Design, Preparation and Synergistic Optimization of Mechanical Properties and Thermal Neutron Shielding Performance of Mg-Dy-Sm-Zr Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization
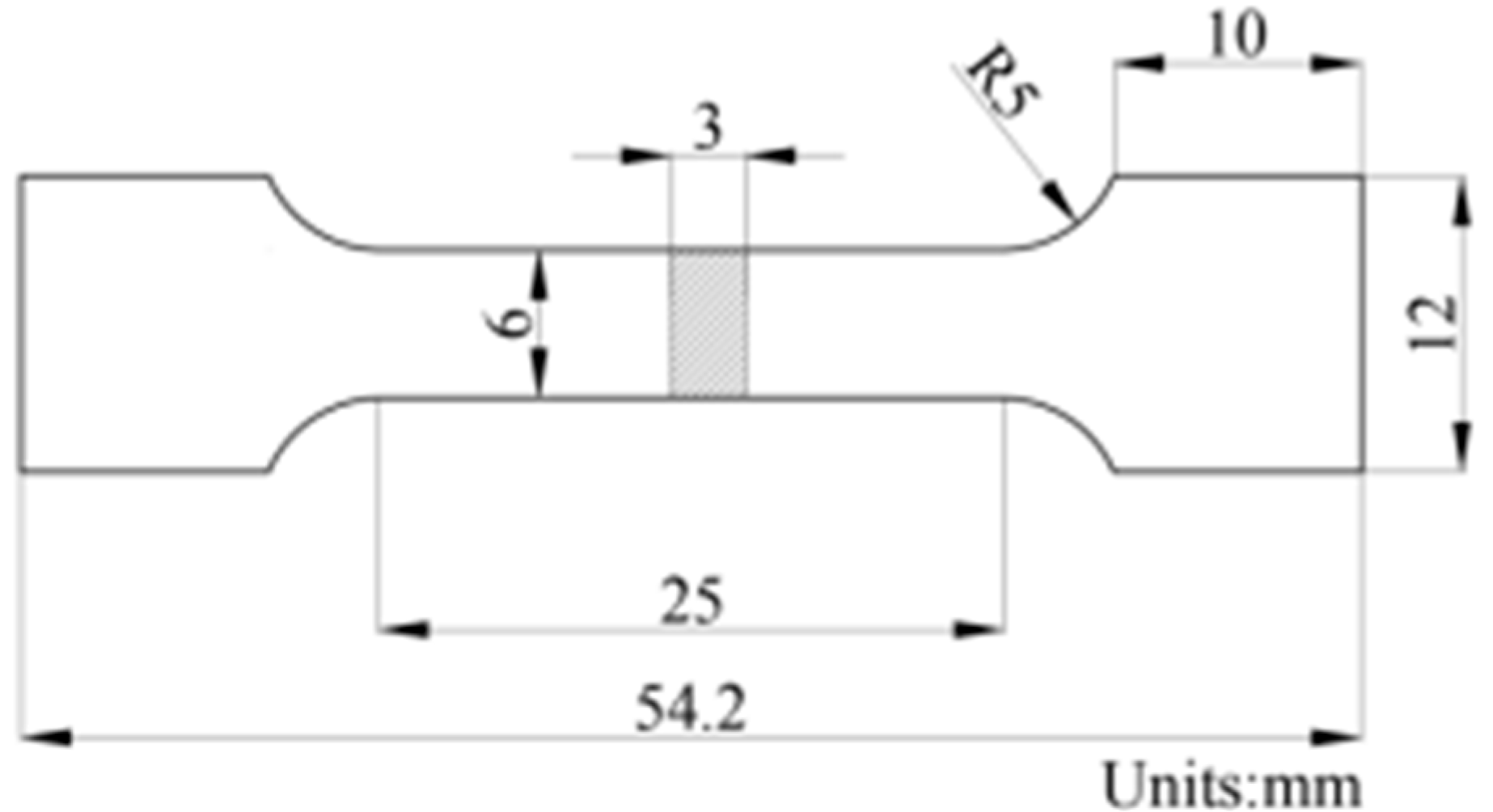
2.3. Mechanical Properties
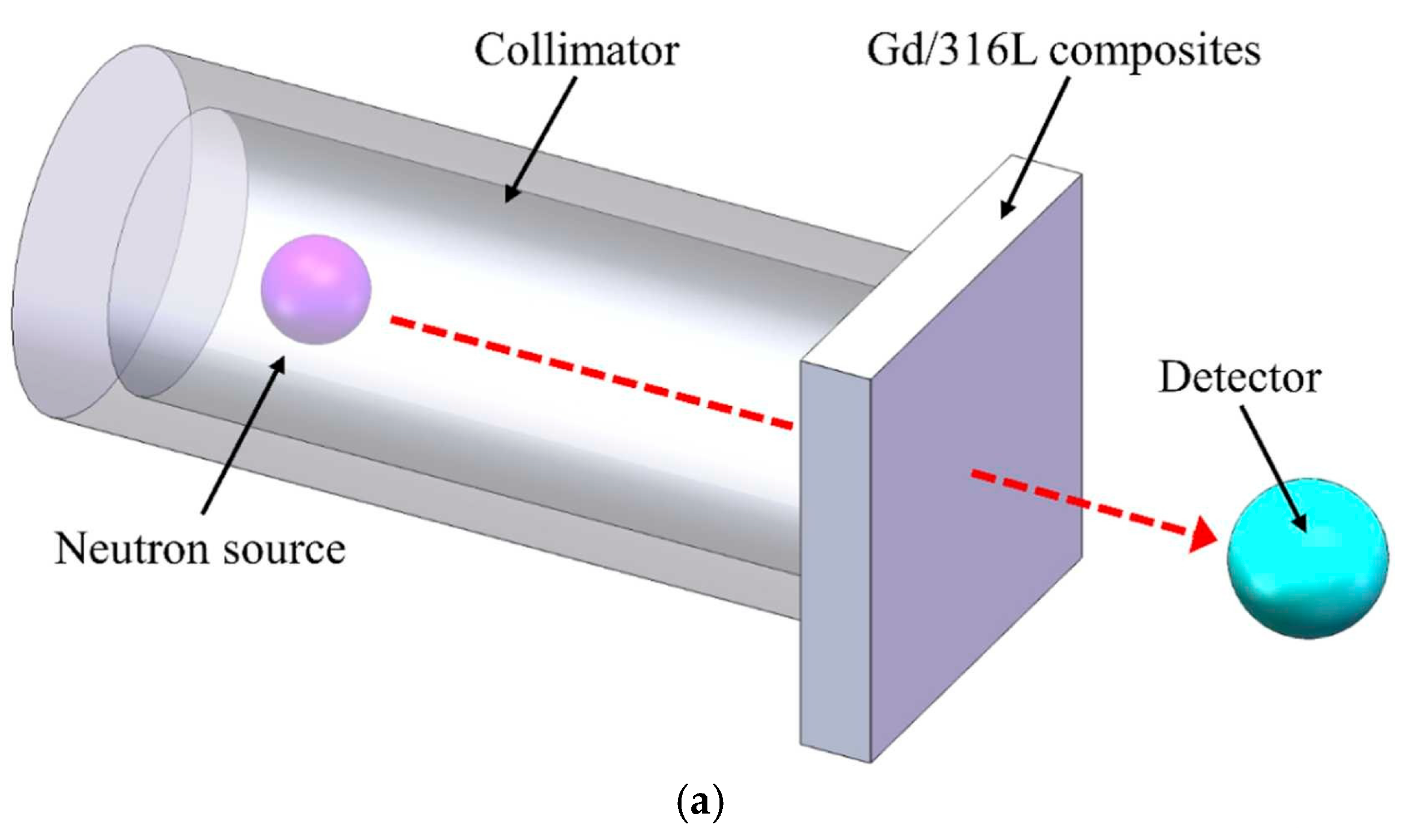
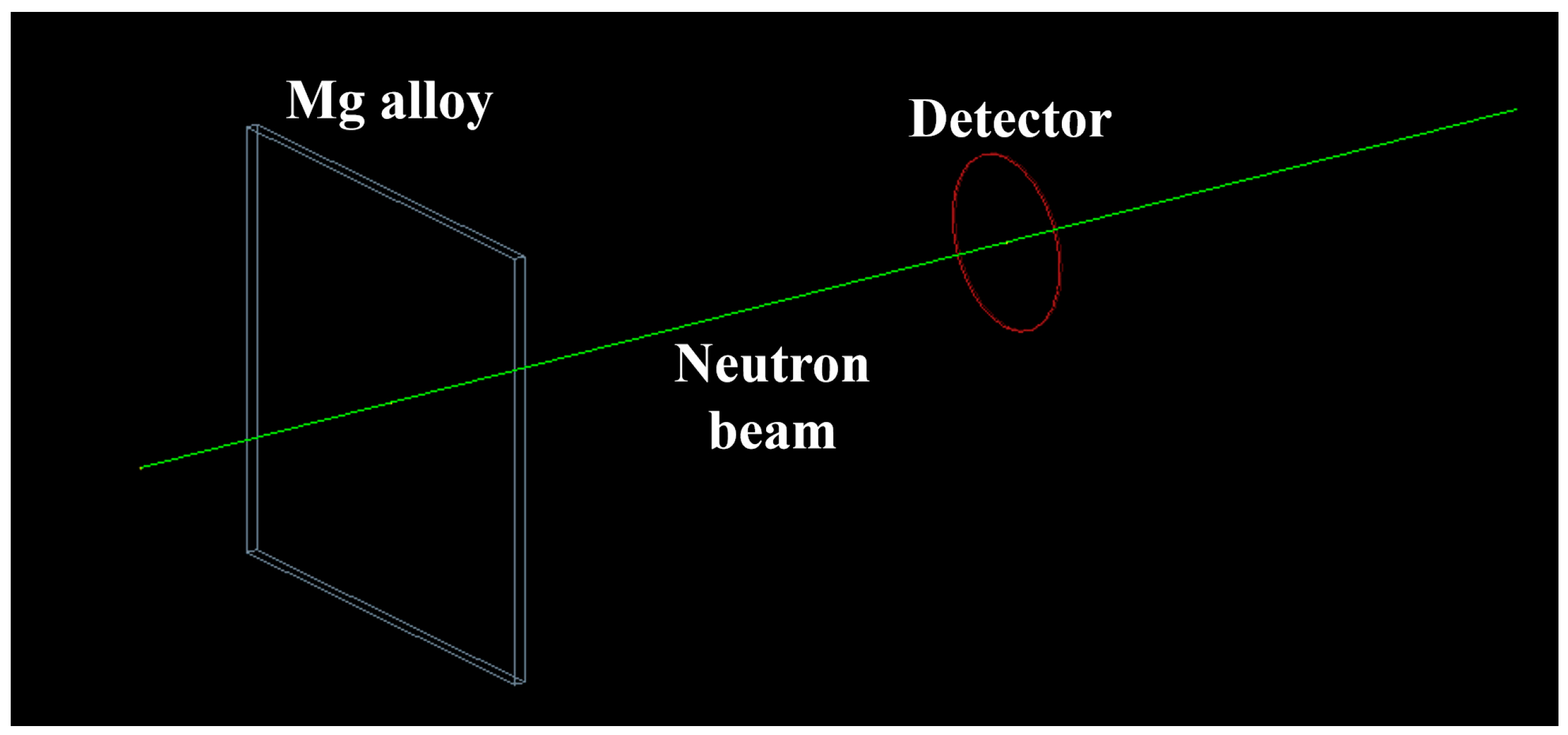
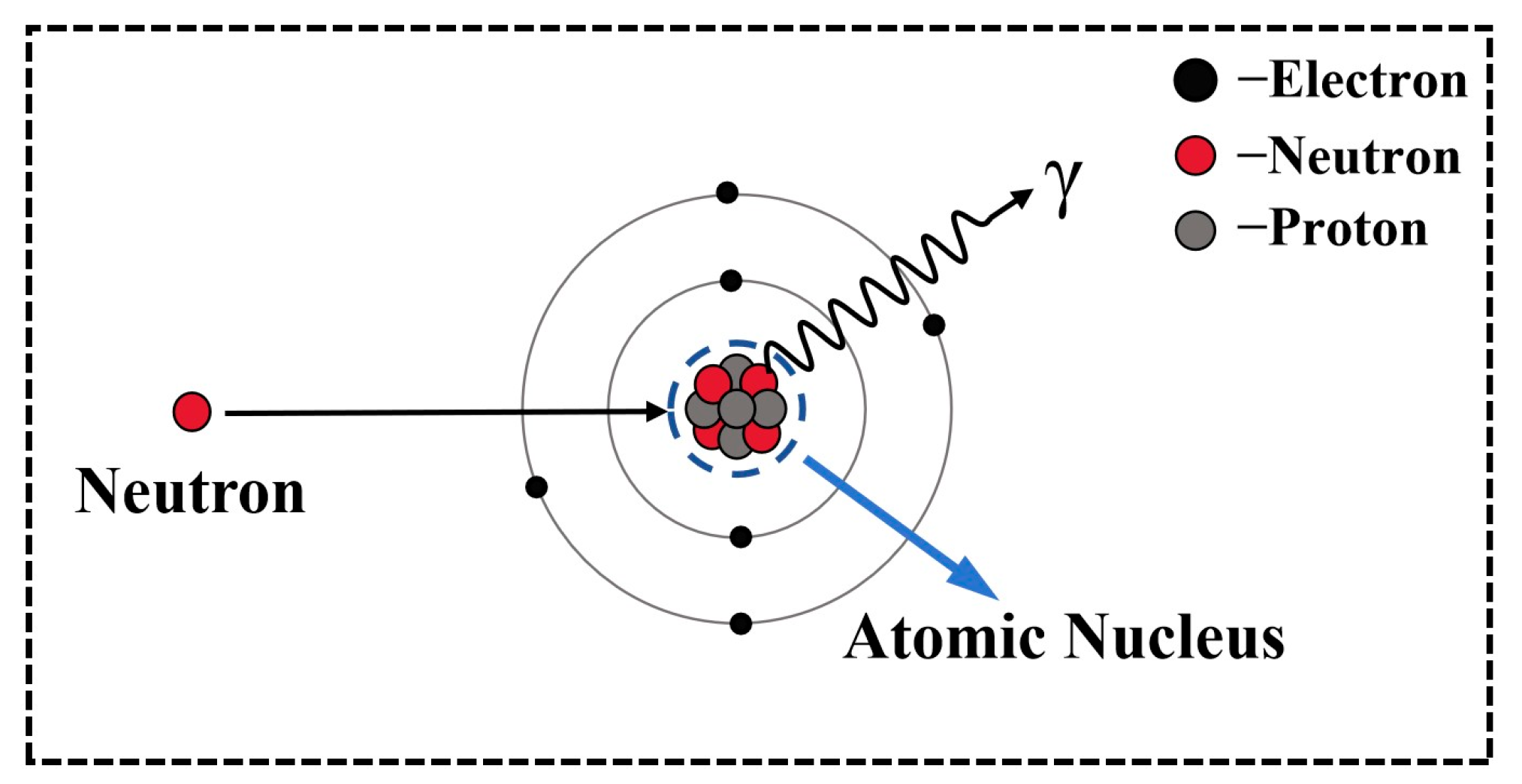
2.4. Neutron Shielding Performance
3. Results and Discussion
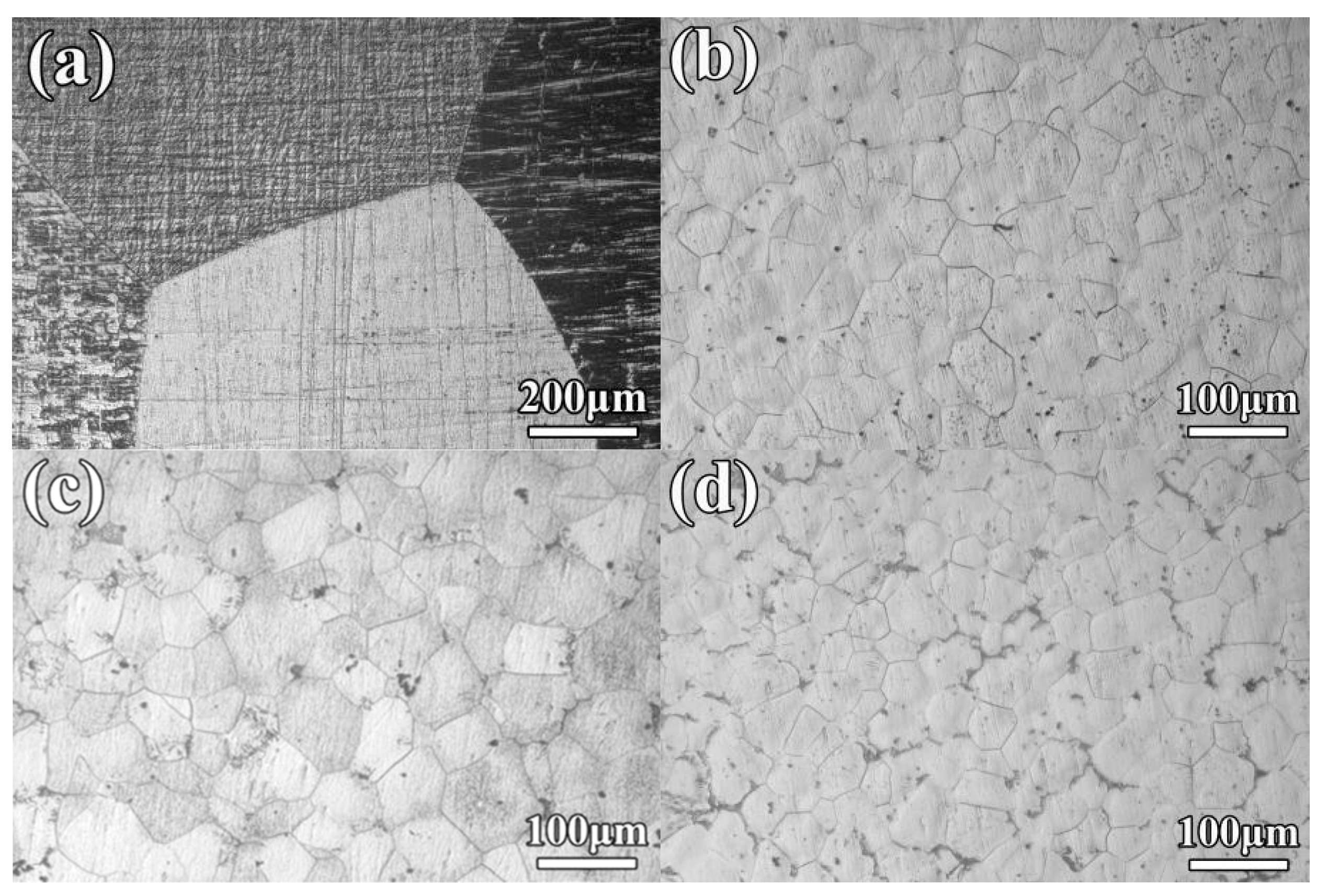
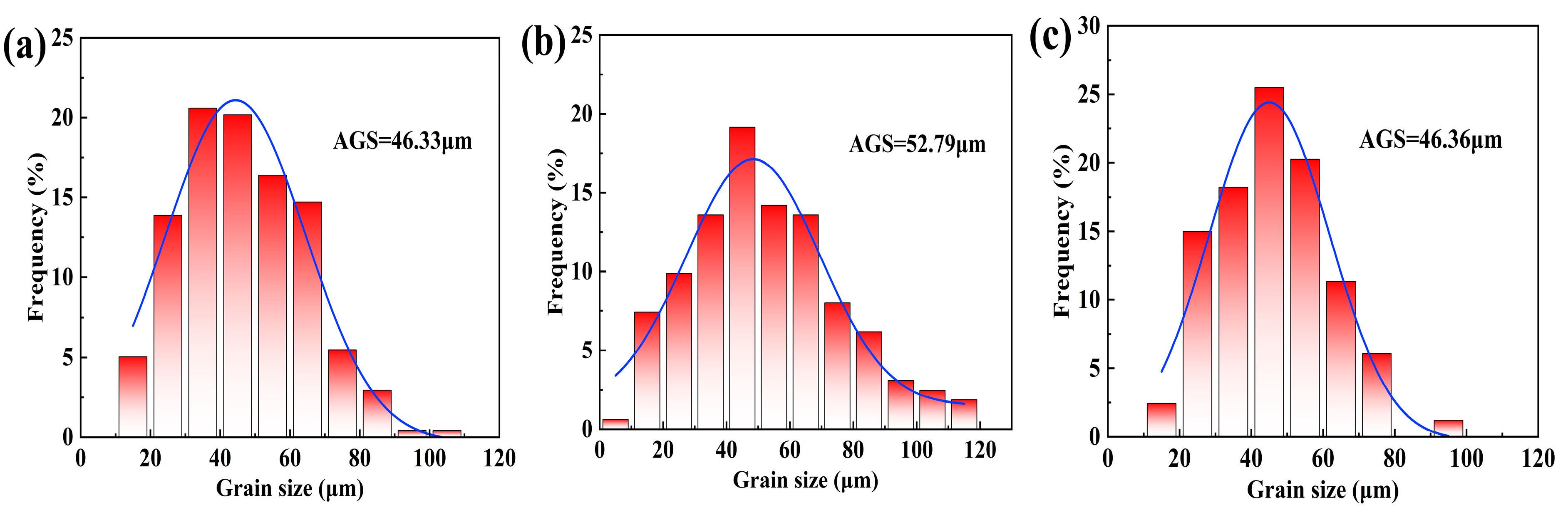
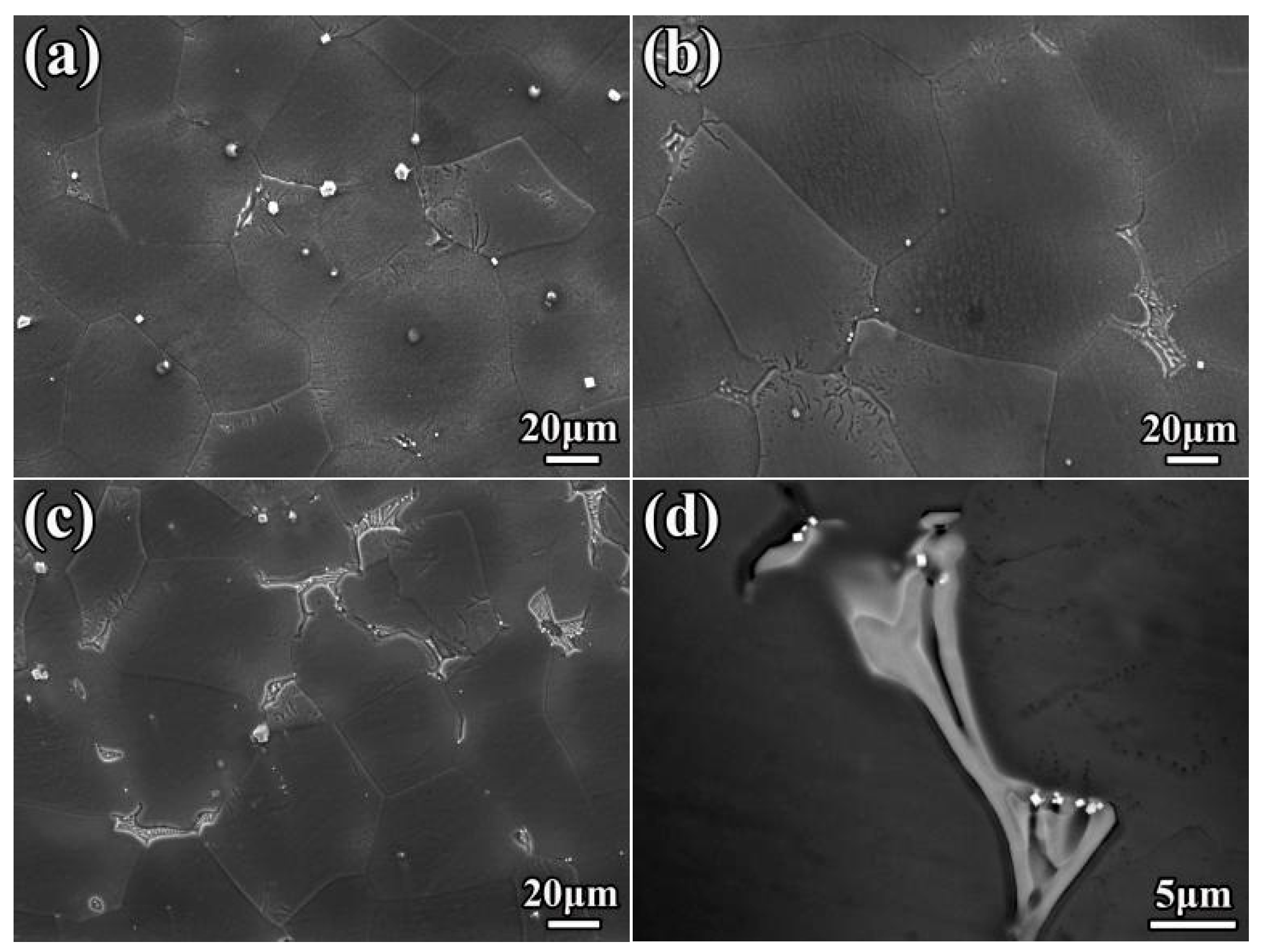
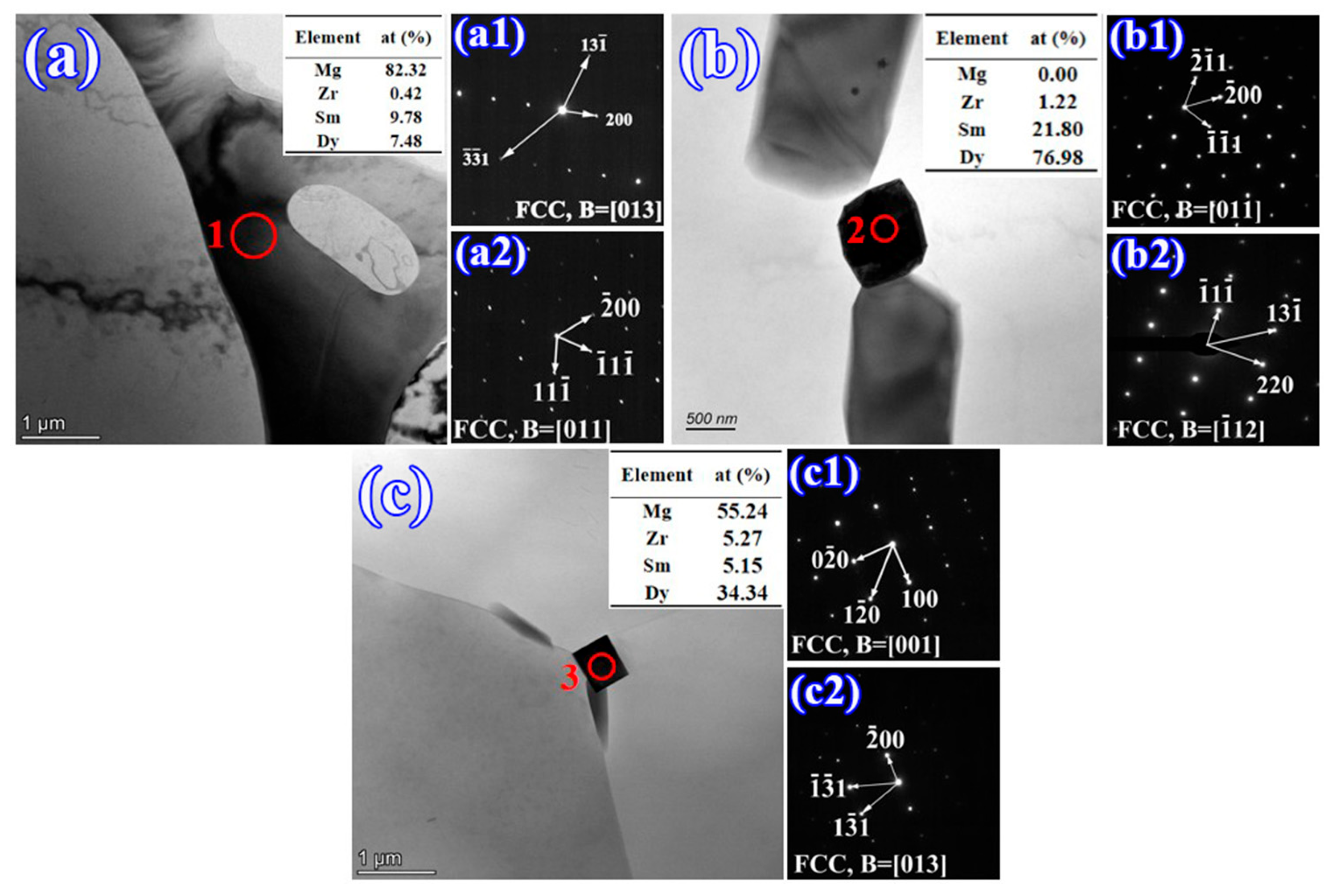
3.1. Microstructure
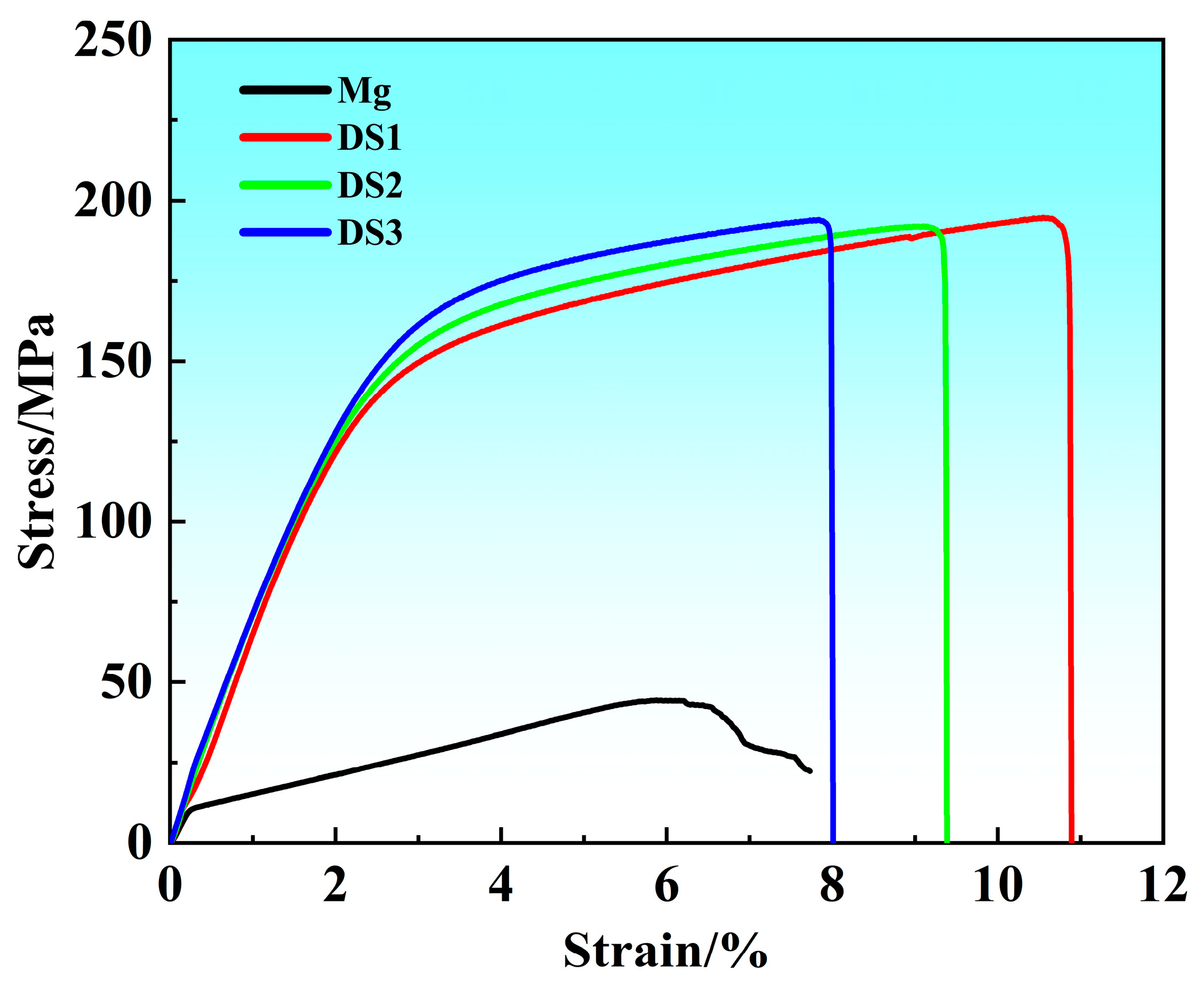
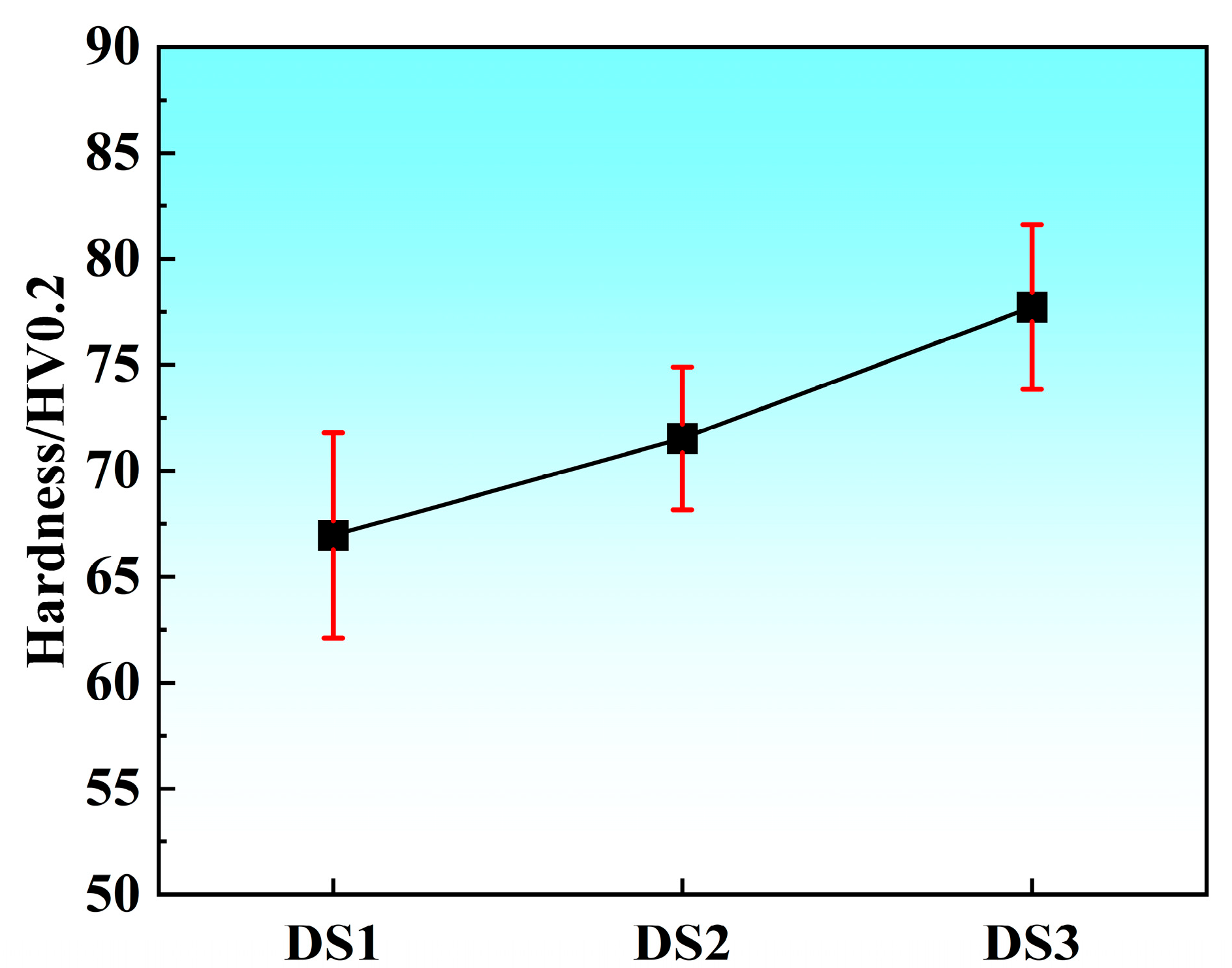
3.2. Mechanical Properties
3.3. Fracture Behavior
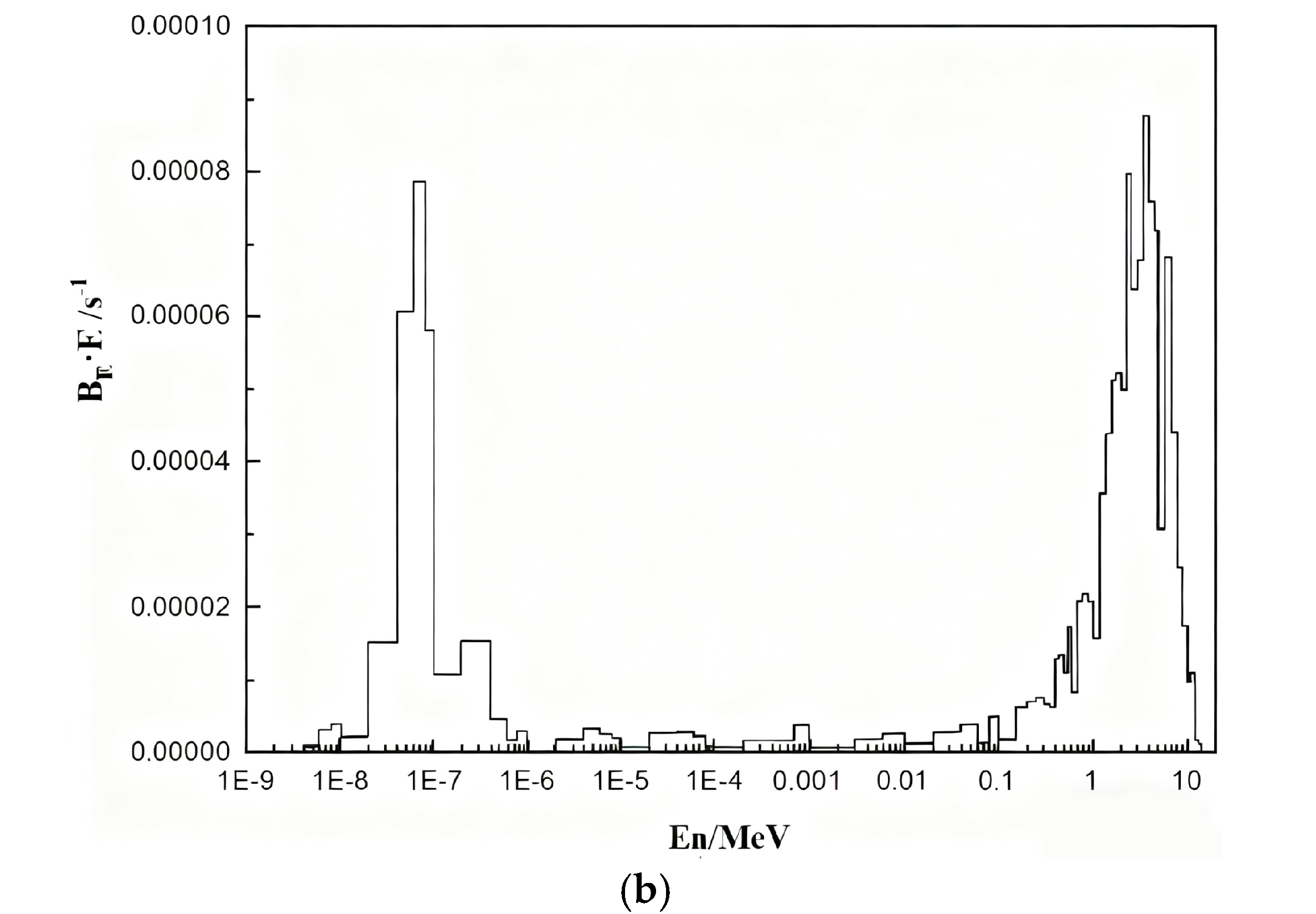
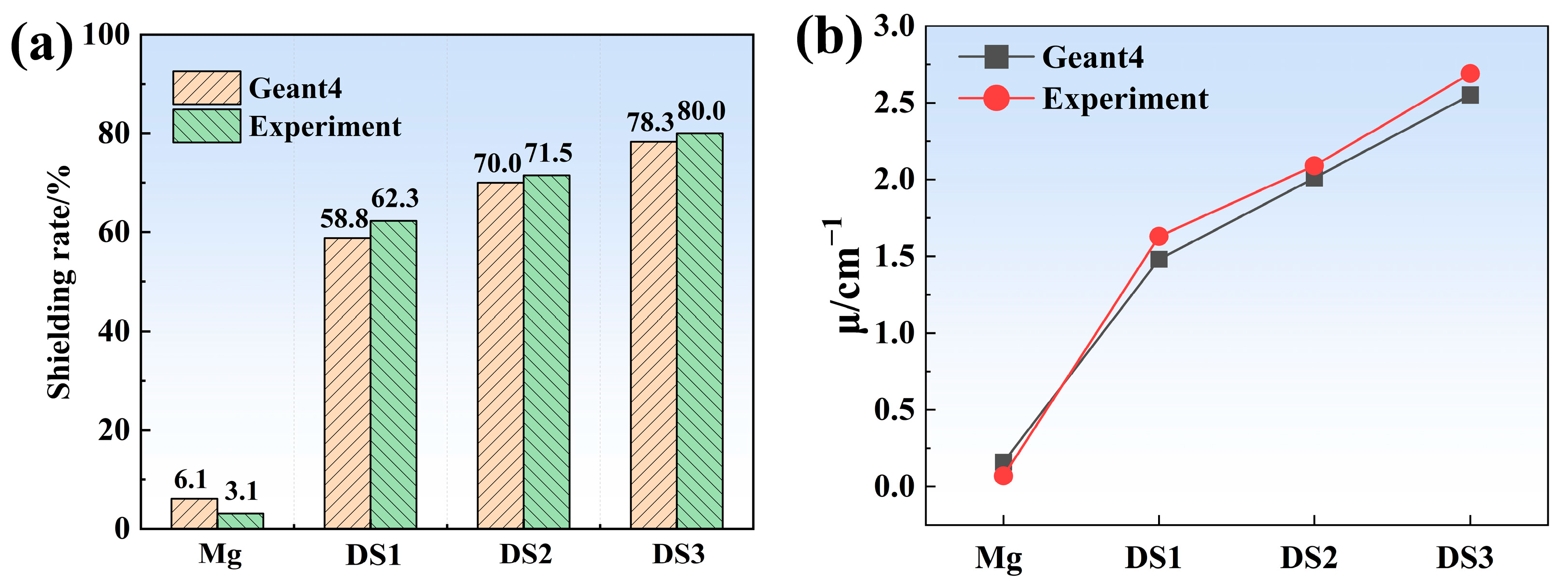
3.4. Neutron Shielding Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Addo, E.K.; Kabo-bah, A.T.; Diawuo, F.A.; Debrah, S.K. The Role of Nuclear Energy in Reducing Greenhouse Gas (GHG) Emissions and Energy Security: A Systematic Review. Int. J. Energy Res. 2023, 2023, 8823507. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Xiao, W.; Pan, D.; Wu, W. Advances in Gadolinium-Based Composite Materials for Neutron and Gamma-Ray Shielding. Front. Mater. 2025, 12, 1561198. [Google Scholar] [CrossRef]
- Gan, B.; Liu, S.; He, Z.; Chen, F.; Niu, H.; Cheng, J.; Tan, B.; Yu, B. Research Progress of Metal-Based Shielding Materials for Neutron and Gamma Rays. Acta Metall. Sin.-Engl. Lett. 2021, 34, 1609–1617. [Google Scholar] [CrossRef]
- Bakri, F.; Gareso, P.L.; Armynah, B.; Tahir, D. A Comparative Study of Glass-Based Material Counter to X-Ray, Gamma-Ray, and Proton and Neutron Radiation: A Review. Radiat. Phys. Chem. 2025, 226, 112270. [Google Scholar] [CrossRef]
- Hao, Z.; Gong, J.; Liang, C. Polymer-Based Neutron Shielding Materials: A Critical Review on Application-Specific Performance Trade-Offs and Optimization Strategies. Polym. Compos. 2025, early view. [Google Scholar]
- Piotrowski, T. Neutron Shielding Evaluation of Concretes and Mortars: A Review. Constr. Build. Mater. 2021, 277, 122238. [Google Scholar] [CrossRef]
- Yilmaz, D.; Aktas, B.; Calik, A.; Aytar, O.B. Boronizing Effect on the Radiation Shielding Properties of Hardox 450 and Hardox HiTuf Steels. Radiat. Phys. Chem. 2019, 161, 55–59. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, W.; Gao, Z.; Wang, B. The Design, Fabrication and Properties of B4C/Al Neutron Absorbers. J. Nucl. Mater. 2013, 437, 350–358. [Google Scholar] [CrossRef]
- Arslan, G.; Kara, F.; Turan, S. Quantitative X-Ray Diffraction Analysis of Reactive Infiltrated Boron Carbide–Aluminium Composites. J. Eur. Ceram. Soc. 2003, 23, 1243–1255. [Google Scholar] [CrossRef]
- Fu, X.; Ji, Z.; Lin, W.; Yu, Y.; Wu, T. The Advancement of Neutron Shielding Materials for the Storage of Spent Nuclear Fuel. Sci. Technol. Nucl. Install. 2021, 2021, 5541047. [Google Scholar] [CrossRef]
- Tasnim, A.; Sahadath, M.H.; Khan, M.N.I. Development of High-Density Radiation Shielding Materials Containing BaSO4 and Investigation of the Gamma-Ray Attenuation Properties. Radiat. Phys. Chem. 2021, 189, 109772. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, G.; Kumar, R.; Kumar, U.; Singh, K.; Kumar, M.; Bala, R.; Meena, R.; Kumar, A. Boron Carbide Nanostructures: A Prospective Material as an Additive in Concrete. In Proceedings of the 2nd International Conference on Condensed Matter and Applied Physics (ICC), Bikaner, India, 24–25 November 2017; AIP Publishing: Melville, NY, USA, 2018; p. 030278. [Google Scholar]
- Pan, F.; Yang, M.; Chen, X. A Review on Casting Magnesium Alloys: Modification of Commercial Alloys and Development of New Alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Mounier, C. Thermal Neutron Cross-Sections of Magnesium. Ann. Nucl. Energy 2004, 31, 911–921. [Google Scholar] [CrossRef]
- Jing, H.; Geng, L.; Qiu, S.; Zou, H.; Liang, M.; Deng, D. Research Progress of Rare Earth Composite Shielding Materials. J. Rare Earths 2023, 41, 32–41. [Google Scholar] [CrossRef]
- Lu, Y.; Huo, Z.; Zhong, G.; Zhang, H.; Hu, L. Rare Earth Based Neutron and Gamma Composite Shielding Materials. Prog. Chem. 2023, 35, 1214–1228. [Google Scholar]
- Hossain, M.K.; Raihan, G.A.; Akbar, M.A.; Kabir Rubel, M.H.; Ahmed, M.H.; Khan, M.I.; Hossain, S.; Sen, S.K.; Jalal, M.I.E.; El-Denglawey, A. Current Applications and Future Potential of Rare Earth Oxides in Sustainable Nuclear, Radiation, and Energy Devices: A Review. ACS Appl. Electron. Mater. 2022, 4, 3327–3353. [Google Scholar] [CrossRef]
- Vinothkumar, P.; Mohapatra, M.; Dhavamurthy, M.; Murugasen, P.; Suresh, A.A. The Effect of Rare Earth on the Radiation Shielding Properties of Transparent Lead-Free Alumino-Borophosphate Glass System. Radiat. Phys. Chem. 2022, 193, 109941. [Google Scholar]
- Wang, K.; Ma, L.; Yang, C.; Bian, Z.; Zhang, D.; Cui, S.; Wang, M.; Chen, Z.; Li, X. Recent Progress in Gd-Containing Materials for Neutron Shielding Applications: A Review. Materials 2023, 16, 4305. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Sun, M.; Ding, W. Recent Developments and Applications on High-Performance Cast Magnesium Rare-Earth Alloys. J. Magnes. Alloys 2021, 9, 1–20. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent Research and Developments on Wrought Magnesium Alloys. J. Magnes. Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Le, Y.; She, J.; Mao, J.; Jing, X.; Yang, J.; Meng, X.; Tan, J.; Wu, L.; Zhang, W.; Yang, W.; et al. Novel Integrated Structure and Function of Mg-Gd Neutron Shielding Materials. Nanotechnol. Rev. 2024, 13, 20240007. [Google Scholar] [CrossRef]
- Gaylan, Y.; Dag, I.E.; Caglar, S.; Avar, B. Investigation of Mechanical and Radiation Shielding Properties of Sm-Sm2O3 Reinforced Al-B4C Composite. Radiat. Phys. Chem. 2025, 226, 112325. [Google Scholar] [CrossRef]
- Zeng, Z.; Pan, H.; Pan, Z.; Wang, S.; Huang, Y.; Tang, W.; Yang, C.; Ren, Y.; Qin, G. Effect of Sm and Ce Content on Microstructure and Mechanical Property of Newly Developed Mg-Sm-Ce-Mn Based Alloy. Mater. Charact. 2023, 206, 113420. [Google Scholar] [CrossRef]
- Pan, J.; Liu, A.; Wang, C.; Li, J.; Wu, Z.; Xiao, X. Fabrication and Characterization of Novel High-Efficiency and High-Durability Neutron-Absorbing Ni-Based Alloys. Nucl. Mater. Energy 2024, 41, 101768. [Google Scholar] [CrossRef]
- An, R.; Cao, J.; Su, X.; Feng, Z.; Li, Y.; Li, Z.; Xiong, J. Research Status of Microstructure and Mechanical Properties of Cast Magnesium Alloys Containing Dy. Spec. Cast Nonferrous Alloys 2021, 41, 1374–1380. [Google Scholar]
- Li, S.; Du, W.; Wang, X.; Li, J.; Wu, Z.; Xiao, X. Effect of Zr Addition on the Grain Refinement Mechanism of Mg-Gd-Er Alloys. Acta Metall. Sin. 2018, 54, 911–917. [Google Scholar]
- Sun, M.; Yang, D.; Zhang, Y.; Mao, L.; Li, X.; Pang, S. Recent Advances in the Grain Refinement Effects of Zr on Mg Alloys: A Review. Metals 2022, 12, 1388. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, Z.; Meng, X.; Yang, X.; Liang, M.; Li, C.; Dai, Y. Microstructure, Thermophysical Properties and Neutron Shielding Properties of Gd/316 L Composites for Spent Nuclear Fuel Transportation and Storage. Mater. Today Commun. 2023, 37, 107315. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; El-Agawany, F.I.; Sriwunkum, C.; Akyildirim, H.; Arslan, H.; Tonguc, B.T.; El-Mallawany, R.; Rammah, Y.S. Influence of Bi2O3/PbO on Nuclear Shielding Characteristics of Lead-Zinc-Tellurite Glasses. Phys. B Condens. Matter 2020, 581, 411946. [Google Scholar] [CrossRef]
- Shi, H.; He, J.; Wen, J.; Li, H.; Xu, D.; Liu, Q. Effect of Rare Earth Element Dy on Microstructure and Properties of Mg-2Zn-0.5Zr Bio-Magnesium Alloy. Mater. Heat Treat. 2020, 41, 12–18. [Google Scholar]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards Developing Mg Alloys with Simultaneously Improved Strength and Corrosion Resistance via RE Alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Yu, M.; Guo, E.; Feng, Y.; Wang, L.; Wang, C. Effect of Sm on Microstructure and Mechanical Properties of As-Cast Mg-0.4Zn-0.8Zr Alloys. Heat Treat. Met. 2016, 41, 78–80. [Google Scholar]
- Zhu, L.; Li, Q.; Zhou, Y.; Yan, J. Effect of Sm on Microstructures and Mechanical Properties of Mg-10Gd-0.5Zr Alloy. Rare Met. Mater. Eng. 2019, 48, 171–176. [Google Scholar]
- Yuan, M.; He, C.; Dong, Z.; Jiang, B.; Song, B.; Guo, N.; Liu, T.; Guo, S.; Pan, F. Effect of Sm Addition on the Microstructure and Mechanical Properties of Mg-xSm-0.4Zr Alloys. J. Mater. Res. Technol. 2023, 23, 4814–4827. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Q.; Fu, S.; Zhang, X.; Cheng, X.; Yan, J. Microstructure and Properties Analysis of As Cast Mg-11Gd-2Y-xSm-0.5Zr Alloy. Foundry 2017, 66, 178–181. [Google Scholar]
- Zhang, Z. Study on the Microstructure, Mechanical Properties and Precipitation Phase Transformation in Mg(-Gd)-Sm-Zr Alloy. Ph.D. Thesis, Shanghai Jiao Tong University, Shanghai, China, 2009. [Google Scholar]
- Lei, W.; Wang, H.; Liang, W. Microstructure and Mechanical Properties of Pure Magnesium Subjected to Hot Extrusion. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 1193–1196. [Google Scholar] [CrossRef]
- Jamel, M.; Lopez, H.; Schultz, B.; Otieno, W. The Effect of Solidification Rate on the Microstructure and Mechanical Properties of Pure Magnesium. Metals 2021, 11, 1264. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, J.; Wu, H.; Liu, X.; Liu, H.; Fan, Z.; Lv, S.; Wang, K.; Wang, Z. Microstructure and Mechanical Properties of a Cast Heat-Resistant Rare-Earth Magnesium Alloy. China Foundry 2023, 20, 289–298. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, S.; Easton, M.A.; Zhang, M.; Qiu, D.; Wu, G.; Liu, W.; Ding, W. Heat treatment, microstructure and mechanical properties of a Mg–Gd–Y alloy grain-refined by Al additions. Mater. Sci. Eng. A 2013, 576, 298–305. [Google Scholar] [CrossRef]
- Liu, D.; Yin, X.; Pang, X.; Hu, S.; Ding, Y. Effects of Dy, Sr and Die Casting on Microstructure, Mechanical and Corrosion Properties of Mg-Dy-Sr-Nd-Zr Alloys. J. Mater. Eng. Perform. 2017, 26, 3983–3992. [Google Scholar] [CrossRef]
- Li, Z.; Xu, M.; Cao, Y.; Zhao, Y. Current Progress in Strengthening, Plastic Deformation and Thermal Stability of Mg Alloys. J. Alloys Compd. 2025, 1018, 179151. [Google Scholar] [CrossRef]
- Wu, G.; Tong, X.; Jiang, R.; Ding, W. Grain Refinement of As-Cast Mg-RE Alloys: Research Progress and Future Prospect. Acta Metall. Sin. 2022, 58, 385–399. [Google Scholar]
- Yang, L.; Huang, Y.; Peng, Q.; Feyerabend, F.; Kainer, K.U.; Willumeit, R.; Hort, N. Mechanical and Corrosion Properties of Binary Mg-Dy Alloys for Medical Applications. Mater. Sci. Eng. B 2011, 176, 1827–1834. [Google Scholar] [CrossRef]
- Bao, J.; Li, Q.; Chen, X.; Zhang, Q.; Chen, Z. Microstructure and Texture Evolution with Sm Addition in Extruded Mg-Gd-Sm-Zr Alloy. Mater. Res. Express 2021, 8, 096523. [Google Scholar] [CrossRef]
- Karparvarfard, S.M.H.; Shaha, S.K.; Behravesh, S.B.; Jahed, H.; Williams, B. Microstructure, Texture and Mechanical Behavior Characterization of Hot Forged Cast ZK60 Magnesium Alloy. J. Mater. Sci. Technol. 2017, 33, 907–918. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Wu, L.; Jiang, F.; Fu, D.; Teng, J.; Zhang, H. Effect of Heat Treatments on the Corrosion Resistance of a High Strength Mg-Gd-Y-Zn-Zr Alloy. Materials 2022, 15, 2813. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Li, D.H.; Dong, J.; Lu, C.; Ding, W. Effect of Solution Treatment and Extrusion on Evolution of Microstructure in a Mg-12Dy-3Nd-0.4Zr Alloy. J. Alloys Compd. 2008, 456, 419–424. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Wang, W.; Shen, F.; Zheng, X.; Wei, X.; Liang, J.; Liang, X.; Zhu, D.; Zhang, Z. Enhanced Strength-Ductility Synergy of Mg-Nd-Zn-Zr Alloys Fabricated by Wire-Arc Directed Energy Deposition and Subsequent Heat Treatment. J. Alloys Compd. 2025, 1036, 181999. [Google Scholar] [CrossRef]
- Yi, S.; Zhao, S.; Feng, Y.; Wang, L.; Guo, E.; Sun, Q. Effect of Heat Treatment on Microstructure, Mechanical Properties and Corrosion Behavior of Mg-Sm-Zn-Zr-xNd Alloys. J. Mater. Res. Technol. 2025, 36, 5341–5354. [Google Scholar] [CrossRef]
- Doan, T.C.; Majety, S.; Grenadier, S.; Li, J.; Lin, J.; Jiang, H. Fabrication and Characterization of Solid-State Thermal Neutron Detectors Based on Hexagonal Boron Nitride Epilayers. Nucl. Instrum. Methods Phys. Res. A 2014, 748, 84–90. [Google Scholar] [CrossRef]
- Stoto, T.; Housseau, N.; Zuppiroli, L.; Kryger, B. Swelling and Microcracking of Boron-Carbide Subjected to Fast-Neutron Irradiations. J. Appl. Phys. 1990, 68, 3198–3206. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Wang, W.; Duan, Y. Effect of Boron Segregation on the Surface Crack of Low Carbon Boron-Bearing Steel. Results Phys. 2019, 13, 102153. [Google Scholar] [CrossRef]
- Pan, J.; Wang, C.; Wang, Z.; Zhang, C.; Fang, L.; Li, J.; Mei, Q.; Gao, J.; Wang, M.; Li, H.; et al. Microstructure Characteristics and Properties of a Novel Ni-Based Alloy for Thermal Neutron and Gamma Ray Co-Shielding. Mater. Charact. 2024, 210, 113840. [Google Scholar] [CrossRef]
- Chen, Z.; Huo, Z.; Zhang, H.; Zhong, G. Gamma Ray Shielding Composite Material with High Z Number. Prog. Chem. 2024, 36, 1102–1116. [Google Scholar]



| Sample | Dy (wt%) | Sm (wt%) |
|---|---|---|
| Mg-12Dy-1Sm-0.4Zr | 12.38 ± 0.05 | 1.03 ± 0.05 |
| Mg-12Dy-2Sm-0.4Zr | 12.65 ± 0.05 | 2.11 ± 0.05 |
| Mg-12Dy-3Sm-0.4Zr | 12.54 ± 0.05 | 3.06 ± 0.05 |
| Sample | UTS (MPa) | YS (MPa) | EL (%) |
|---|---|---|---|
| Mg | 44.1 | 11.9 | 7.7 |
| Mg-12Dy-1Sm-0.4Zr | 194.6 | 138.2 | 10.9 |
| Mg-12Dy-2Sm-0.4Zr | 191.9 | 128.0 | 9.4 |
| Mg-12Dy-3Sm-0.4Zr | 193.4 | 125.9 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Duan, C.; Niu, E.; Xu, X.; She, J.; Tan, J.; Zhang, W.; Mao, J. Design, Preparation and Synergistic Optimization of Mechanical Properties and Thermal Neutron Shielding Performance of Mg-Dy-Sm-Zr Alloys. Crystals 2025, 15, 894. https://doi.org/10.3390/cryst15100894
Lu H, Duan C, Niu E, Xu X, She J, Tan J, Zhang W, Mao J. Design, Preparation and Synergistic Optimization of Mechanical Properties and Thermal Neutron Shielding Performance of Mg-Dy-Sm-Zr Alloys. Crystals. 2025; 15(10):894. https://doi.org/10.3390/cryst15100894
Chicago/Turabian StyleLu, Huabing, Chengzhi Duan, Enci Niu, Xiyu Xu, Jia She, Jun Tan, Wei Zhang, and Jianjun Mao. 2025. "Design, Preparation and Synergistic Optimization of Mechanical Properties and Thermal Neutron Shielding Performance of Mg-Dy-Sm-Zr Alloys" Crystals 15, no. 10: 894. https://doi.org/10.3390/cryst15100894
APA StyleLu, H., Duan, C., Niu, E., Xu, X., She, J., Tan, J., Zhang, W., & Mao, J. (2025). Design, Preparation and Synergistic Optimization of Mechanical Properties and Thermal Neutron Shielding Performance of Mg-Dy-Sm-Zr Alloys. Crystals, 15(10), 894. https://doi.org/10.3390/cryst15100894








