Abstract
Micrometeorites (MMs), which are cosmic dust grains ranging from 10 microns to 2 mm in size, can reach the Earth’s surface through collisions with asteroids or by fragmentation of comets in space. When MMs enter the atmosphere, they are heated to varying degrees depending on their size, mass, speed, and angle of entry. As a result of friction during atmospheric entry, MMs undergo partial melting and subsequently recrystallize during undercooling. In this study, we focused on molten micrometeorites and identified four main types: silicate, glassy, ferruginous, and intermediate forms. The mineralogical compositions of MMs were determined using Raman spectroscopy, while their chemical compositions and phase changes were analyzed using SEM-EDX and LA-ICP-MS methods. The primary silicate phases include olivine, pyroxene, and plagioclase, whereas the opaque mineral phases comprise magnetite, troilite, and kamacite (Fe-Ni alloys). Olivine exhibits Fo values ranging from 41 to 96 mol%, and the pyroxenes consist of enstatite and pigeonite compositions (Wo3–8En79–97Fs2–19). Olivine and magnetite display dendritic and skeletal crystal morphologies due to melting and undercooling during atmospheric entry.
1. Introduction
Micrometeorites (MMs) are defined as extraterrestrial dust particles with grain sizes smaller than 2 mm that can pass through atmospheric entry without completely burning up, ultimately reaching the Earth’s surface [1]. MMs typically reach the Earth’s surface as a result of asteroid collisions in space or, occasionally, by breaking off from comets. They are collected from ice and snow, aeolian deposits in Antarctica [2,3,4,5], and deep-sea sediments [6]. The most pristine cosmic dust collections, which are least affected by terrestrial weathering, come from Antarctica. Most MMs are thought to represent primitive asteroid materials, primarily from carbonaceous and ordinary chondritic meteorites [1,7,8,9,10,11,12,13,14].
During atmospheric entry, micrometeorites experience heating, resulting in the loss of their original mineralogical and textural characteristics. Many particles undergo partial melting, forming vesicular “scoriaceous” micrometeorites (MMs), while a significant number melt almost completely, resulting in the formation of cosmic spherules. MMs, ranging in size from 50 microns to 2 mm, form a continuous series between interplanetary dust particles (IDPs) smaller than 50 microns and meteorites larger than 2 mm, representing extraterrestrial materials arriving on Earth [15]. The distinction between MM types reflects variations in their parent body sources and, consequently, in the petrology of the materials depending on size [16]. Micrometeorites can originate from both asteroids and, less commonly, a variety of objects such as comets [8,17,18,19]. This diversity makes MMs a captivating area of research. Additionally, MMs are abundant, relatively easier to find compared to meteorites, and have been studied less extensively than larger meteorites.
Due to the high temperatures experienced by MMs during atmospheric entry, most grains undergo melting and subsequently recrystallize through rapid cooling (undercooling); these grains are referred to as molten MMs. This study focuses on molten MMs. These cosmic grains, which reached Earth as MMs, were examined using various analytical techniques to determine their mineralogical and elemental compositions. The mineralogical compositions of MMs were determined using Raman spectroscopy, while their chemical compositions and phase changes were analyzed using SEM-EDX and LA-ICP-MS methods. The results obtained were compared with the existing literature data, and the micrometeorites were classified accordingly.
2. Materials and Methods
2.1. Materials and Sample Preparation
The MMs used in this study were selected from samples taken from frozen sediments under the snow and ice of the Widero Flejellet Mountain in East Antarctica, as part of the TUBITAK 1001-POLAR project (119N207). Sediment samples were initially washed with pure water through a 63-micrometer sieve to eliminate fine clay and mud particles. Following this process, the sediments were dried in an oven at 60 °C for 24 h to remove moisture. The dried sediments were sieved with a vibrating device using a 2 mm–63 µm sieve set for size separation. The size-fractionated sediments were placed in different Petri dishes. Each sediment size fraction was examined under a binocular microscope. Candidate particles were selected using a Leica S8APO stereomicroscope at the ESAN Central Laboratory (İstanbul, Turkey). The identified MMs were carefully extracted with tweezers and placed on heat-resistant double-sided tape attached to glass. Seventy molten micrometeorite grains were selected from the sediments (Figure 1). The selected cosmic grains were embedded in epoxy resin for mounting. The mounts were polished using a Retch polishing device with felt pads and diamond solutions to achieve a smooth surface. Metallic phases in the MMs were identified using reflection microscopy, completing the preparation for analysis.
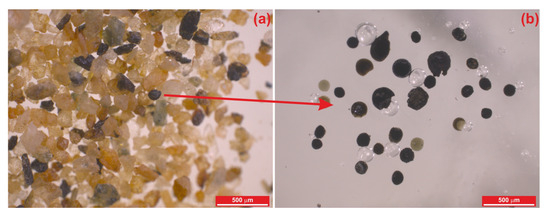
Figure 1.
Binocular microscope images: (a) appearance of MMs in sediment, and (b) embedded in epoxy resin.
2.2. Methods
2.2.1. Scanning Electron Microscopy–Energy-Dispersive X-Ray (SEM-EDX)
BSE imaging, SEM-EDX analyses, and elemental mapping of the MM samples were performed using a Hitachi SU4000 SEM (Tokyo, Japan) equipped with an Oxford XACT EDX spectrometer (Oxford, UK) at the TETRA Technological Systems laboratories (İstanbul, Turkey). The acceleration voltage was set to 15 kV, while the beam current was adjusted based on grain size. SRM NIST 612 glass was used as the control standard to ensure analytical precision during EDS analyses. The measurement uncertainty for major oxides, checked every ten points, remained below 0.1 wt.%.
2.2.2. Raman Spectroscopy
Raman spectroscopy data were acquired at the Şişecam Science and Technology Center (İstanbul, Türkiye) using a WiTec Alpha300R confocal Raman imaging micro spectroscopy system. The system is equipped with a 600 g/mm grating, a 532 nm Nd:YAG laser, and a 50× objective (NA = 0.8). The spectrograph was calibrated with a silicon wafer substrate before measurements. Individual Raman spectra were collected over the 0–4000 cm−1 range, with 60 accumulations and a 1 s integration time. The laser power was approximately 1–3 mW on the sample surface throughout data acquisition. Intensity distribution maps of individual chemical components were generated using the WITec Project software (v. 2.10) by integrating signals between the spectral endpoints of Raman peaks. Reference spectra of mineral components were obtained from the RRUFF database [20].
2.2.3. LA-ICP-MS Analysis
Bulk-rock geochemical analyses of MMs were conducted at the Geochronology and Geochemistry Laboratory of İstanbul University-Cerrahpasa (IUC-GGL), Türkiye. Bulk-rock major element analyses were performed using a Perkin Elmer NexION 2000 mass spectrometer (Waltham, MA, USA) coupled with an ESI NWR-213 solid-state laser ablation system at IUC-GGL. A laser spot size of 40 microns was used for mineral chemistry analyses. For each spot analysis, background acquisition lasted 20 s, followed by 30 s of ablation and 30 s of washout time. The laser repetition rate was set to 5 Hz, with an energy density of 5 J/cm2. Helium was used as the carrier gas at a flow rate of 0.6 L/s. The instrument was calibrated before mineral chemistry analyses using the ThO/Th ratio, which remained below 0.05%. The quadrupole ion deflector (QID) settings were optimized to maximize signals for light, medium, and heavy masses. The ICP-MS system features two detectors (analog and pulse) with a wide dynamic range (up to 109), enabling the measurement of both major and trace elements. LA-ICP-MS accuracy was validated by independently measuring the 43Ca and 29Si contents of the BCR-2g glass standard using SEM-EDX. The error margin for major oxides in repeatable measurements was <1 wt.%. The NIST 612 standard was used for drift correction and calibration. Additionally, BCR-2g and AGV-2g were used as calibration reference standards. Data reduction was carried out using the ICPMSDATACAL software package (V9.5), following the methodology of [21].
3. Results
Detailed mineralogical and petrographic descriptions, along with the classification of the studied MMs, are presented below. The classification is based on the textures and mineral compositions of the molten MMs. These MMs are categorized into four groups: silicate, glassy, ferrous, and intermediate forms.
3.1. Barred Olivine (BO) Spheres
The dark-colored barred olivine sphere is a molten MM approximately 300 µm in size, exhibiting irregular edges and melting cavities on its surface. The barred olivine chondrule structure is clearly visible in BSE images (Figure 2a). It has a texture characterized by parallel and partially crystallized olivine chains, along with glassy phases aligned parallel to the crystals. Some olivine chains are long and parallel to each other, while others are arranged perpendicularly or at 45-degree angles. Additionally, opaque minerals, including magnetite, chromite, troilite, and kamacite, appear as bright reflective phases in BSE images. Raman analyses indicate that the olivine phase is predominantly forsteritic, while opaque minerals are represented by magnetite (Figure 2b). Elemental mapping using SEM-EDX shows that the MM is primarily composed of Si, Fe, Mg, and O (Figure 2c). The EDX analysis of the entire MM reveals the following composition: 29.67 wt.% SiO2, 1.47 wt.% Al2O3, 42.05 wt.% FeO, 22.33 wt.% MgO, 0.81 wt.% CaO, 2.28 wt.% NiO, and 0.81 wt.% Cr2O3, with minor amounts of MnO. These values indicate that the micrometeorite consists mainly of magnetite and forsteritic olivine. The relatively high NiO and Cr2O3 contents suggest the presence of iron–nickel alloys such as kamacite, as well as troilite and minor amounts of chromite minerals as inclusions. Additionally, the small amounts of Al2O3 and CaO suggest that the glassy (maskelyenite) phase may correspond to plagioclase.
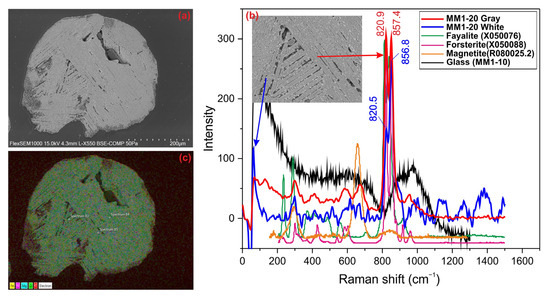
Figure 2.
(a) BSE image, (b) Raman spectrum, and (c) EDX mapping of barred olivine sphere.
3.2. Porphyritic Olivine (PO) Spheres
The porphyritic olivine spheres are dark-colored and have a metallic luster. They are approximately 150 µm in size and exhibit a structure with numerous cracks and melting cavities on its surface (Figure 3a). The main silicate phases of porphyritic olivine spheres consist of olivine, pyroxene, and plagioclase, while the opaque mineral phase is represented by magnetite, troilite, and kamacite. BSE images and Raman spectra reveal that the gray crystals are forsterite, while the bright white crystals are magnetite (Figure 3b). The micrometeorite displays a typical porphyritic chondrule structure, with dendritic and skeletal olivine minerals, as well as skeletal magnetite crystals, identified in the BSE images. Elemental distribution maps obtained via SEM-EDX show that Mg, Fe, and Si elements are concentrated in the large olivine crystals (Figure 3c).
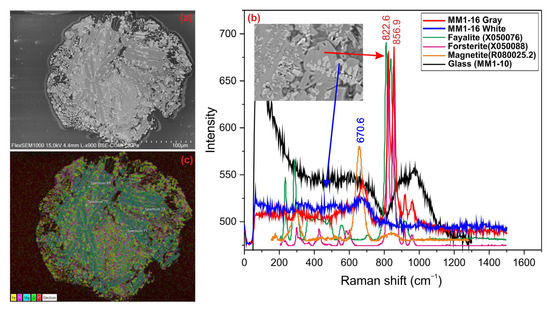
Figure 3.
(a) BSE image, (b) Raman spectrum, and (c) EDX mapping of porphyritic olivine sphere.
3.3. Cryptocrystalline Olivine (CC) Spheres
These are dark-colored, irregularly shaped MM grains, approximately 150 µm in size, with irregular edges. They were identified as cryptocrystalline olivine spheres in BSE images (Figure 4a) and were determined to consist of dendritic olivine minerals of varying sizes and opaque minerals (magnetite, chromite, troilite, etc.) in the bright regions of the image. Raman analysis revealed that the gray areas correspond to forsteritic olivine, while the bright areas represent magnetite minerals (Figure 4b). In cryptocrystalline spheres, the elemental distributions are almost homogeneous for all elements. As in other micrometeorites, Fe, Mg, and Si are the predominant elements, primarily representing minerals such as olivine and magnetite (Figure 4c).
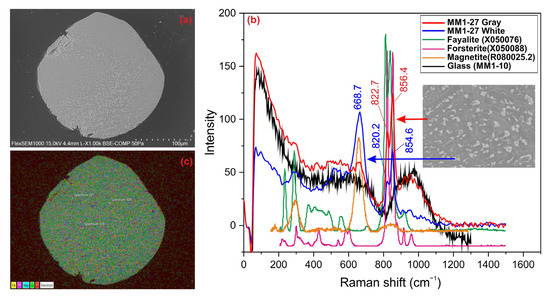
Figure 4.
(a) BSE image, (b) Raman spectrum, and (c) EDX mapping of cryptocrystalline olivine sphere.
3.4. Glassy (Vitreous: V) Spheres
Glassy spheres are transparent, yellowish in color, and completely spherical; some glassy spheres contain large gas cavities. These are silica (S type), vitreous (V), molten MMs. In BSE images, these spheres appear completely glassy and amorphous. Magnetite minerals that produced bright white reflections were detected, albeit rarely (Figure 5a). No clear peaks were obtained in the Raman spectrum (Figure 5b). Since the sample had an amorphous structure like a glassy phase, the peaks were not well-defined. However, it is believed that the peak at 974.7 cm−1 may have corresponded to the olivine mineral (RRUFF-ID: R040018). The possible peak belonging to the fayalite endmember of olivine may have shifted from its characteristic position due to the processes the MMs underwent. These processes vitrified the grain and resulted in a noisy spectrum with an amorphous-phase appearance. Glassy spheres exhibited a homogeneous elemental distribution due to their amorphous structure. However, in these spheres, the Al element was commonly found in addition to the Mg and Si elements (Figure 5c).
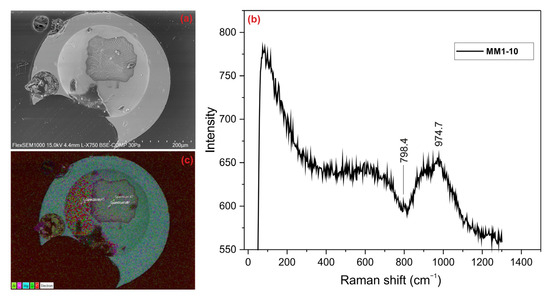
Figure 5.
(a) BSE image, (b) Raman spectrum, and (c) EDX mapping of glassy sphere.
3.5. Mineral Chemistry
To determine the chemical composition of the mineral phases in the MMs, measurements were performed using both EDX and LA-ICP-MS techniques. The results of these measurements revealed the presence of olivine, pyroxene, magnetite minerals, and glassy phases. The data are presented in Supplementary Table S1. Analyses of olivine minerals generally indicated compositions between forsterite and fayalite, with some approaching the forsteritic end member. Olivine minerals in the MMs exhibited Fo values ranging from 41 to 96 mol%. When applying the Di-Hd-En-Fs classification of Morimoto et al. [22], all of the studied pyroxenes from the MMs (Wo3–8En79–97Fs2–19) fell into the enstatite and pigeonite fields (Figure 6). Considering the dominant mineral ratios in the defined regions, rare earth element (REE) contents were normalized to chondrite. The chondrite-normalized element patterns closely resembled the chondrite composition in the olivine- and pyroxene-rich areas. The glassy phase exhibited a pattern that was enriched relative to chondrite, with a distinct negative Eu anomaly. Magnetite-rich areas were significantly depleted in rare earth elements (Figure 7).
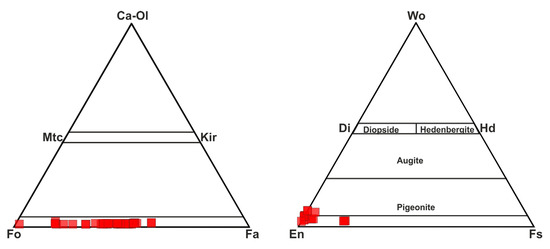
Figure 6.
Classification of olivine and pyroxene minerals. Abbreviations: Fo: forsterite; Fa: fayalite; En: enstatite, Fs: ferrosilite, Mtc: monticellite, Kir: kirschsteinite, Ol: olivine, Di: diopside, Hd: hedenbergite, Wo: wollastonite.
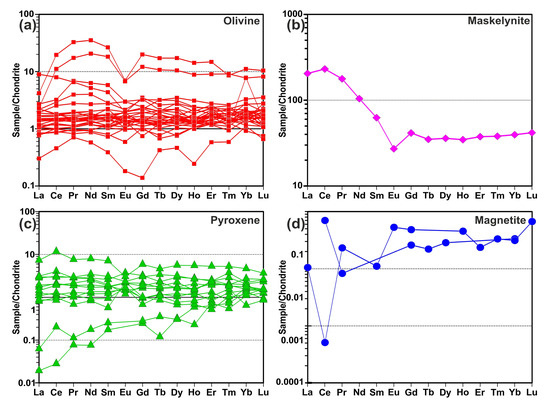
Figure 7.
Chondrite [23]-normalized REE patterns: (a) olivine, (b) maskelyenite, (c) pyroxene and (d) magnetite mineral phases of the MMs.
4. Discussion
4.1. Description and Origin of MMs
MM samples, processed through multiple stages, were analyzed using various spectroscopic methods to characterize their internal structures. First, the MMs were classified based on BSE images. It was determined that the selected MMs belonged to four types: barred olivine spheres, porphyritic olivine spheres, cryptocrystalline spheres, and glassy spheres. The barred olivine spheres and porphyritic olivine spheres are primarily similar to the chondrule types found in chondritic meteorites and likely correspond to dust particles formed by collisions and/or fragmentation of chondritic meteorites in space. According to the mineral chemistry and Raman analyses of both barred and porphyritic spheres, the olivines are primarily forsteritic, just as in chondritic meteorites. Similarly, both mineral chemistry and Raman analyses clearly show that the olivines in the cryptocrystalline spheres are also forsteritic. In the case of glassy spheres, olivine and a similar mineral phase have been identified, along with a completely glassy mesostasis. Raman spectra of solid solutions of olivine group minerals vary depending on their chemical composition and pressure–temperature conditions [24]. Utilizing Raman spectroscopy, investigations into synthetic end members Mg2SiO4, Fe2SiO4, Mn2SiO4, and Co2SiO4, as well as natural samples from the forsterite–fayalite and tephroite–montiselite series, revealed that the Mg2SiO4-Ca(Mg, Fe)SiO4 solid solution series—with non-transition elements occupying the M2 site—displayed distinct peaks within the 847 cm−1 to 857 cm−1 and 815 cm−1 to 825 cm−1 ranges, respectively [24]. However, the study found that fayalite, tephroite, and other olivine group minerals containing transition elements in the M2 site exhibited peak values in the range of 836–839 cm−1, while also demonstrating values between 808 and 819 cm−1. The Raman shift values in these two peak regions in MMs were determined as 820–823 and 855–857 cm−1, and these values were found to be compatible with forsterite in the RRUFF database. Moreover, it was observed that the Raman spectra obtained from different MM types contained forsterite, magnetite, and partially glassy phases (Figure 2, Figure 3 and Figure 4). It has been determined that forsteritic olivines possess low-Ti contents but relatively high-Ni contents (see Supplementary Table S1). In the experimental studies conducted by Bloise et al. [25], forsterite doped with Ti and Ni single crystals was synthesized through the flux growth technique within the temperature range of 750–1350 °C, utilizing lithium–vanadium–molybdate as a fluxing agent. Their experimental findings demonstrated that the optimal conditions for producing higher quantities of Ti-forsterite and Ni-forsterite involved the slow cooling of an appropriate starting mixture from 1350 °C to 750 °C at a cooling rate of 1.8 K/h [25]. Bloise et al. [25] concluded that variations in crystal size and morphology were solely due to the differing experimental conditions under which Ti-forsterite was synthesized, with the amount of Ti substitution for magnesium (Mg) in the structure being deemed insignificant. In contrast, changes in the crystal size of Ni-forsterite were attributed to the extent of nickel substitution for magnesium. Additionally, Ni-forsterite crystals were consistently idiomorphic and larger than the hypidiomorphic Ti-forsterite crystals, indicating non-equilibrium growth conditions for the latter [24].
Chemical analyses of olivine-bearing MMs performed using LA-ICP-MS revealed that olivine-rich MMs present a pattern closely aligned with a line in the chondrite-normalized rare earth element (REE) spider diagrams. This pattern closely resembles that of other chondritic meteorites found in the asteroid belt and suggests that the origin of these cosmic dusts may be similar to that of chondritic meteorites. As a result, it is shown that the MMs collected from Antarctica and used in this study are largely similar to other chondritic meteorites found in the asteroid belt, suggesting they may have originated from a comparable source.
4.2. Crystal Morphology of MMs
Crystal morphology is influenced by various physicochemical factors such as temperature, pressure, cooling rate, nucleation, diffusion, volatile composition, and viscosity [26]. The development of distinct crystal morphologies is observed in both natural and artificial materials (e.g., glassy volcanic rocks, micrometeorites, archaeometallurgical slags, ceramics, and polymers) as a result of the cooling rate [26]. The morphology of crystals is governed by the degree of undercooling (ΔT), and euhedral crystals, which reflect their crystallographic internal structure, form under specific physicochemical conditions [26]. MMs undergo partial and/or complete melting due to their entry speed and trajectory during atmospheric entry. This melting and subsequent solidification process occur rapidly and are characterized by textures indicative of undercooling, as clearly seen in the barred olivine spheres (Figure 8a–c). A large olivine crystal begins to form; however, due to the rapid cooling, skeletal olivine chains form and link together, leaving glassy mesostasis between them. Occasionally, the barred olivine spheres also contain different forms of olivine chains that intersect at various angles (Figure 2a).
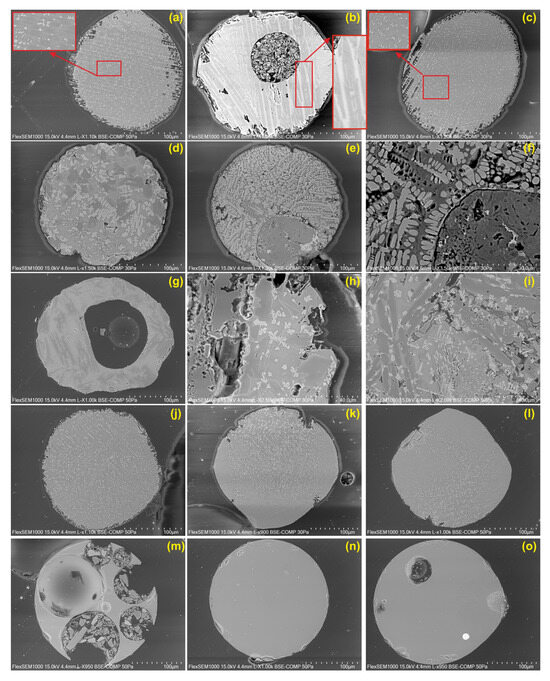
Figure 8.
BSE images of the various type of MMs: (a–c) skeletal and dendritic olivine and magnetites in the barred olivine spheres; (d–i) skeletal and dendritic olivine and magnetites in the porphyritic olivine spheres; (j–l) skeletal and dendritic magnetites in the cryptocrystalline spheres; (m–o) glassy spheres.
Experimental petrology studies show that olivine chains consist of longitudinal skeletons of interconnected subunits [27]. Donaldson [27] suggested that these subunits grow either in random or parallel orientations. In sections parallel to (001) or (100), the subunits exhibit H-shaped (hourglass or hopper crystal) features, with some displaying “tongues” connecting the H-bar to adjacent units. Growth occurs by nucleation of a new unit on top of the previous one [27]. In porphyritic olivine spheres, larger crystalline olivines are visible, clearly reflecting textures of undercooling. Skeletal and dendritic olivine crystals are clearly distinguishable in BSE images (Figure 8d–i). While euhedral crystals are observed in a temperature-controlled cooling system, a crystal formation sequence is observed that transforms into diffusion-controlled skeletal > baby swallowtail > swallowtail > dendrite undercooling conditions where temperature control is disabled [26].
The formation of dendrites into crystals requires very specific thermodynamic conditions [28]. Experimental studies by Faure et al. [29,30] have shown that olivine develops a dendritic habit with rapid growth at a strong undercooling (−ΔT > 60 °C, i.e., when the temperature drops below the liquidus) and for any cooling rate ≥ 47 °C/h in the magma (Figure 9). Welsh et al. [28] suggested that olivine crystals develop a skeletal habitus at moderate undercooling (−ΔT < 20–60 °C) and a polyhedral habit at low undercooling (−ΔT < 20 °C). They also emphasized that when the undercool degree is much higher than 60 °C, the morphology of olivine rapidly changes from a hollow (swallowtail habit: grasshopper with few parallel copies) to a true dendrite (swallowtail habit: grasshopper with many parallel, reduced-sized copies). As a result, MMs rapidly became molten during atmospheric intrusion and formed skeletal and dendritic crystals under the extreme cooling. Similar processes are observed in basalts that erupt on the Moon and on Earth [27,31,32].
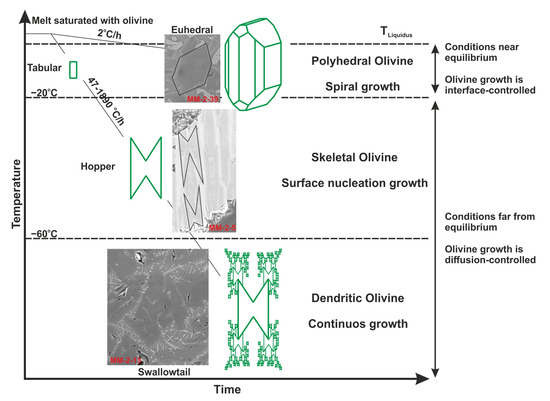
Figure 9.
Different olivine crystal morphologies developed as a function of the cooling rate and nominal degree of undercooling in the CaO-MgO-Al2O3-SiO2 system (modified from [28]).
Skeletal and dendritic crystal structures are not exclusive to olivine; similar structures are also observed in magnetite minerals. Magnetite, a common opaque accessory mineral in both terrestrial and extraterrestrial rocks, forms dendritic crystals by propagating growth layers from two-dimensional nucleation points (Volmer method) or from emerging screw dislocations (Frank method) during successive growth stages (Figure 10) [33]. Dendritic magnetite is also found in MMs that experience rapid heating and quenching during atmospheric entry [33]. Isobe and Gondo [34] proposed that the habit, crystal size, and texture of magnetite are primarily influenced by the thermal history of crystallization and the chemical composition of the crystallization system. They also emphasized that euhedral magnetite crystals, commonly found as high-temperature liquidus minerals, are present in the quenched glass fraction of silicate melt phases; the characteristic dendritic and skeletal magnetite crystals reflect relatively rapid cooling rates during nucleation and crystal growth steps in the crystallization process.
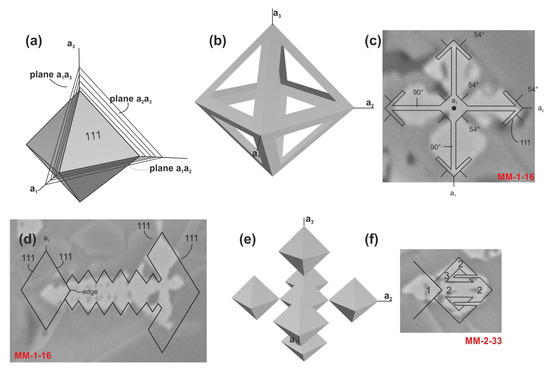
Figure 10.
The order of formation of magnetite dendrites: (a) Formation of primary sheets shown as three incompletely drawn planes. (b) Model of magnetite dendrite. (c) Horizontal section of magnetite dendrite. (d) Section of magnetite dendrite in which all {111} planes are vertical. (e) Idealized cluster of magnetite octahedral crystals. (f) Schematic growth of a satellite of the cluster to produce basement mass coves (modified from [33]).
Dendritic magnetite crystals have also been observed at the magmatic margin of MMs derived from fine interplanetary particles falling to Earth [1,35]. Isobe and Gondo [34] suggested that the nucleation density, final crystal size, and habit at the end of the cooling process may provide crucial insights into the thermal history of various cases. For example, in the chondrule formation process, crystallographic restrictions on crystal growth are common in barred olivine and radial pyroxene chondrule. As demonstrated in this study, the barred olivine texture is also prevalent in molten MM globules [9]. Isobe and Gondo [34] extended their cooling rate experiments up to 2 × 106 °K/h in their fine particle drop apparatus to simulate the thermal history of atmospheric heating in micrometeorites. All these textural features suggest that MMs underwent undercooling. The situation in cryptocrystalline and glassy spheres differs somewhat from the other two types. In cryptocrystalline and glassy spheres, the grain is nearly completely molten and undercooled within a short period. In cryptocrystalline spheres, minerals can be partially identified in Raman and BSE images. In glassy spheres, an almost isotropic glass formation occurs without any crystal development. This phenomenon is similar to the mesostasis phases (such as the maskelyenite phase in plagioclases) found in meteorites affected by high degrees of shock metamorphism.
Elemental mappings in barred and porphyritic olivine spheres show that the distribution of elements is controlled by the minerals, with Fe, Mg, and Si elements enriched in olivine-bearing areas. However, in cryptocrystalline and glassy spheres, the elements are almost uniformly distributed. This pattern is expected due to the melting and undercooling processes.
5. Conclusions
MMs selected from the frozen sediments of Antarctica were thoroughly studied using various spectroscopic methods, primarily mineralogical and petrographic analyses. The detailed results contribute significantly to the literature. SEM imaging revealed undercooling textures, such as olivine chains and dendritic olivine structures. The spherical and semi-spherical shapes of the MMs, along with these undercooling textures, confirm that the studied MMs are molten. The examined MMs were classified into four distinct subclasses: barred olivine (BO), porphyritic olivine (PO), cryptocrystalline (CC), and glassy (V) MMs. Raman analysis identified the mineral composition, showing that the MMs mainly consist of olivines, metallic minerals (magnetite, troillite, etc.), pyroxenes, and glassy phases. EDX and LA-ICP-MS analyses provided detailed major oxide, trace element, and rare earth element compositions. Olivines were predominantly found to be forsteritic, and pyroxenes were mainly enstatite. This study highlights the need for further detailed analysis of MM samples, particularly in terms of mineral chemistry. The small size of the grains and the complexity of the textures present challenges to this area of study.
Supplementary Materials
The following supporting information can be downloaded at: https://zenodo.org/records/14806385, 10.5281/zenodo.14720767 (accessed on 20 December 2024). Supplementary Table S1: Geochemical analysis for the various phases in the MMs.
Author Contributions
Conceptualization, T.S. and N.A.; methodology, all authors; data curation, T.S. and N.A.; writing—original draft preparation, T.S. and N.A.; writing—review and editing, all authors; visualization, T.S. and N.A.; project administration, N.A.; funding acquisition N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research Projects Coordination Unit of İstanbul University-Cerrahpasa (project no: FYL-2023-37201), and the Scientific and Technological Research Council of Türkiye (project no: 119N207).
Data Availability Statement
The data supporting the findings of this study were taken from Taki Sönmez’s master’s thesis.
Acknowledgments
Many thanks go to Mehmet Yeşiltaş, who provided us with the opportunity to obtain Antarctic micrometeorites within the scope of the TÜBİTAK project and supported the thesis both intellectually and financially. In addition, the authors would like to thank Fatma Şişman Tükel and Fulya Uzun for their support and contributions during the implementation and evaluation of laboratory analyses, and astrogeologist Ersin Kaygisiz for the evaluation of our spectroscopic analyses. The authors’ final thanks go to Lida Taherzadeh for the English proofreading. This article is derived from Taki Sönmez’s master’s thesis.
Conflicts of Interest
Author Taki Sönmez was employed by the company Esan Eczacıbaşı Industrial Raw Materials Industry and Trade Inc. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Genge, M.J.; Engrand, C.; Gounelle, M.; Taylor, S. The classification of micrometeorites. Meteorit. Planet. Sci. 2008, 43, 497–515. [Google Scholar] [CrossRef]
- Maurette, M.; Olinger, C.; Michel-Levy, M.C.; Kurat, G.; Pourchet, M.; Brandstatter, F.; Bourot-Denise, M.A. Collection of diverse micrometeorites recovered from 100 tonnes of Antarctic blue ice. Nature 1991, 351, 44–47. [Google Scholar] [CrossRef]
- Taylor, S.; Lever, J.H.; Harvey, R.P. Numbers, types, and compositions of an unbiased collection of cosmic spherules. Meteorit. Planet. Sci. 2000, 55, 651–666. [Google Scholar] [CrossRef]
- Duprat, J.; Engrand, C.; Maurette, M.; Kurat, G.; Gounelle, M.; Hammer, C. Micrometeorites from Central Antarctic snow: The Concordia collection. Adv. Space Res. 2007, 39, 605–611. [Google Scholar] [CrossRef]
- Rochette, P.; Folco, L.; Suavet, C.; van Ginneken, M.; Gattacceca, J.; Perchiazzi, N.; Braucher, R.; Harvey, R.P. Micrometeorites from the Transantarctic Mountains. Proc. Natl. Acad. Sci. USA 2008, 105, 8206–18211. [Google Scholar] [CrossRef]
- Brownlee, D.E. Cosmic dust: Collection and research. Annu. Rev. Earth Planet. Sci. 1985, 13, 147–173. [Google Scholar] [CrossRef]
- Kurat, G.; Koeberl, C.; Presper, T.; Brandstätter, F.; Maurette, M. Petrology and geochemistry of Antarctic micrometeorites. Geochim. Cosmochim. Acta 1994, 58, 3879–3904. [Google Scholar] [CrossRef]
- Genge, M.J.; Grady, M.M.; Hutchison, R. The textures and compositions of fine-grained Antarctic micrometeorites—Implications for comparisons with meteorites. Geochim. Cosmochim. Acta 1997, 61, 5149–5162. [Google Scholar] [CrossRef]
- Cordier, C.; Folco, L.; Taylor, S. Vestoid cosmic spherules from the South Pole Water Well and Transantarctic Mountains (Antarctica): A major and trace element study. Geochim. Cosmochim. Acta 2011, 75, 1199–1215. [Google Scholar] [CrossRef]
- Goderis, S.; Soens, B.; Huber, M.S.; McKibbin, S.; van Ginneken, M.; Van Maldeghem, F.; Debaille, V.; Greenwood, R.C.; Franchi, I.A.; Cnudde, V.; et al. Cosmic spherules from Widerøefjellet, Sør Rondane Mountains (East Antarctica). Geochim. Cosmochim. Acta 2020, 270, 112–143. [Google Scholar] [CrossRef]
- Noguchi, T.; Matsumoto, R.; Yabuta, H.; Kobayashi, H.; Miyake, A.; Naraoka, H.; Okazaki, R.; Imae, N.; Yamaguchi, A.; Kilcoyne, A.L.D.; et al. Antarctic micrometeorite composed of CP and CS IDP-like material: A micro-breccia originated from a partially ice-melted comet-like small body. Meteorit. Planet. Sci. 2022, 57, 2042–2062. [Google Scholar] [CrossRef]
- Rojas, J.; Duprat, J.; Engrand, C.; Dartois, E.; Delauche, L.; Godard, M.; Gounelle, M.; Carrillo-Sánchez, J.D.; Pokorný, P.; Plane, J.M.C. The micrometeorite flux at Dome C (Antarctica), monitoring the accretion of extraterrestrial dust on Earth. Earth Planet. Sci. Lett. 2021, 560, 116794. [Google Scholar] [CrossRef]
- Fernandes, D.; Rudraswami, N.G.; Pandey, M.; Singh, V.P. Chemical compositions of Fe-rich relict olivines from cosmic spherules, understanding their links with ordinary and carbonaceous chondrites. Meteorit. Planet. Sci. 2024, 59, 605–625. [Google Scholar] [CrossRef]
- Rudraswami, N.G.; Fernandes, D.; Pandey, M. Probing the nature of extraterrestrial dust reaching the Earth’s surface collected from the Maitri station, Antarctica. Meteorit. Planet. Sci. 2020, 55, 2256–2266. [Google Scholar] [CrossRef]
- Zolensky, M.; Bland, P.; Brown, P.; Halliday, I. Flux of extra-terrestrial materials. Meteor. Early Sol. Syst. 2006, 943, 869–888. [Google Scholar]
- Zolensky, M.E.; Zega, T.J.; Yano, H.; Wirick, S.; Westphal, A.J.; Weisberg, M.K.; Weber, I.; Warren, J.L.; Velbel, M.A.; Tsuchiyama, A.; et al. Mineralogy and petrology of comet 81P/Wild 2 nucleus samples. Science 2006, 314, 1735–1739. [Google Scholar] [CrossRef]
- Nesvorny, D.; Bottke, W.F.; Levison, H.F.; Dones, L. Recent origin of the solar system dust bands. Astrophys. J. 2003, 591, 486–497. [Google Scholar] [CrossRef]
- Nesvorny, D.; Vokrouhlicky, D.; Bottke, W.F.; Sykes, M. Physical properties of asteroid dust bands and their sources. Icarus 2006, 181, 107–144. [Google Scholar] [CrossRef]
- Noguchi, T.; Ohashi, N.; Tsujimoto, S.; Mitsunari, T.; Bradley, J.P.; Nakamura, T.; Toh, S.; Stephan, T.; Iwata, N.; Imae, N. Cometary dust in Antarctic ice and snow: Past and present chondritic porous micrometeorites preserved on the Earth’s surface. Earth Planet. Sci. Lett. 2015, 410, 1–11. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Güther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LAICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifert, F.A.; Zussman, J. Nomenclature of pyroxenes. Mineral. Mag. 1988, 52, 535–550. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Mouri, T.; Enami, M. Raman spectroscopic study of olivine-group minerals. J. Mineral. Petrol. Sci. 2008, 103, 100–104. [Google Scholar] [CrossRef]
- Bloise, A.; Barrese, E.; Apollaro, C.; Miriello, D. Flux growth and characterization of Ti- and Ni-doped forsterite single crystals. Cryst. Res. Technol. 2009; 44, 463–468. [Google Scholar]
- Aysal, N.; Kurt, Y.; Öztürk, H.; Ildiz, G.O.; Yesiltas, M.; Laçin, D.; Öngen, S.; Nikitin, T.; Fausto, R. Crystallization Kinetics: Relationship between Crystal Morphology and the Cooling Rate—Applications for Different Geological Materials. Crystals 2023, 13, 1130. [Google Scholar] [CrossRef]
- Donaldson, C.H. An experimental investigation of olivine morphology. Contrib. Miner. Petrol. 1976, 57, 187–213. [Google Scholar] [CrossRef]
- Welsch, B.; Faure, F.; Famin, V.; Baronnet, A.; Bachèlery, P. Dendritic crystallization: A single process for all the textures of olivine in basalts? J. Petrol. 2013, 54, 539–574. [Google Scholar] [CrossRef]
- Faure, F.; Trolliard, G.; Nicollet, C.; Montel, J.M. A developmental model of olivine morphology as a function of the cooling rate and the degree of undercooling. Contrib. Miner. Petrol. 2003, 145, 251–263. [Google Scholar] [CrossRef]
- Faure, F.; Schiano, P.; Trolliard, G.; Nicollet, C.; Soulestin, B. Textural evolution of polyhedral olivine experiencing rapid cooling rates. Contrib. Miner. Petrol. 2007, 153, 405–416. [Google Scholar] [CrossRef]
- Scarani, A.; Zandonà, A.; Di Fiore, F.; Valdivia, P.; Putra, R.; Miyajima, N.; Bornhöft, H.; Vona, A.; Deubener, J.; Romano, C.; et al. A chemical threshold controls nanocrystallization anddegassing behaviour in basalt magmas. Commun. Earth Environ. 2022, 3, 284. [Google Scholar] [CrossRef]
- Arzilli, F.; Polacci, M.; La Spina, G.; Le Gall, N.; Llewellin, E.; Brooker, R.A.; Torres-Orozco, R.; Di Genova, D.; Neave, D.A.; Hartley, M.; et al. Dendritic crystallization in hydrous basaltic magmas controls magma mobility within the Earth’s crust. Nat. Commun. 2022, 13, 3354. [Google Scholar] [CrossRef]
- Kretz, R. Dendritic magnetite and ilmenite in 590 Ma Grenville dikes near Otter Lake, Quebec, Canada. Can. Mineral. 2003, 41, 1049–1059. [Google Scholar] [CrossRef]
- Isobe, H.; Gondo, T. Dendritic magnetite crystals in rapid quenched fine spherules produced by falling experiments through the high temperature furnace with controlled gas flow. J. Mineral. Petrol. Sci. 2013, 108, 227–237. [Google Scholar] [CrossRef]
- Genge, M.J. Igneous rims on micrometeorites. Geochim. Cosmochim. Acta 2006, 70, 2603–2621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).