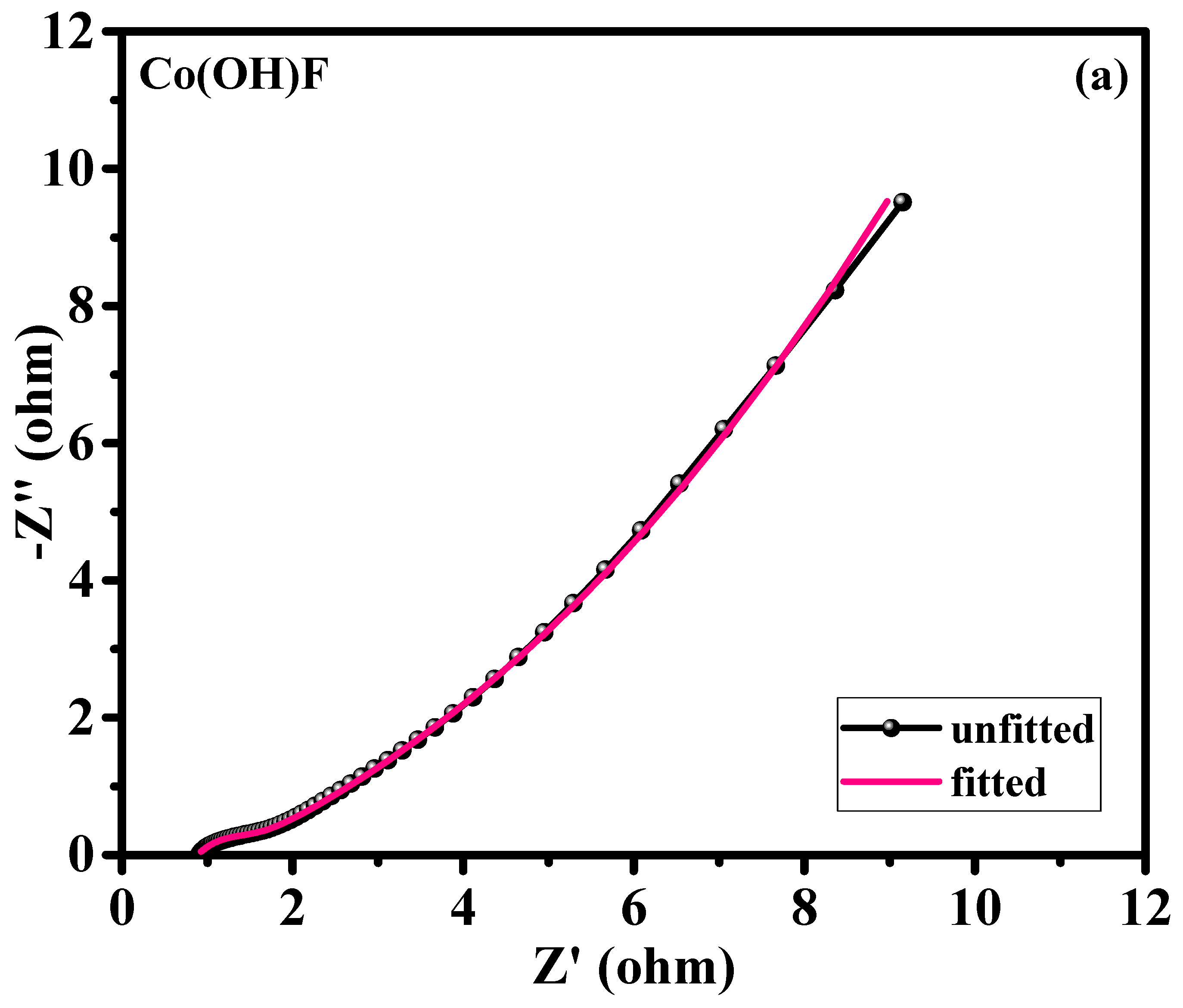
Electrochemical impedance spectroscopy (EIS) analysis offered important insights into the charge transport characteristics of the Co(OH)F/NF and Co
0.85Se/NF electrodes.
Figure 7a,b show Nyquist plots for both samples measured across a frequency spectrum of 0.01–100 kHz. These Nyquist diagrams were analyzed using the suggested equivalent circuit model (
Figure S2), and the obtained parameters are summarized in
Table 1. The Co
0.85Se/NF electrode exhibited lower internal resistance (Rs) and charge transfer resistance (Rct) values of 0.48 Ω and 0.154 Ω, respectively, compared to the Co(OH)F/NF electrode (0.87 Ω and 0.43 Ω). This result indicates superior electrical conductivity and faster electron transport in the Co
0.85Se/NF electrode. The reduction in Rct for Co
0.85Se/NF is due to the selenization process, which enhances the intrinsic conductivity of the electrode by incorporating selenium into the structure. This modification facilitates more efficient electron transfer at the interface between the electrode and the electrolyte, which is crucial to improving energy storage performance. Additionally, the Rs values representing the combined resistance of the electrolyte, electrode, and contact interfaces, was slightly lower for Co
0.85Se/NF than for Co(OH)F/NF. The slightly lower Rs value for Co
0.85Se/NF reflects improved contact and ion transport pathways. The Nyquist plots corroborate these findings, as evidenced by the reduced semicircle diameter in the high-frequency region for Co
0.85Se/NF, which corresponds to its lower Rct. In summary, the lower Rs and Rct values for Co
0.85Se/NF indicate enhanced conductivity and superior charge transport dynamics, which significantly contribute to its superior electrochemical performance compared to Co(OH)F/NF. These EIS findings align with the CV and GCD results presented in the previous sections, further supporting the improved performance of the Co
0.85Se/NF electrode.
The cycling stability of the Co(OH)F/NF and Co
0.85Se/NF electrodes was assessed at a current density of 20 A g
−1 over 10,000 cycles, as shown in
Figure 8a,b. The Co(OH)F/NF electrode retained 70.2% of its capacity, while the Co
0.85Se/NF electrode exhibited superior retention of 87.1%. The higher stability of Co
0.85Se/NF can be attributed to its enhanced electrical conductivity, which facilitates efficient charge transport, and its robust spike-like structure, which accommodates ion insertion and extraction with minimal degradation. Furthermore, Co
0.85Se/NF benefits from highly reversible Faradaic reactions, mitigating side reactions and structural fatigue. By contrast, Co(OH)F’s lower conductivity may result in greater stress and degradation during extended cycling, leading to comparatively reduced retention.
Building on the promising results obtained from the three-electrode system, an HSC device was assembled with Co
0.85Se/NF as the positive electrode and AC as the negative electrode. The electrochemical performance of the HSC device was evaluated in a 2M KOH aqueous electrolyte, leveraging the synergistic combination of a Faradaic electrode (Co
0.85Se) for high energy density and a non-Faradaic electrode (AC) for excellent power density. Before evaluating the HSC device, the electrochemical properties of the AC electrode were assessed in a three-electrode setup using 2M KOH as the electrolyte. The CV curves in
Figure S3a exhibit a nearly rectangular shape characteristic of electric double-layer capacitance (EDLC) behavior. Charge storage occurs through the electrostatic adsorption of ions at the electrode–electrolyte interface without Faradaic reactions. The high surface area of the AC electrode (2700–3000 m
2 g
−1) enhances ion adsorption and electrolyte accessibility, maintaining a rectangular shape across scan rates of 10–50 mV s
−1. GCD measurements conducted at various current densities ranging from 1–5 A g
−1 further evaluated the specific capacitance of the AC electrode (
Figure S3b). The GCD profiles exhibit nearly linear and symmetric charge-discharge curves, consistent with EDLC behavior and efficient energy storage mechanisms. The specific capacitance was calculated from the discharge curves using
Equation (S2), yielding 287.3 F g
−1 at a current density of 1 A g
−1. These results confirm that AC is an exceptional material for EDLC-type electrodes, offering high specific capacitance and remarkable reversibility, making it an ideal choice for the negative electrode in HSC devices.
Figure 9a presents the comparative CV curves of the positive (Co
0.85Se) and negative (AC) electrodes, recorded at a scan rate of 10 mV s
−1 in a three-electrode configuration. The CV profiles highlight the distinct electrochemical characteristics of the two electrodes. The Co
0.85Se/NF electrode exhibited Faradaic behavior, as evidenced by the presence of redox peaks, which indicate reversible redox reactions associated with Faradaic processes. In contrast, the AC electrode exhibited non-Faradaic behavior, characterized by a nearly rectangular CV curve, which is characteristic of EDLC.
The CV curves of the Co
0.85Se/NF//AC HSC device were evaluated over a range of potential windows from 1.2 to 1.7 V at a constant scan rate of 50 mV s
−1 (
Figure 9b). The CV profiles remained stable across this range, with no evidence of the oxygen evolution reaction (OER) observed up to 1.7 V. The absence of an OER is critical for a suitable electrochemical window and stable energy storage without parasitic reactions. Based on these findings, the optimal operating potential window for an HSC device was established as 1.7 V.
Figure 9c shows the CV curves of the Co
0.85Se/NF//AC HSC device at scan rates ranging from 10 to 50 mV s
−1. The CV curves exhibit a combination of redox peaks attributed to the Co
0.85Se electrode and a rectangular shape characteristic of the AC electrode, highlighting the hybrid nature of the device. This combination reflects the contributions of both Faradaic and non-Faradaic capacitance. As expected, the area under the CV curves increased with the scan rate, indicating a greater current response for the HSC device. The consistent increase in the curve area indicates the device’s excellent electrochemical performance and rate capability, even at higher scan rates.
Figure 9d shows the GCD curves of the HSC device measured at different potential windows (1.2–1.7 V) under a constant current density of 5 A g
−1. The non-linear charge-discharge profiles indicate a hybrid charge storage mechanism that combines redox and EDLC behaviors. The presence of both asymmetric and plateau regions reflects the Faradaic charge storage from the Co
0.85Se electrode and the non-Faradaic charge storage from the AC electrode.
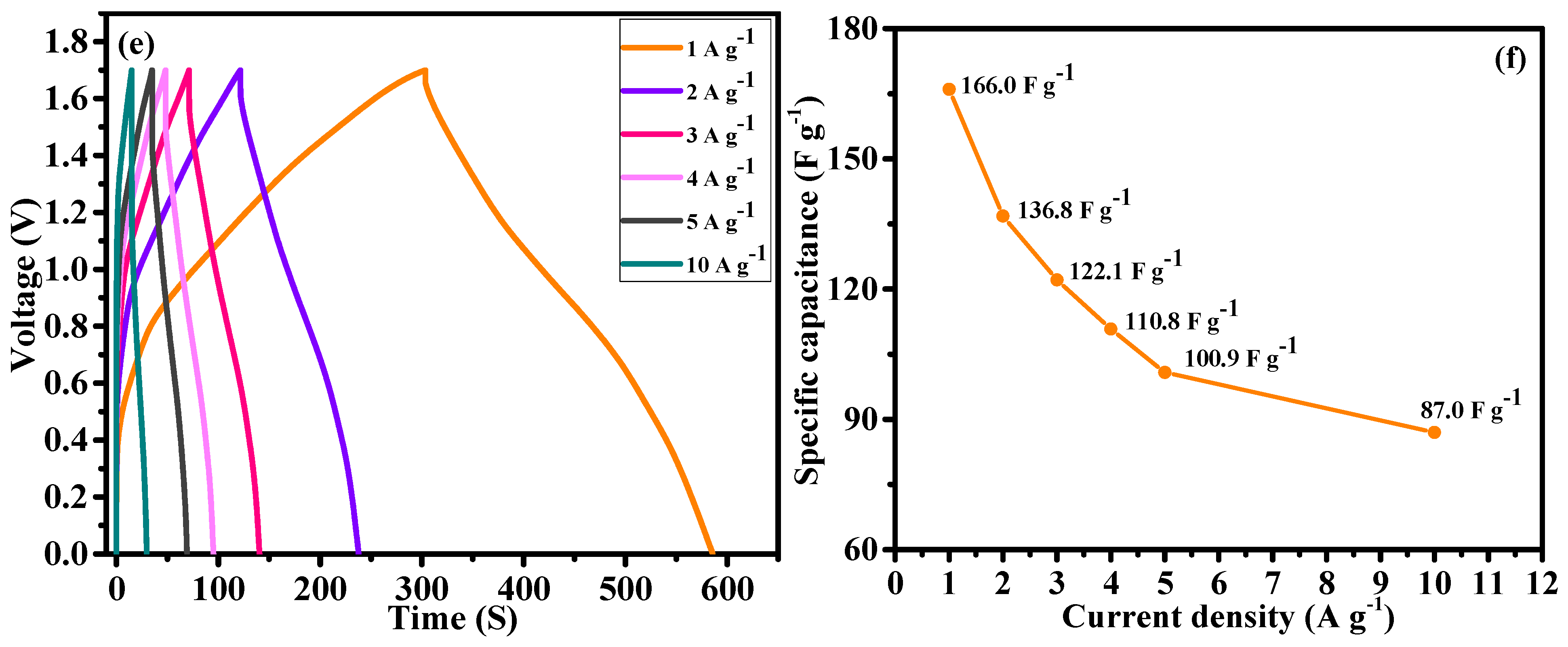
Figure 9e shows the GCD curves of the HSC device measured at current densities ranging from 1 to 10 A g
−1. As the current density increased, the curves exhibited asymmetry and distinct plateau regions, reaffirming the hybrid nature of the device. The specific capacitance of the HSC device was calculated from the discharge curves using
Equation (S2), as shown in
Figure 9f. At a current density of 1 A g
−1, the device achieved a specific capacitance of 166 F g
−1, highlighting the efficient charge storage capability of the hybrid system. As expected, the specific capacitance decreased slightly with increasing current density due to the limited time for ion diffusion and charge transfer. The specific capacitance values at various current densities were as follows: 166 F g
−1 at 1 A g
−1, 136.8 F g
−1 at 2 A g
−1, 122.1 F g
−1 at 3 A g
−1, 110.8 F g
−1 at 4 A g
−1, 100.9 F g
−1 at 5 A g
−1, and 87 F g
−1 at 10 A g
−1. This result indicates a gradual decrease with increasing current density. The HSC device exhibited excellent rate capability, retaining 87 F g
−1 of its initial capacitance at 10 A g
−1, proving its ability to maintain a significant portion of its charge storage capacity under fast charge-discharge conditions.
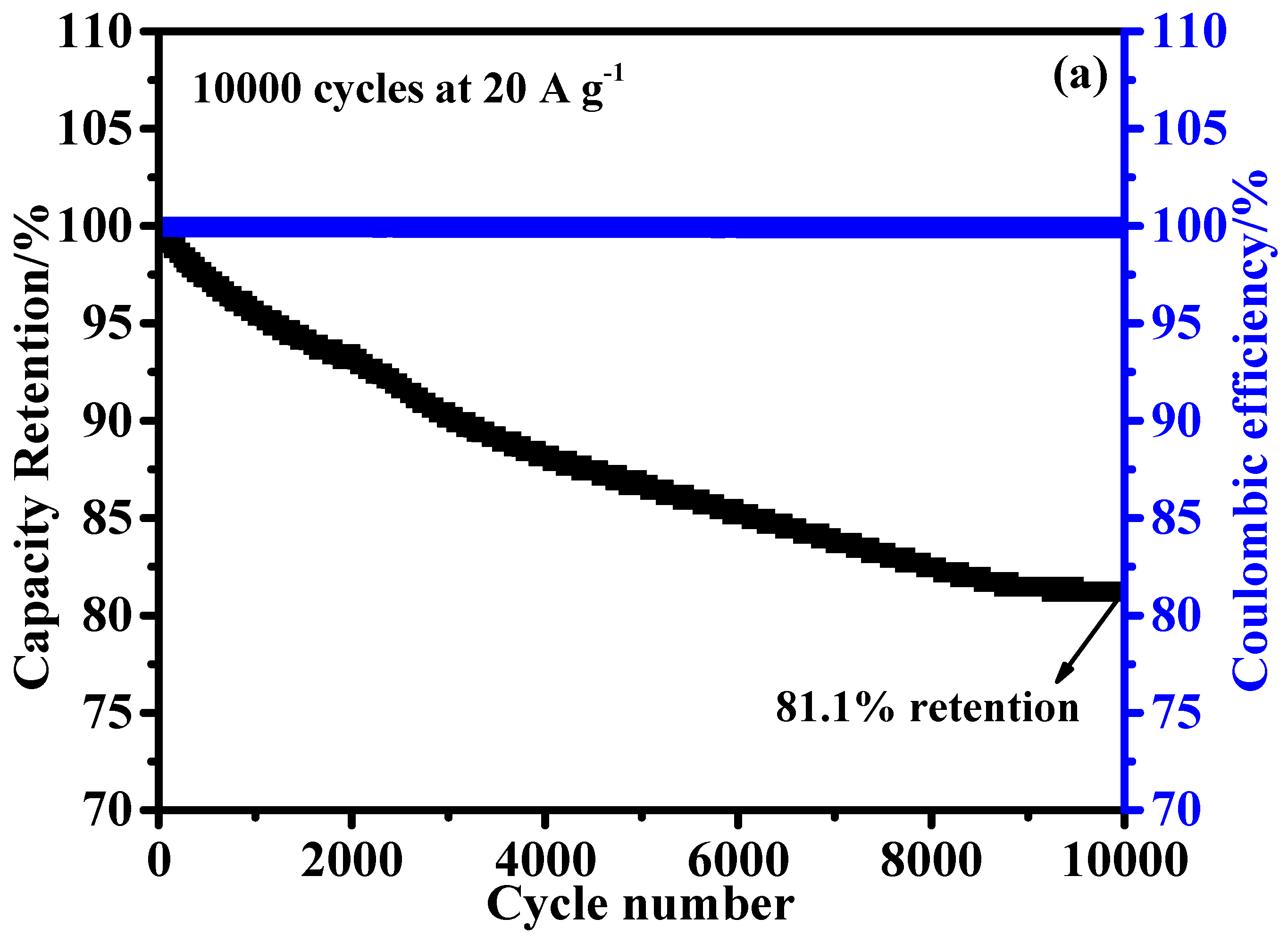
The long-term stability of HSC devices is critical for practical applications. To assess its cycling stability, the device underwent continuous charge-discharge tests for 10,000 cycles at a current density of 20 A g
−1 (
Figure 10a). The device retained approximately 81.1% of its initial capacitance after 10,000 cycles, demonstrating remarkable electrochemical stability. The high capacitance retention highlights the robustness and durability of the HSC device, underscoring its potential for long-term use in energy storage applications. The energy and power densities of the HSC device were calculated using
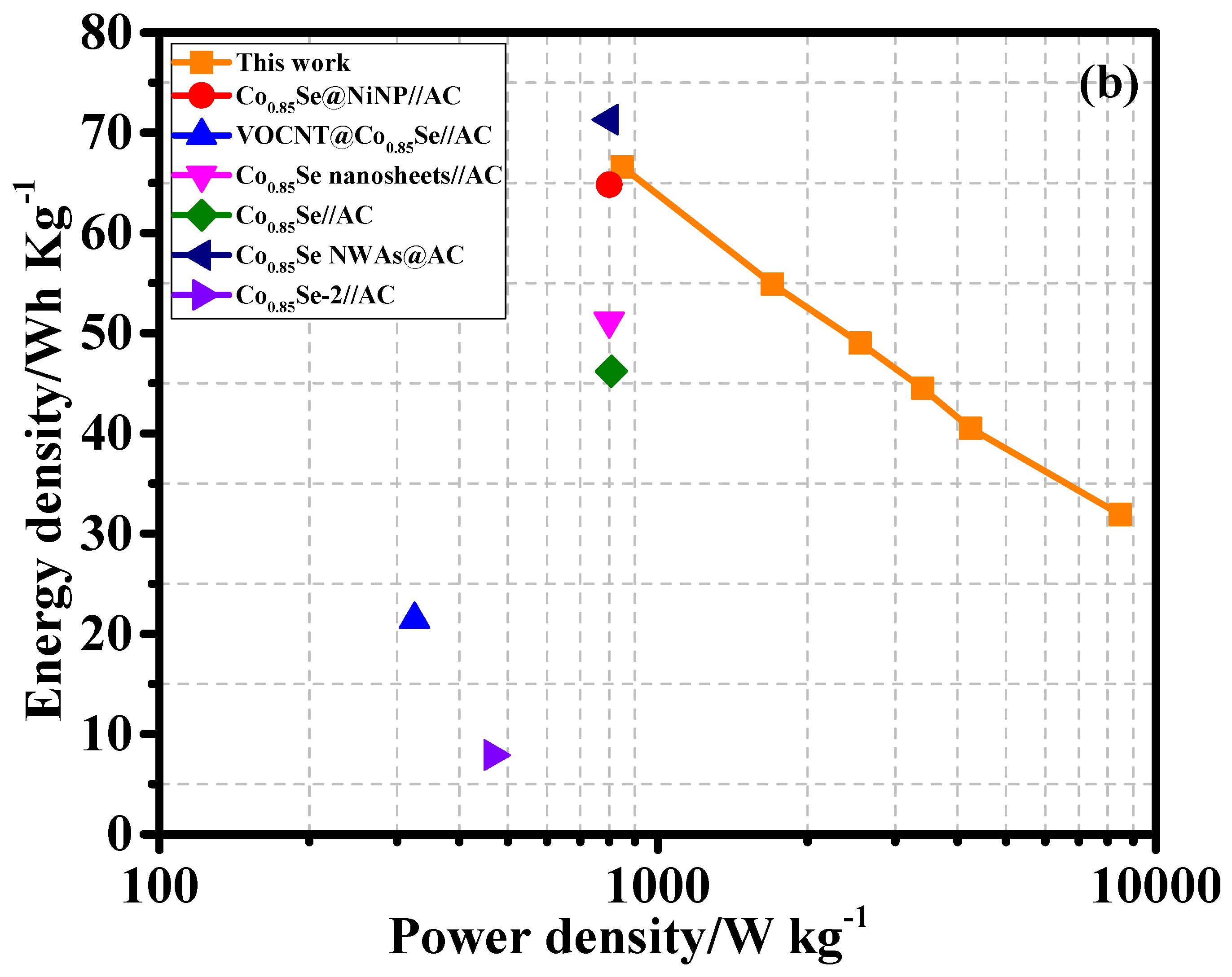
Equations (S5) and (S6). The results are shown in the Ragone plot (
Figure 10b). The Co
0.85Se/NF//AC HSC device achieved a high energy density of 66.6 Wh kg
−1 at a power density of 849.3 W kg⁻¹. Even at a significantly higher power density of 8489.2 W kg
−1, the device maintained a respectable energy density of 31.9 Wh kg
−1. These results indicate that the Co
0.85Se/NF//AC HSC device outperforms several previously reported HSC devices, highlighting the superior electrochemical performance of the Co
0.85Se/NF//AC configuration. A comparative analysis (
Table 2) further highlights the superior energy and power densities of the Co
0.85Se/NF//AC HSC device compared to state-of-the-art HSCs. For instance, Co
0.85Se@NiNP//AC achieved 64.81 Wh kg
−1 at 800 W kg
−1 [
38], Co
0.85Se hollow spheres//AC yielded 54.6 Wh kg
−1 at 1600 W kg
−1 [
26], VOCNT@Co
0.85Se//AC delivered 21.47 Wh kg
−1 at 325 W kg
−1 [
27], Co
0.85Se nanosheets//AC achieved 51.2 Wh kg
−1 at 800 W kg
−1 [
28], Co
0.85Se NWAs//AC reached 71.3 Wh kg
−1 at 800 W kg
−1 [
29], Co
0.85Se//AAC yielded 23.95 Wh kg
−1 at 800 W kg
−1 [
30], and CoSe
2/CFF//AC/CFF achieved 22.43 Wh kg
−1 at 823.12 W kg
−1 [
39]. These comparisons emphasize the outstanding performance of the Co
0.85Se/NF//AC HSC device in terms of energy and power densities.