Abstract
Impurities significantly constrain the production of high-purity magnesium sulfate crystals, essential for advanced magnesium-based materials. Sodium–magnesium co-precipitation affects the crystals’ purity and surface smoothness, with NaCl embedding into crystal surfaces during cooling crystallization in the MgSO4-NaCl-H2O system. Trace impurities, however, inhibit NaCl adhesion, alter SO42− coordination structures, and enhance the purity and morphology of MgSO4·6H2O crystals. Adding 300 mmol/L K2SO4 reduces sodium content in crystals from 0.57% to 0.03% and surface roughness from 2.76 nm to 0.415 nm. The binding energies of Na+ on MgSO4·6H2O crystal planes are lower than those of impurity ions, which compete for active growth sites and prevent Na+ nucleation. This finding challenges the assumption that higher-purity solutions yield higher-purity crystals.
1. Introduction
Salt lakes are a major source of magnesium sulfate; however, the magnesium sulfate crystals extracted from brine often exhibit significant issues, including surface impurities, irregular morphologies, and rough surfaces. These deficiencies reduce the surface smoothness and overall purity of the crystals, thereby compromising the quality of downstream products, such as basic magnesium sulfate. This, in turn, impacts the compatibility [1] and interfacial bonding strength of these materials with other components [2,3]. For instance, in construction materials and adhesives [4], smoother surfaces enhance interfacial bonding properties [5]. Similarly, in photocatalytic composite materials [6], smoother surfaces reduce frictional resistance, improve magnesium sulfate dispersion within the matrix, and enhance both catalytic efficiency and reaction selectivity. At present, the quality of magnesium sulfate products derived from salt lakes requires substantial improvement. Achieving the desired purity and morphology in magnesium sulfate crystals is essential for advancing the salt-lake magnesium industry.
An ideal crystal surface should be smooth and defect-free, enabling efficient crystal growth, minimizing impurity accumulation, and enhancing overall crystal purity. The surface morphology and purity of a crystal are closely linked to its nucleation and growth processes. During nucleation, classical nucleation theory describes crystallization in aqueous solutions as the aggregation of constituent ions [7,8]. Structural details in the pre-nucleation stage play a foundational role in determining the composition and structure of the resulting crystals. For example, Hu [9] demonstrated via Raman spectroscopy that altering solution conditions can modify ionic structures, thereby influencing the composition and morphology of precipitated crystals. Similarly, Kollias [10] employed molecular dynamics simulations to show that the ionic composition and concentration in a solution significantly affect the morphology of ion clusters, which subsequently impacts nucleation rates and the final crystal morphology. In the MgSO4 system, the association structures of SO42− ions are influenced by other ions, leading to the formation of distinct MgSO4 crystal nuclei [11].
During crystal growth, the anisotropic surface energy of nuclei drives solute molecules to preferentially attach and accumulate on low-energy lattice planes, thereby promoting directional growth [12]. Dobberschütz [13] identified that the inhibitory effects of inorganic substances on crystallization are primarily due to surface adsorption, which blocks active growth sites. Other studies [14] have suggested that impurities may bind to specific crystal surface sites via charge adsorption [15] or chelation with lattice functional groups [16]. These interactions alter the adsorption layer at the crystal–solution interface [17], modify energy barriers during crystallization, and interfere with nucleation, aggregation, and growth processes [18]. Such disruptions ultimately impact the morphology and purity of the final crystalline products [19]. Impurity ions, therefore, play a critical role in disrupting crystal habits. Accumulated impurities during growth result in defect sites or boundaries, adversely affecting the crystal’s overall quality. The specific effects of impurities vary depending on the crystallization system [20]. In the context of cooling crystallization for magnesium sulfate production, the mechanisms through which impurity ions influence nucleation and growth remain poorly understood, necessitating further investigation.
This study focused on the effects of common brine ions, such as K+, Li+, and Ca2+, on solution ion structures and crystallization products. Molecular dynamics simulations were employed to analyze these effects at the atomic scale, exploring impurity attachment mechanisms and their interactions with magnesium sulfate crystal surfaces. The ultimate goal was to control magnesium sulfate crystal morphology and provide guidance for the production of high-quality magnesium sulfate.
2. Materials and Methods
2.1. Materials
The reagents used in the experiments included magnesium sulfate heptahydrate, sodium chloride, potassium chloride, potassium sulfate, calcium sulfate dihydrate, lithium chloride, and lithium sulfate monohydrate, all of which were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Additionally, nitric acid (analytical grade) was sourced from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). Ultrapure water with a conductivity of less than 0.1 μS/cm was utilized throughout the experiments to ensure minimal contamination and consistent results.
Crystallization experiments were performed in a 500 mL borosilicate glass reactor under ambient atmospheric conditions (25 °C, 1 atm). The reactor remained open to air throughout the process to simulate industrial crystallization environments, with no active control of oxygen ingress.
The instruments employed in this study included an X-ray diffractometer (X-ray diffraction (XRD) measurements were performed using a D/teX-Ultra, Rigaku, Japan, diffractometer with Cu Ka radiation (λ = 1.5406 Å) operated at 40 kV and 40 mA. Scans were collected over a 2θ range of 10°–80° with a step size of 0.02°) for crystallographic analysis, a high-vacuum scanning electron microscope equipped with an energy-dispersive spectrometer (JSM-IT500HR, JEOL, Tokyo, Japan) for morphological and elemental composition analysis, and a focused ion beam scanning electron microscope (Scios 2 HiVac, Thermo Fisher Scientific, Bothell, WA, USA) for detailed structural imaging. An atomic force microscope (AFM, Bruker Dimension Icon, Karlsruhe, Germany) was used to measure surface roughness, while an inductively coupled plasma optical emission spectrometer (5800VD, Agilent Technologies, Santa Clara, CA, USA) provided elemental quantification. Additionally, an in situ infrared spectrometer (Nicolet iS50, Thermo Fisher Scientific, Bothell, WA, USA) was used for the chemical bond analysis.
In an aqueous solution environment, oxygen may exist as dissolved oxygen (DO) or peroxides (e.g., H2O2). While dissolved oxygen could theoretically interact with Mg2+ and SO42− ions during nucleation and growth, our experiments were conducted in an open crystallizer under ambient atmospheric conditions (25 °C). Atmospheric oxygen diffusion was not actively controlled here, as the study prioritized impurity incorporation. Nevertheless, oxygen-related effects on crystal morphology and doping—including potential mitigation strategies such as sealed crystallizers—will be systematically investigated in future work.
2.2. Experimentai Procedure
Given the relatively high NaCl content in the cooling crystallization process of magnesium sulfate, it was considered the primary component of the salt solution. To obtain high-purity magnesium sulfate hydrates, a quaternary phase diagram of the Na+, Mg2+//Cl−, SO42−-H2O system (Figure 1) [1] was used as a reference. A co-saturated solution of MgSO4·6H2O-NaCl at the M1 phase point (containing 31.19 g/L Na+, 82.41 g/L Mg2+, 48.21 g/L Cl−, and 327.68 g/L SO42−) was prepared in a constant-temperature water bath maintained above 60 °C. The SO42− to Cl− ratio was adjusted, and the solution was gradually cooled to 60 °C under static conditions to form a metastable saturated solution. Crystallization was initiated by further cooling the solution from M1 to M2, down to room temperature, resulting in the precipitation of magnesium sulfate hydrates.
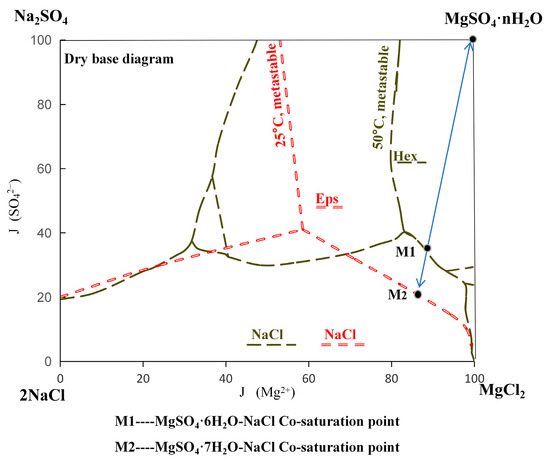
Figure 1.
Phase diagram of Na+,Mg2+//Cl−, SO42−-H2O quaternary water–salt system.
2.3. Molecular Dynamics Simulation
During crystal growth, the attachment of solute molecules and impurities is influenced by both the anisotropic surface energy and the atomic structure of the crystal faces. While low-energy lattice planes may promote preferential attachment, the specific arrangement of atoms on each face also plays a critical role in determining impurity capture. For example, in KDP crystals, the (100) and (101) faces both exhibit low surface energy but capture impurities differently due to their distinct atomic structures [21]. Therefore, the binding energy of impurities on the crystal surface was calculated using the (100) and (010) planes of MgSO4·6H2O as examples.
Molecular dynamics simulations were conducted to investigate the cooling crystallization process of MgSO4-NaCl co-saturated solutions. A mixed solution model of MgSO4-NaCl was constructed, and energy minimization was performed to ensure system neutrality, as shown in Figure 2a. The system was cooled from 333.15 K to 298.15 K with a timestep of 2 femtoseconds, and energy values were obtained over a 365 ps stabilization period to monitor changes in system energy over time. Optimized MgSO4·6H2O (100) and (010) crystal planes were prepared, and NaCl crystals were added to the processed crystal surfaces. Layer models of MgSO4·6H2O (100) and (010) with NaCl were developed; the (100) plane is characterized by the hexahydrated Mg octahedra, while the (010) plane simultaneously features both Mg octahedra and sulfate tetrahedra. The following equations were applied to calculate the surface energy of the two planes without NaCl, the formation energy of NaCl on the product surface, and the interaction energy between impurity ions and Na+ on the magnesium sulfate crystal planes, to analyze the competition between impurity ions and Na+.
where Esurf is the surface energy; Eslab is the total energy of the surface model, including the energy of surface atoms and additional energy due to the surface presence; n is the number of atoms in the slab model; Ebulk is the average energy of each atom in the bulk phase; A is the surface area of the slab model.
where E(crystallographicnplane) is the total energy of the crystal plane; ENaCl is the energy of NaCl; and Eslab is the total energy of the surface model.
where ΔE is the binding energy; Etotal is the total energy of the system with impurity ions on the crystal surface; Esurface is the total energy of the surface; and Eion is the energy of the ions.
Esurf = (Eslab − nEbulk)/2A
E = E(crystallographicnplane) – EnaCl − Eslab
ΔE = Etotal − (Esurface + Eion)
Figure 2.
Model schematic diagram. (a) Schematic of the solution model. (b) Crystal structure model of MgSO4·6H2O.
3. Results
3.1. Morphology and Composition of Cryogenic Crystallization Products
To evaluate the crystallization effects of the MgSO4-NaCl saturated solution under cooling conditions, experiments were conducted, and the results are summarized in Figure 3. The XRD spectrum in Figure 3a confirms that the primary solid product was MgSO4·6H2O. The presence of co-precipitated impurities or additional crystalline phases in the product resulted in extra diffraction peaks in the XRD spectrum compared to the standard reference pattern. Therefore, as shown in the SEM image in Figure 3b, small square-shaped particles were observed on the crystal surface. The EDS analysis in Figure 3c identified these particles as NaCl, with sodium and chlorine present in a 1:1 ratio. AFM measurements in Figure 3d revealed that the surface roughness (Ra) of the crystal within a 2.5 μm × 2.5 μm region was 2.76 nm. Further analysis using FIB-SEM in Figure 3e demonstrated that NaCl particles were embedded within the MgSO4·6H2O crystal surface, which was further confirmed by 3D imaging and EDS analysis in Figure 3f. These results indicate that NaCl co-precipitates with MgSO4 during the crystallization process, significantly affecting the surface roughness and overall quality of the crystals.
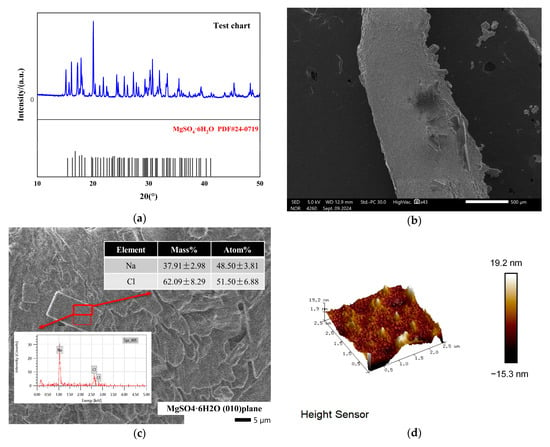
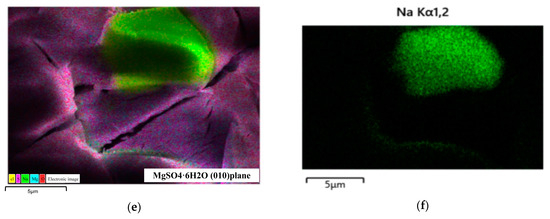
Figure 3.
Model schematic diagram. Morphology and composition of cryogenic crystallization products. (a) Crystalline XRD pattern. (b) Crystal morphology. (c) Crystalline EDS spectrum. (d) Atomic force microscope 3D diagram. (e) EDS analysis of FIB-SEM profile. (f) Na element distribution.
These results indicate that the cryogenic crystallization products of MgSO4-NaCl saturated solutions have poor quality. The primary reason is the embedding of NaCl particles into the crystal surface during crystallization, which significantly impacts the morphology and quality of the crystals.
3.2. Effects of Impurity Ions on Crystallization Product Morphology and Composition
To explore the influence of impurity ions in the solution on cryogenic crystallization products, commonly found impurity ions in brine were added to the MgSO4-NaCl saturated solution. The chemical composition, phase composition, and morphology of the resulting crystalline products were analyzed, with the results shown in Figure 4, Figure 5, Figure 6 and Figure 7. As shown in Figure 4, the addition of trace impurity ions significantly reduced the sodium content in the crystallized product. For example, when 300 mmol/L K2SO4 was added, the sodium mass fraction in the product decreased from 0.57% to 0.03%, resulting in the highest-purity product.
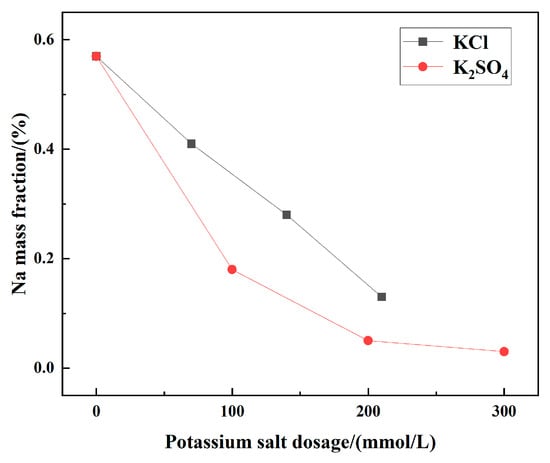
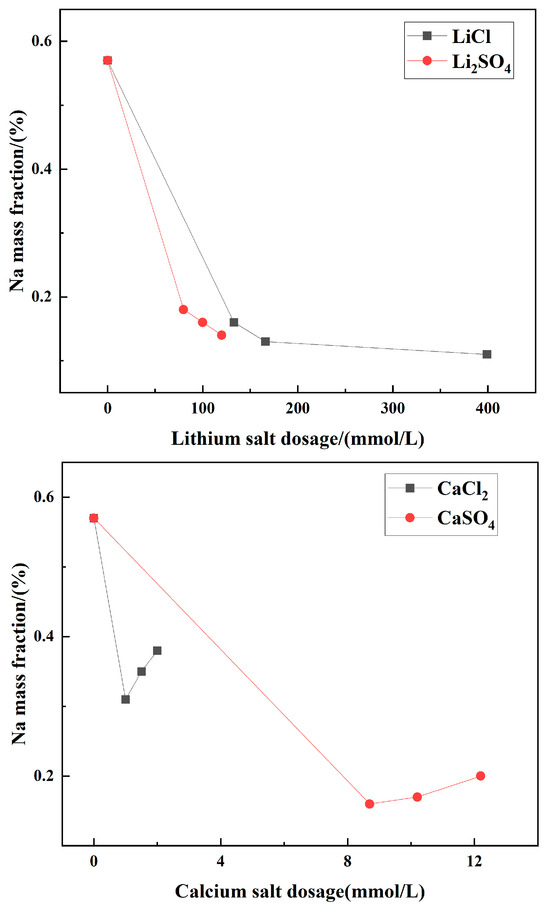
Figure 4.
Mass fraction of Na+ in crystalline products with different impurity ion contents.
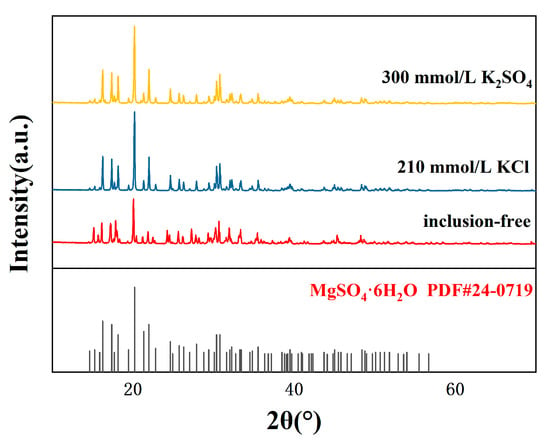
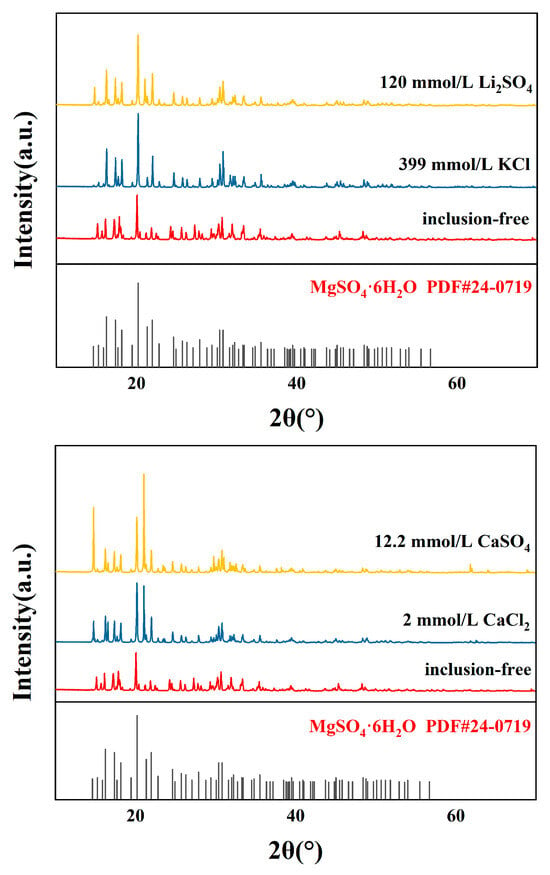
Figure 5.
XRD patterns of crystalline products with different impurity ion contents.
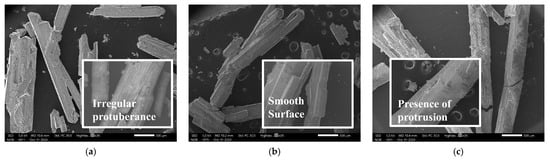

Figure 6.
Morphology of crystals obtained under different impurity ion contents: (a) 210 mmol/L KCl; (b) 300 mmol/L K2SO4; (c) 399 mmol/L LiCl; (d) 120 mmol/L Li2SO4; (e) 2.0 mmol/L CaCl2; (f) 12.2 mmol/L CaSO4.
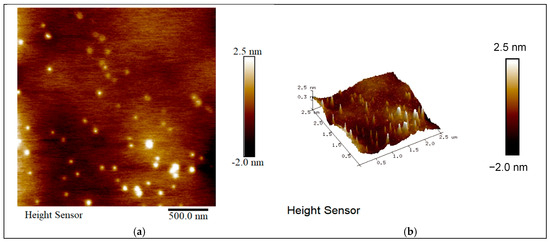
Figure 7.
Morphology of crystals containing 300mmol/L K2SO4. (a) AFM figure. (b) AFM 3D figure.
The XRD analysis (Figure 5) of the crystalline products indicated that the addition of impurity ions did not alter the phase composition of the product, which remained MgSO4·6H2O. The sharp diffraction peaks and absence of additional peaks in the XRD spectra confirmed the product’s phase purity. However, slight shifts in peak positions and intensities suggested that impurity ions slightly influenced the crystal lattice structure.
The SEM and AFM analysis (Figure 6 and Figure 7) demonstrated that the addition of impurity ions significantly reduced the number of square-shaped NaCl particles on the crystal surface, resulting in smoother and more uniform crystal morphologies. When 300 mmol/L K2SO4 was added, the surface roughness was reduced to 0.415 nm, as observed in the AFM images.
These results indicated that reducing the sodium content improved the regularity of the crystal morphology and enhanced product purity. Within the experimental range, the addition of impurity ions optimized the morphology of MgSO4-NaCl cooling crystallization products, thereby improving product quality. It is hypothesized that the addition of impurity ions would increase the solubility of sodium chloride, thereby reducing the Na content in the crystal [22].
3.3. Effects of Impurity Ions on the Structure of Saturated Solutions
In aqueous solutions, Mg2+ exhibits strong electrostatic interactions with water molecules due to its high charge density and small ionic radius (0.72 Å). Beyond its primary hydration shell, Mg2+ interacts with surrounding sulfate ions (SO42−) through ion pairing and hydrogen bonding networks. The relative proportions of these species directly impact the nucleation and growth mechanisms of MgSO4·6H2O crystals.
Previous studies have demonstrated that impurity ions enhance the morphology and purity of MgSO4·6H2O crystals. To further elucidate the microscopic mechanisms behind this improvement, in situ Raman spectroscopy was utilized to analyze the influence of impurity ions on the SO42− coordination structures in the MgSO4 system [23,24]. As shown in Figure 8, the characteristic peak at 983 cm−1 corresponded to solvent-separated ion pairs (Mg2+-H2O-SO42−), while the peak at 1145 cm−1 represented complex SO42− ionic clusters [25]. An increased transmittance at these peaks indicates a higher concentration of the respective components [26]. After introducing impurity ions, the transmittance at both 983 cm−1 and 1145 cm−1 increased significantly, suggesting that impurity ions promote the formation of Mg2+-H2O-SO42− complexes, facilitating the nucleation of MgSO4·6H2O crystals. These findings confirm that impurity ions alter the ratio of SO42− coordination structures in the solution, making the system more conducive to the precipitation of MgSO4·6H2O. This conclusion aligns with the improved purity and morphology of magnesium sulfate crystals observed in Figure 4 and Figure 6.
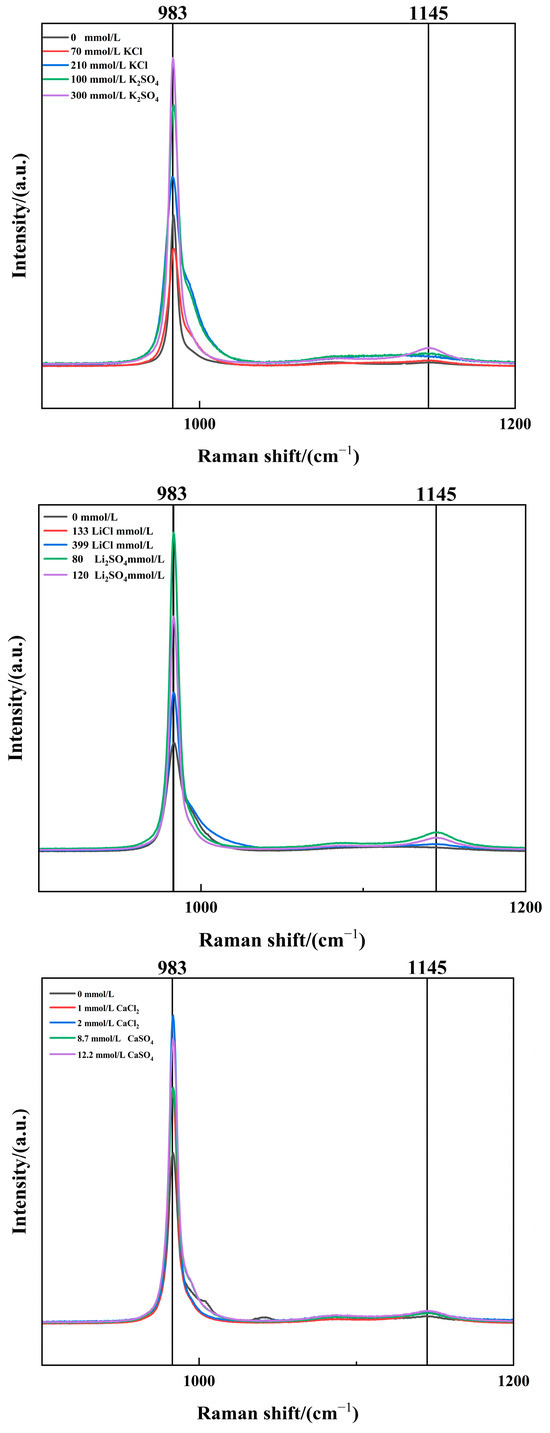
Figure 8.
Raman Spectra of Saturated Solutions with Different Impurity Ion Contents.
3.4. Competitive Modification Effects of Impurities During Cryogenic Crystallization
To investigate the atomic-scale mechanisms of NaCl co-precipitation with MgSO4 and the role of impurities in the reduction of Na content in crystals, molecular dynamics simulations were conducted.
The calculated formation energies of NaCl on the (100) and (010) planes of MgSO4·6H2O were negative, as shown in Table 1, indicating a thermodynamic tendency for NaCl precipitation under current cooling and pressure conditions. Furthermore, the presence of sulfate tetrahedra on the (010) plane creates energetically favorable sites for NaCl attachment, which is why NaCl preferentially attaches to the (010) plane.

Table 1.
Calculation of NaCl formation energy.
This suggests that Na+ and Cl− ions in the system, having reached saturation, occupy active growth sites on MgSO4·6H2O surfaces [27]. Figure 9a illustrates how Na+ and Cl− diffuse and are adsorbed onto MgSO4·6H2O clusters via electrostatic interactions, leading to the formation of local NaCl nuclei [28,29]. These nuclei modify the relative growth rates of different crystallization sites, altering the crystal morphology [30].
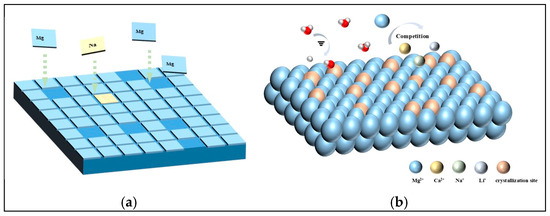
Figure 9.
Mechanism diagram of influence of impurity ions on crystallization products. (a) Na+ and Cl− occupy active sites on the surface of MgSO4·6H2O crystal. (b) Impurity ions compete with Na+ for active crystallization sites on the crystal surface.
Impurity ions exhibited stronger binding affinities to MgSO4·6H2O crystal surfaces compared to Na+, as shown in Figure 10. This competitive advantage allowed impurity ions to occupy active growth sites, effectively inhibiting NaCl nucleation and growth.
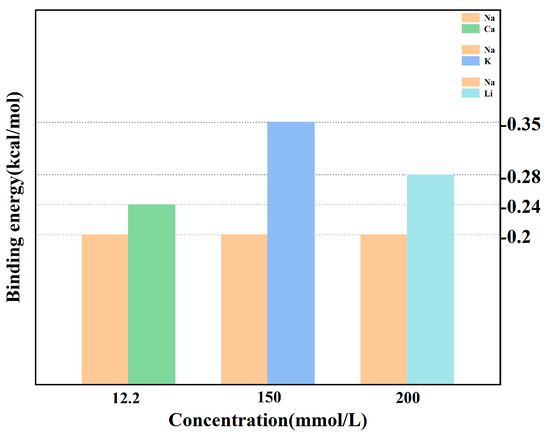
Figure 10.
Calculation results of binding energy.
The microscopic mechanism of NaCl crystal nucleation and growth on the surface of MgSO4·6H2O crystals involves the migration of Na+ ions to occupy crystallization sites on the MgSO4·6H2O crystal surface. Simultaneously, impurity ions compete with Na+ ions for the active crystallization sites, exhibiting a preferential adsorption behavior that inhibits the formation of NaCl crystals. This observation highlights the competitive modification effect of impurities on crystal growth and provides an atomic-level explanation for the phenomenon whereby solutions containing trace impurities are more likely to precipitate high-purity crystals.
4. Conclusions
The magnesium sulfate crystals produced through salt lake cooling crystallization processes are often characterized by high surface roughness and low purity, which significantly limit their applicability in downstream processes. The primary reason for the poor quality of crystals derived from MgSO4-NaCl saturated solutions is the attachment of numerous square-shaped NaCl particles onto the crystal surface during cooling crystallization. However, within a specific range, the presence of impurity ions does not alter the composition of the crystallization product. Instead, it significantly reduces the occurrence of square-shaped NaCl particles on the crystal surface, resulting in smoother and more uniform crystals. For instance, the addition of 300 mmol/L K2SO4 reduced the sodium content in the final product from 0.57% to 0.03% and decreased surface roughness from 2.76 nm to 0.415 nm.
The Raman spectroscopy analysis of the solution structure revealed that trace impurities promoted the binding of Mg2+ and SO42− to form Mg2+-H2O-SO42− complexes, thereby facilitating the nucleation and precipitation of MgSO4·6H2O. Molecular dynamics simulations further elucidated the cooling crystallization process at the atomic level. The simulations demonstrated that Na+ and Cl− ions occupy active growth sites on MgSO4·6H2O crystal surfaces, leading to nucleation and growth that result in rougher surfaces and reduced crystal purity. However, the introduction of impurity ions into the system allows them to compete with Na+ ions for these active growth sites, effectively inhibiting NaCl nucleation and optimizing the crystal morphology.
These findings highlight the critical role of impurity competition in modifying the surface of magnesium sulfate crystals. Remarkably, solutions containing trace impurities are more conducive to the precipitation of high-purity crystals, challenging the conventional understanding of crystallization mechanisms. This work provides a novel perspective on the interplay between impurities and crystal growth, offering theoretical insights and practical strategies for improving the quality of magnesium sulfate products.
Author Contributions
Conceptualization, M.Y. and H.C.; software, H.C.; methodology, M.Y.; validation, M.Y., H.C., and J.Z.; formal analysis, M.Y.; investigation, M.Y. and J.Z.; resources, H.C.; data curation, M.Y.; writing—original draft preparation, M.Y.; writing—review and editing, H.C. and W.C.; visualization, M.Y. and W.C.; supervision, H.C.; project administration, H.C. and W.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science [U20A20149, 22478232]; Qinghai provincial central government local science and technology development funds [2024ZY006]; The Open Project of Salt Lake Chemical Engineering Research Complex, Qinghai University, 2024-DXSSKF-Z02, and the National Natural Science Foundation Youth Project (22208198).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We appreciate Suan Chou (suan-chou.com) for the theoretical study on the formation energy of NaCl on the surface of magnesium sulfate during the cooling process.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pojnar, K.; Pilch-Pitera, B. Correlation between the Chemical Structure of (Meth) Acrylic Monomers and the Properties of Powder Clear Coatings Based on the Polyacrylate Resins. Materials 2024, 17, 1655. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Sawangrat, C.; Kanthiya, T.; Thipchai, P.; Kaewapai, K.; Suhr, J.; Worajittiphon, P.; Tanadchangsaeng, N.; Wattanachai, P.; Jantanasakulwong, K. Effect of Plasma Treatment on Bamboo Fiber-Reinforced Epoxy Composites. Polymers 2024, 16, 938. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shang, J.; Abdurexit, A.; Jamal, R.; Abdiryim, T.; Li, Z.; You, J.; Wei, J.; Su, E.; Huang, L. Effect of Chemical Treatment of Cotton Stalk Fibers on the Mechanical and Thermal Properties of PLA/PP Blended Composites. Polymers 2024, 16, 1641. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Cui, Z.; Yan, Y. Research Status of Surface Modification Technology for Medical Magnesium Alloy. Guangdong Chem. Ind. 2023, 50, 104–106. [Google Scholar]
- Zhao, Y.; Yang, X.; Cheng, Z.; Lau, C.H.; Ma, J.; Shao, L. Surface manipulation for prevention of migratory viscous crude oil fouling in superhydrophilic membranes. Nat. Commun. 2023, 14, 2679. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, H.; Ma, L.; Lu, M.; Wu, C.; Liu, Q.; Yu, X.; Liu, H.; Ye, X.; Ma, Z.; et al. Basic magnesium sulfate@TiO2 composite for efficient adsorption and photocatalytic degradation of 4-dodecylmorpholine in brine. Sci. Rep. 2024, 14, 9315. [Google Scholar] [CrossRef]
- Kékicheff, P.; Heinrich, B.; Hemmerle, A.; Fontaine, P.; Lambour, C.; Beyer, N.; Favier, D.; Egele, A.; Emelyanenko, K.A.; Modin, E.; et al. Condensation or Desublimation: Nanolevel Structural Look on Two Frost Formation Pathways on Surfaces with Different Wettabilities. ACS Nano 2024, 18, 15067–15083. [Google Scholar] [CrossRef]
- Armstrong, T.; Schmid, J.; Niemelä, J.P.; Utke, I.; Schutzius, T.M. Nanostructured Surfaces Enhance Nucleation Rate of Calcium Carbonate. Small 2024, 20, 2402690. [Google Scholar] [CrossRef]
- Hu, Y.J.; Knope, K.E.; Skanthakumar, S.; Kanatzidis, M.G.; Mitchell, J.F.; Soderholm, L. Understanding the role of aqueous solution speciation and its application to the directed syntheses of complex oxidic Zr chlorides and sulfates. J. Am. Chem. Soc. 2013, 135, 14240–14248. [Google Scholar] [CrossRef]
- Kollias, L.; Rousseau, R.; Glezakou, V.A.; Salvalaglio, M. Understanding Metal-Organic Framework Nucleation from a Solution with Evolving Graphs. J. Am. Chem. Soc. 2022, 144, 11099–11109. [Google Scholar] [CrossRef]
- Sui, Y.; Scida, A.M.; Li, B.; Chen, C.; Fu, Y.; Fang, Y.; Greaney, P.A.; Popp, T.M.O.; Jiang, D.; Fang, C.; et al. The Influence of Ions on the Electrochemical Stability of Aqueous Electrolytes. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401555. [Google Scholar] [CrossRef] [PubMed]
- Schiele, S.A.; Haider, T.; Briesen, H. Growth of broken crystals tracked in 4D using X-ray computed tomography and its influence on impurity incorporation. Sci. Rep. 2024, 14, 21999. [Google Scholar] [CrossRef] [PubMed]
- Dobberschütz, S.; Nielsen, M.R.; Sand, K.K.; Civioc, R.; Bovet, N.; Stipp, S.L.S.; Andersson, M.P. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors. Nat. Commun. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, L.; Steendam, R.R.E.; Blijlevens, M.A.R.; Pishnamazi, M.; Frawley, P.J. Influence of impurities on the solubility, nucleation, crystallization, and compressibility of paracetamol. Cryst. Growth Des. 2019, 19, 4193–4201. [Google Scholar] [CrossRef]
- Su, Y.; Li, S.; Li, X.; Zhou, J.Y.; Chauhan, V.P.; Li, M.; Su, Y.H.; Liu, C.M.; Ren, Y.F.; Yin, W.; et al. Tartronic Acid as a Potential Inhibitor of Pathological Calcium Oxalate Crystallization. Adv. Sci. 2024, 11, e2400642. [Google Scholar] [CrossRef]
- Seydioglu, T.; Kurnaz, S.; Tokeşer, E.A.; Yildirim, G.; Ozturk, O. Effect of foreign impurity and growth temperatures on hexagonal structure and fundamental properties of ZnO nanorods. Microsc. Res. Tech. 2024, 87, 2687–2700. [Google Scholar] [CrossRef]
- Jia, H.; Yang, W.; Zhang, X.; Zhou, X.; Qiu, H.; Qin, H.; Lu, S.; Bian, L. Effects and mechanisms of In surfactant on high Al-content AlGaN grown by plasma-assisted molecular beam epitaxy. Opt. Express 2022, 30, 1782–1792. [Google Scholar] [CrossRef]
- Wu, P.; Liu, J.; Li, F.; Ren, X.; Tian, A.; Zhou, W.; Zhang, F.; Li, X.; Zhou, B.; Ikeda, M.; et al. Effects of Miscut on Step Instabilities in Homo-Epitaxially Grown GaN. Nanomaterials 2024, 14, 748. [Google Scholar] [CrossRef]
- Widjaja, T.; Altway, A.; Nurkhamidah, S.; Rahmawati, Y.; Meka, W.; Alifatul, A.; Hartanto, D.; Sari, R. Effectiveness study of recrystallisation method in pharmaceutical salt production from processed salt with zero waste concept. Heliyon 2024, 10, e30472. [Google Scholar] [CrossRef]
- Kırboğa, S.; Mualla, Ö. The role of vinyl sulfonic acid homopolymer in calcium oxalate crystallization. Colloids Surf. B Biointerfaces 2010, 78, 357–362. [Google Scholar] [CrossRef]
- de Vries, S.A.; Goedtkindt, P.; Bennett, S.L.; Huisman, W.J.; Zwanenburg, M.J.; Smilgies, D.-M.; De Yoreo, J.J.; van Enckevort, W.J.P.; Bennema, P.; Vlieg, E. Surface Atomic Structure of KDP Crystals in Aqueous Solution: An Explanation of the Growth Shape. Phys. Rev. Lett. 1998, 80, 2229–2232. [Google Scholar] [CrossRef]
- Glikin, A.E. Polymineral-Metasomatic Crystallogenesis; Springer Nature: Dordrecht, GX, Netherlands, 2009; ISBN 978-1-4020-8982-4. [Google Scholar]
- Buchner, R.; Rudolph, W.W.; Hefter, G.T. Comment on “Dynamic ion association in aqueous solutions of sulfate” [J. Chem. Phys. 123, 034508 (2005)]. J. Chem. Phys. 2006, 124, 247101. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Hamaguchi, H.O. Ion association dynamics in aqueous solutions of sulfate salts as studied by Raman band shape analysis. J. Chem. Phys. 2005, 123, 034508. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, W.W.; Irmer, G.; Hefter, G.T. Raman spectroscopic investigation of speciation in MgSO4(aq). Phys. Chem. Chem. Phys. 2003, 5, 5253–5261. [Google Scholar] [CrossRef]
- Dos Santos, P.F.; Lassin, A.; Gaona, X.; Garbev, K.; Altmaier, M.; Madé, B. Thermodynamics of the Eu(III)-Mg-SO4-H2O and Eu(III)-Na-SO4-H2O systems. Part I: Solubility experiments and the full dissociation Pitzer model. Dalton Trans. 2024, 53, 6289–6299. [Google Scholar] [CrossRef]
- Jia, S.; Gao, Z.; Yao, T.; Wang, J.; Gong, J. Fractal model-based crystal pillar structure simulation and mechanism analysis of impurity migration process in layer melt crystallization. Chem. Eng. Sci. 2023, 275, 118722. [Google Scholar] [CrossRef]
- Kırboğa, S.; Öner, M. Inhibition of calcium oxalate crystallization by graft copolymers. Cryst. Growth Des. 2009, 9, 2159–2167. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, H.B.; Piao, J.N.; Ye, S.R.; Huang, J. Crystallization behavior of poly (trimethylene terephthalate)/multi-walled carbon nanotube composites. J. Mater. Sci. 2008, 43, 417–421. [Google Scholar] [CrossRef]
- Qiao, M.; Li, F.; Wang, M.; Zhu, H.; Zhang, Y.; Yuan, J. Application of molecular dynamics simulation and Raman spectroscopy to study the hydration phenomena of calcium, magnesium and chloride ions. Sci. Bull. 2022, 67, 520–528. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).