Color Mechanism of Blue Myanmar Jadeite Jade: The Role of Trace Elements and Mineralogical Characteristics
Abstract
1. Introduction
2. Materials and Methods
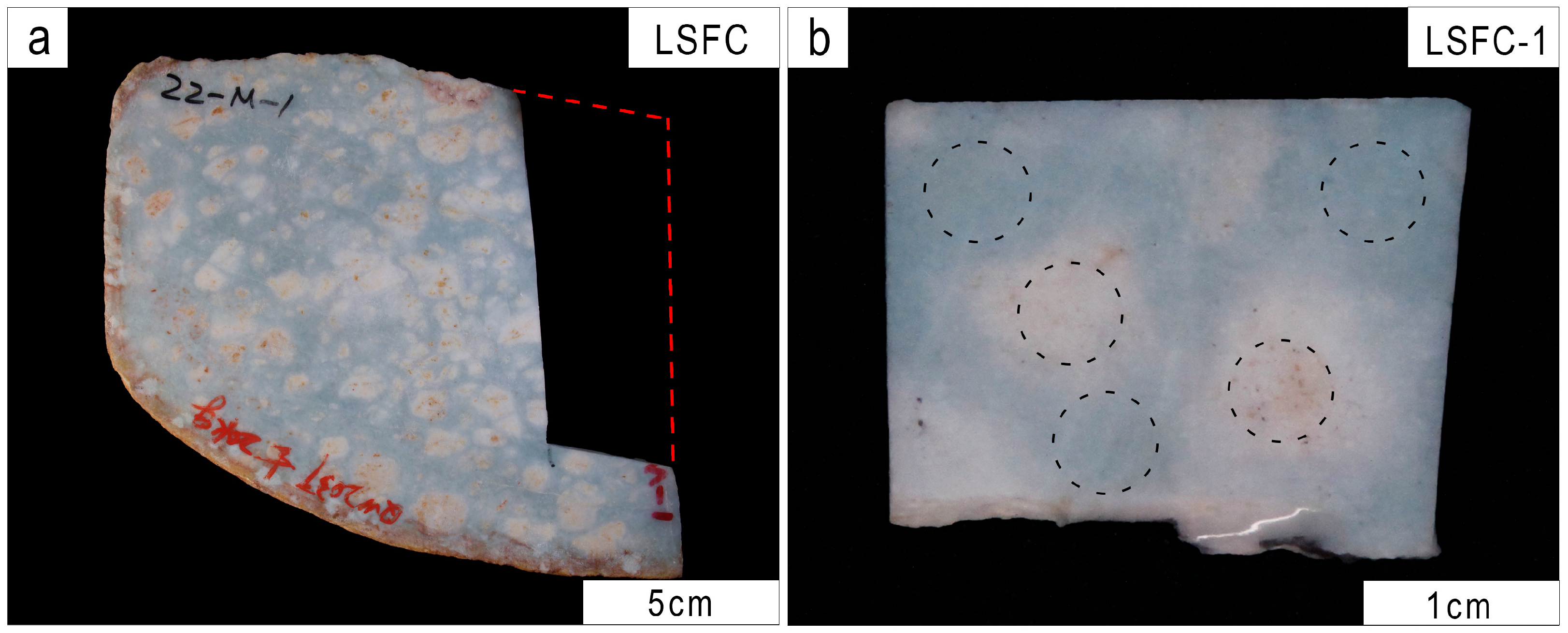
2.1. Samples
2.2. Methods of Analysis
2.3. Color Testing
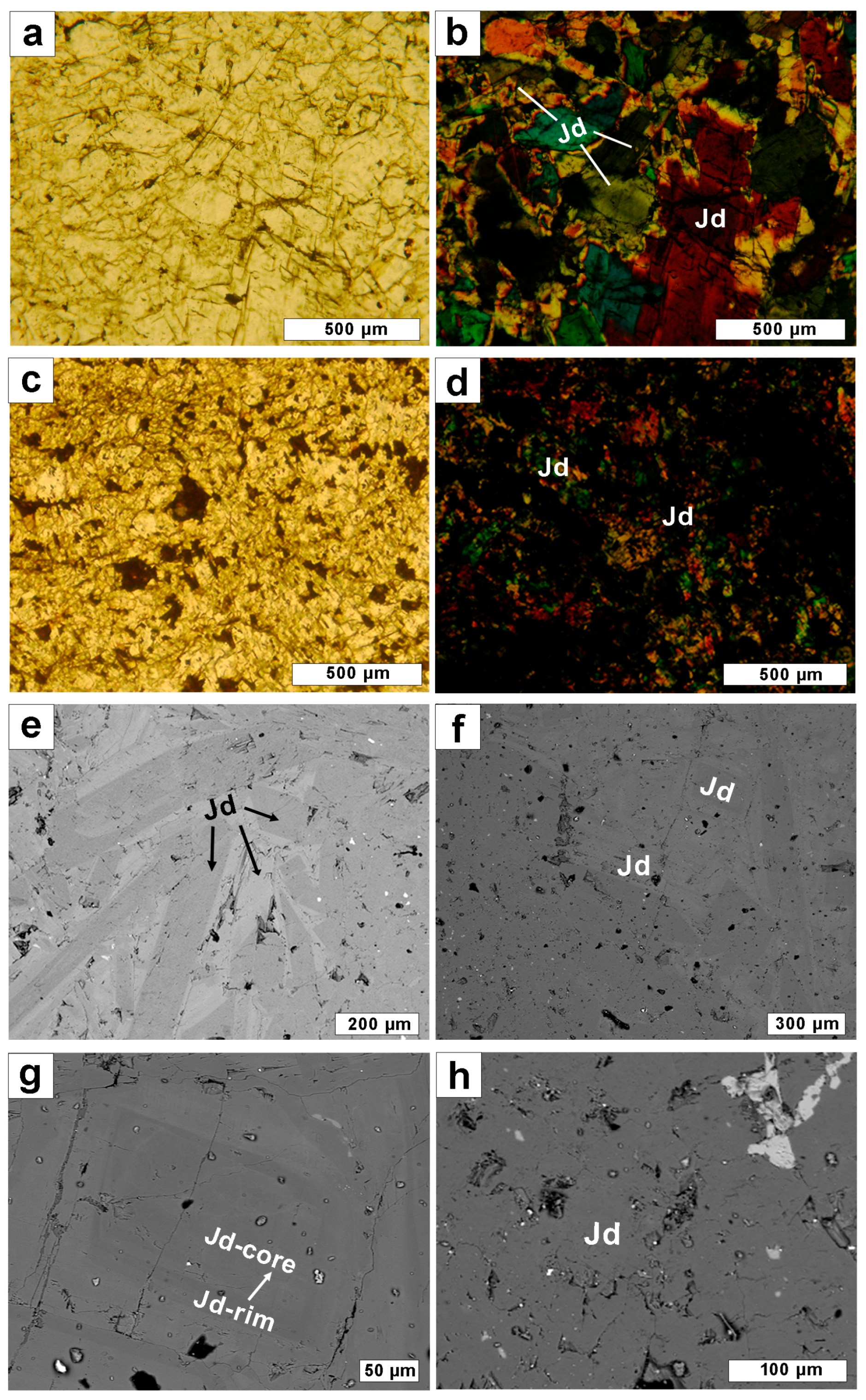
2.4. Petrography and Main Mineral Composition
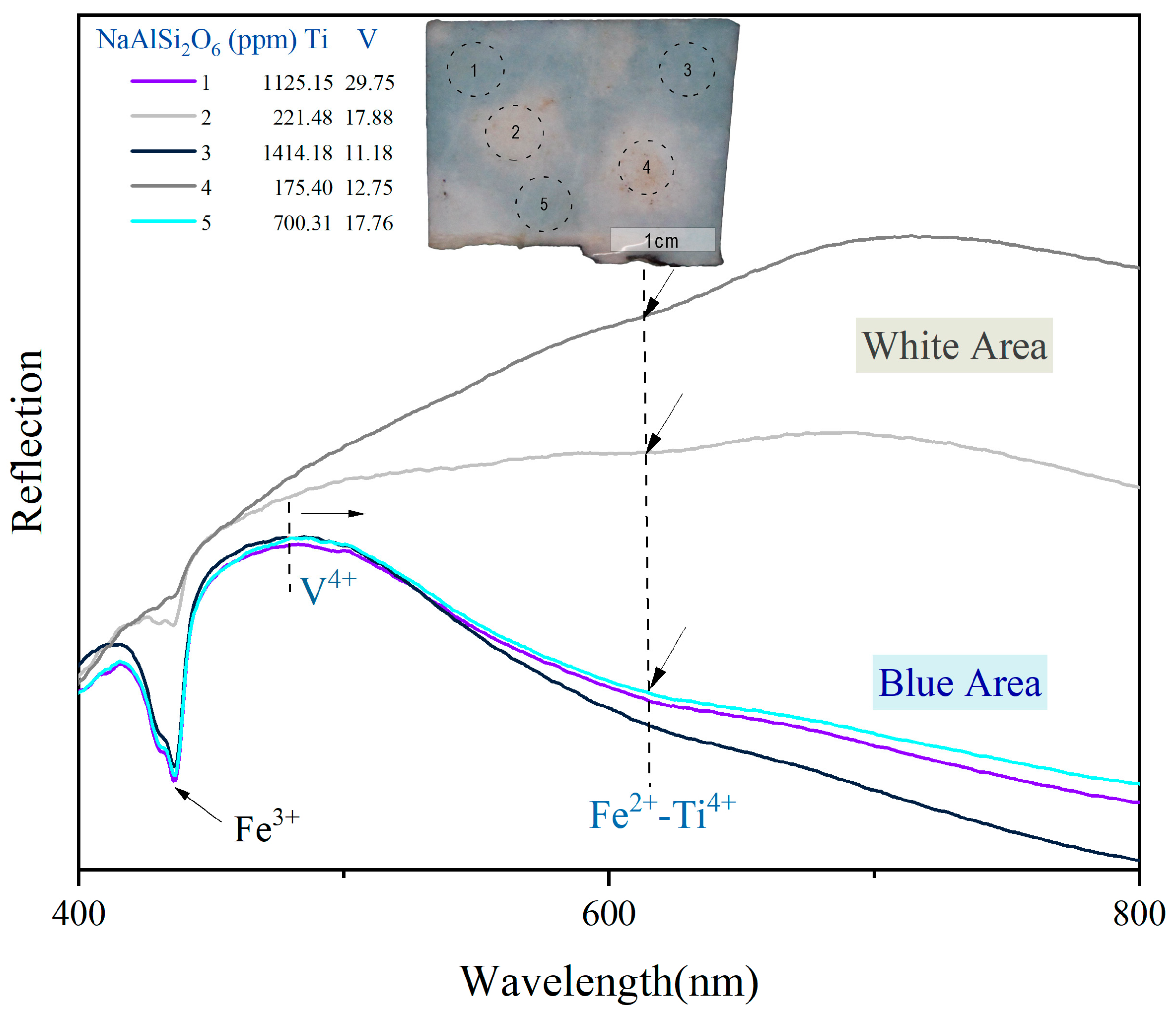
2.5. UV–Vis Spectroscopy and Trace Element Composition (LA-ICP-MS)
3. Discussion
3.1. Color and Chemical Composition of Blue Jadeite from Myanmar
3.2. Coloring Elements and Coloration Mechanism
3.3. Blue Jadeite Jade Formation Process
3.4. Gemmological Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, X.; Shi, G.H.; He, M.Y. Jadeitized omphacitite from Myanmar jadeite area. Acta Petrol. Sin. 2006, 22, 971–976. [Google Scholar]
- Shi, G.H.; Stöckhert, B.; Cui, W.Y. Kosmochlor and chromian jadeite aggregates from the Myanmar jadeitite area. Minerals 2007, 69, 1059–1075. [Google Scholar] [CrossRef]
- Shi, G.H.; Harlow, G.E.; Wang, J.; Wang, J.; Ng, E.; Wang, X.; Cao, S.M.; Cui, W.Y. Mineralogy of jadeitite and related rocks from Myanmar: A review with new data. Eur. J. Mineral. 2012, 24, 345–370. [Google Scholar] [CrossRef]
- Shi, G.H.; Cui, W.Y.; Tropper, P.; Wang, C.Q.; Shu, G.M.; Yu, H.X. The petrology of a complex sodic and sodic-calcic amphibole association and its implications for the metasomatic processes in the jadeitite area in northwestern Myanmar, formerly Burma. Contrib. Mineral. Petrol. 2003, 145, 355–376. [Google Scholar] [CrossRef]
- Harlow, G.E.; Sisson, V.B.; Sorensen, S.S. Jadeitite from Guatemala: New observations and distinctions among multiple occurrences. Geol. Acta 2011, 9, 363. [Google Scholar]
- Abduriyim, A.; Kazuko, S.; Yusuke, K. Japanese Jadeite: History, Characteristics, and Comparison with Other Sources. Gems Gemol. 2017, 53, 48–67. [Google Scholar] [CrossRef]
- Lesz, S.; Griner, S.; Nowosielski, R. Chemical microstructure of Franciscan jadeite from Pacheco Pass, California. Am. Mineral. 1998, 83, 273–279. [Google Scholar]
- Shi, G.H.; Jiang, N.; Liu, Y.; Wang, X.; Zhang, Z.Y.; Xu, Y.J. Zircon Hf isotope signature of the depleted mantle in the Myanmar jadeitite: Implications for Mesozoic intra-oceanic subduction between the Eastern Indian Plate and the Burmese Platelet. Lithos 2009, 112, 342–350. [Google Scholar] [CrossRef]
- Xing, B.Q.; Shi, G.H.; Zhang, J.H.; Long, C.; Zhang, Y.; He, L.Y.; Hu, R.J. Characteristics of the Guatemalan Feicui and Its Comparison to the Myanmar Feicui. Geoscience 2021, 35, 1769–1788. [Google Scholar]
- Shi, G.H.; Cui, W.Y. Petrology of jadeite-bearing serpentinized peridotite and its country rocks from Northwestern Myanmar (Burma). Acta Petrol. Sin. 2001, 17, 483–490. [Google Scholar]
- Wang, X.; Shi, G.H.; Qiu, D.F.; Wang, J.; Cui, W.Y. Grossular-bearing jadeite omphacite rock in the Myanmar jadeite area: A kind of jadeitized rodingite? Eur. J. Mineral. 2012, 24, 237–246. [Google Scholar] [CrossRef]
- Khomenko, V.M.; Platonov, A.N. Electronic absorption spectra of Cr3+ ions in natural clinopyroxenes. Physics. Chem. Miner. 1985, 11, 261–265. [Google Scholar] [CrossRef]
- Harder, H. Trace elements as coloring agents in jadeites. J. Gemmol. 1995, 24, 508–511. [Google Scholar] [CrossRef]
- Shi, G.H.; Cui, W.Y. Desalinization of Chromium within Myanmar Jadeite Jade and Implications. J. Gems Gemmol. 2005, 7, 7–12. [Google Scholar]
- Zhen, C.L.; Gao, Y.J.; Li, K. Identification Characteristic of Green Feicui from Myanmar and Guatemala. J. Gems Gemmol. 2023, 25, 17–28. [Google Scholar]
- Liu, Z.Y.; Guo, Y.; Shang, Y.R.; Yuan, B. Research on parameters optimization of digital imaging system in red–yellow jadeite color measurement. Sci. Rep. 2022, 12, 3619. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Guo, Y.; Liu, Z.Y.; Zhang, Z.K.; Shi, Y.X. Impact of different standard lighting sources on red jadeite and color quality grading. Earth Sci. Res. J. 2019, 23, 371–378. [Google Scholar] [CrossRef]
- Rossman, G.R. Lavender jade. The optical spectrum of Fe3+ and Fe2+⤍Fe3+ intervalence charge transfer in jadeite from Burma. American. Mineral. 1974, 59, 868–870. [Google Scholar]
- Shang, Y.R.; Wang, W.Z.; Jin, T.L.; Huang, L.M.; Wu, Z.Y.; Guo, Y. Colouration in purple jadeite-jade from Myanmar A spectroscopy and chromaticity investigation. Acta Petrol. Et. Mineral. 2024, 43, 643–651. [Google Scholar]
- Li, X. The Color Causing Mechanism and Influencing Factors of Lavender Feicui in Myanmar. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2012. [Google Scholar]
- Han, W.; Liu, Y.; Zhang, J.; Lu, T.J. Color origins of two types of natural lavender jadeite jades. Acta Mineral. Sin. 2020, 40, 549–555. [Google Scholar]
- Wu, X.; Bao, Z.; Kang, Y.; Han, X.; Liu, X.; Qu, M. Color Origin of Burmese Lavender Jadeite. Laser Optoelectron. Prog. 2019, 56, 073001–073005. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.H. Origin of Blue-Water Jadeite Jades from Myanmar and Guatemala: Differentiation by Non-Destructive Spectroscopic Techniques. Crystals 2022, 12, 1448. [Google Scholar] [CrossRef]
- Yan, W.W.; Wang, J.H. Origin of the blue fog zone and black crust of Myanmar jadeite jade boulder. Acta Sci. Natralium Univ. Sunyatseni 2011, 50, 107–113. [Google Scholar]
- Yu, K.T.; Zhu, Z.B. A study on the chemical state of vanadium in turguoise pigment. Jourcal Ceram. 2000, 21, 186–189. [Google Scholar]
- Wang, D.; Mo, Z.R.; Li, X.M.; Wang, H.Q. Study on Colouring Mechanisms of Blue Jadeite. J. Gems Gemmol. 2013, 15, 14–17. [Google Scholar]
- Sujatha Devi, P.; Ganguli, D. Vanadyl ion stability in silica gels. J. Non-Cryst. Solids 1998, 240, 50–54. [Google Scholar] [CrossRef]
- Li, T.; Zhang, C.; Zhang, H.T.; Chen, Y.Q.; Li, Z.B.; Liu, Y. Color-Causing Mechanisms of Guatemala Jadeite Jade: Constraints from Spectroscopy and Chemical Compositions. Crystals 2023, 13, 1535. [Google Scholar] [CrossRef]
- Xie, Y.H.; Wang, C.Y. Trace Elements in Jadeites with Different Color and Their Infrared Spectra Characteristics. Rock Miner. Anal. 2003, 22, 183–187. [Google Scholar]
- Zhang, X.H. Jade Color Changes to the Color and Type and Content-Relations. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2008. [Google Scholar]
- Oberhänsli, R.; Bousquet, R.; Moinzadeh, H.; Moazzen, M.; Arvin, M. The Field of Stability of blue jadeite: A new occurrence of jadeite at Sorkhan, IRAN, As a case study. Mineralogist 2007, 45, 1501–1509. [Google Scholar] [CrossRef]
- Guo, Y.; Mo, T.; Cheng, S.H. Contribution of Green Jadeite-Jade’s Chroma Difference Based on CIE 1976 L*a*b* Uniform Color Space. Adv. Mater. Res. 2009, 177, 620–623. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Mo, T. Quality Evaluation of Green Jadeite-jade’s Lightness Based on CIE 1976 L*a*b* Uniform Color Space. Bull. Chin. Ceram. Soc. 2010, 29, 560–566. [Google Scholar]
- Morimoto, N. Nomenclature of Pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.H.; Wen, J.B. Chromite and Its Thin Kosmochlor and Cr-Omphacite Cortex in Amphibolite from the Myanmar Jadeite Deposits. Crystals 2025, 15, 79. [Google Scholar] [CrossRef]
- Guo, Y.; Zong, X.; Qi, M.; Zhang, Y.; Wang, H. Feasibility study on color evaluation of jadeite based on GemDialogue color chip images. EURASIP J. Image Video Process 2018, 2018, 95. [Google Scholar] [CrossRef]
- Liu, S.I.; Man, K.Y.; Seneewong-Na-Ayutthaya, M.; Jakkawanvibul, C.; Lee, A.T.-Y. Geographic Origin Deter-mination of High-quality Green Jadeite-Omphacite Jade (Fei Cui) from Myanmar, Guatemala and Italy Using Statistical Processing Coupled with Spectroscopic and Chemical Analyses. J. Gemmol. 2024, 39, 124–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.H.; Wen, J.B. Manganese-Rich Chromite in Myanmar Jadeite Jade: A Critical Source of Chromium and Manganese and Its Role in Coloration. Crystals 2025, 15, 704. [Google Scholar] [CrossRef]
- Coccato, A.; Karampelas, S.; Wörle, M.; Van Willigend, S.; Pétrequin, P. Gem quality and archeological green ‘jadeite jade’ versus ‘omphacite jade’(Article). J. Raman Spectrosc. 2014, 45, 1260–1265. [Google Scholar] [CrossRef]
- Fritsch, E.; Rossman, G.R. An Update on Color in Gems. Part 2: Colors Involving Multiple Atoms and Color Centers. Gems Gemol. 1988, 24, 3–15. [Google Scholar] [CrossRef]
- Pauling, L. The Principles Determining The Structure Of Complex Ionic Crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Geochemische Verteilungsgesetze und kosmische H?ufigkeit der Elemente. Naturwissenschaften 1930, 18, 999–1013. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Harlow, G.H.; Sorensen, S. Jade (Nephrite and Jadeitite) and Serpentinite: Metasomatic Connections. Int. Geol. Rev. 2005, 47, 113–146. [Google Scholar] [CrossRef]
- Zhang, M.; Groat, L.A.; Salje, E.K.H.; Beran, A. The origin of jadeitite-forming subduction-zone fluids: CL-guided SIMS oxygen-isotope and trace-element evidence. Am. Mineral. 2006, 91, 979–996. [Google Scholar]
- Tsujimori, T.; Harlow, G.E. Petrogenetic relationships between jadeitite and associated high-pressure and low-temperature metamorphic rocks in worldwide jadeitite localities: A review. Eur. J. Mineral. 2012, 24, 371–390. [Google Scholar] [CrossRef]
- Shi, G.H.; Wang, X.; Chu, B.B.; Cui, W.Y. Jadeite jade from Myanmar: Its texture and gemmological implications. J. Gemmol. 2010, 31, 185–195. [Google Scholar] [CrossRef]




| Points | Blue | White | |||
|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 1 |
| a* | −2.30 | −5.32 | −5.60 | −6.10 | −0.31 |
| b* | −3.29 | −2.10 | −2.89 | −2.04 | −0.63 |
| L* | 57.97 | 58.28 | 56.49 | 57.31 | 66.44 |
| Points | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Descriptions | Blue | White | ||||||||||||
| Core | Rim | |||||||||||||
| SiO2 | 57.50 | 57.96 | 58.46 | 57.53 | 57.32 | 57.24 | 57.93 | 57.20 | 60.26 | 59.45 | 59.19 | 57.40 | 56.70 | 59.25 |
| TiO2 | 0.05 | 0.07 | 0.27 | 0.25 | 0.14 | 0.17 | 0.05 | 0.00 | 0.01 | 0.00 | 0.17 | 0.07 | 0.03 | 0.00 |
| Al2O3 | 23.56 | 24.71 | 22.26 | 21.35 | 24.49 | 22.66 | 25.26 | 24.40 | 23.65 | 23.67 | 24.50 | 24.31 | 24.66 | 24.18 |
| FeO | 0.61 | 0.29 | 1.05 | 1.17 | 0.29 | 0.57 | 0.14 | 0.05 | 0.07 | 0.02 | 0.08 | 0.06 | 0.28 | 0.06 |
| MnO | 0.03 | 0.01 | 0.00 | 0.10 | 0.04 | 0.00 | 0.01 | 0.02 | 0.05 | 0.01 | 0.07 | 0.00 | 0.04 | 0.02 |
| MgO | 1.08 | 0.55 | 1.61 | 1.75 | 0.58 | 1.66 | 0.16 | 0.37 | 0.02 | 0.16 | 0.21 | 0.16 | 0.26 | 0.03 |
| CaO | 1.48 | 0.77 | 1.96 | 2.02 | 0.61 | 1.91 | 0.26 | 0.37 | 0.08 | 0.23 | 0.28 | 0.21 | 0.46 | 0.04 |
| Na2O | 14.13 | 14.37 | 13.72 | 14.54 | 14.47 | 12.80 | 14.50 | 16.19 | 15.50 | 15.49 | 14.62 | 14.76 | 15.80 | 15.03 |
| Cr2O3 | 0.02 | 0.04 | 0.03 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 |
| NiO | 0.03 | 0.00 | 0.00 | 0.01 | 0.00 | 0.09 | 0.04 | 0.00 | 0.03 | 0.03 | 0.03 | 0.03 | 0.00 | 0.01 |
| V2O5 | 0.05 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.06 | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | 0.05 | 0.00 |
| CuO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.10 | 0.00 | 0.00 | 0.06 | 0.13 |
| Total | 98.66 | 98.93 | 99.49 | 98.78 | 97.98 | 97.18 | 98.55 | 98.37 | 99.78 | 99.18 | 99.61 | 97.12 | 98.41 | 98.76 |
| Si | 1.98 | 1.98 | 2.00 | 1.99 | 1.98 | 2.00 | 1.98 | 1.97 | 2.04 | 2.02 | 2.01 | 1.99 | 1.96 | 2.02 |
| Ti | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Al | 0.96 | 1.00 | 0.90 | 0.87 | 1.00 | 0.93 | 1.02 | 0.99 | 0.94 | 0.95 | 0.98 | 1.00 | 1.00 | 0.97 |
| Fe | 0.02 | 0.01 | 0.03 | 0.03 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Mn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | 0.06 | 0.03 | 0.08 | 0.09 | 0.03 | 0.09 | 0.01 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 |
| Ca | 0.05 | 0.03 | 0.07 | 0.08 | 0.02 | 0.07 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.02 | 0.00 |
| Na | 0.94 | 0.95 | 0.91 | 0.98 | 0.97 | 0.86 | 0.96 | 1.08 | 1.02 | 1.02 | 0.96 | 0.99 | 1.06 | 0.99 |
| Cr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ni | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| V | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cu | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Quad | 0.05 | 0.02 | 0.07 | 0.07 | 0.02 | 0.07 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Jd | 0.93 | 0.95 | 0.03 | 0.90 | 0.97 | 0.91 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 |
| Ae | 0.02 | 0.03 | 0.90 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 |
| Points | 1-1 | 1-2 | 1-3 | 3 | 5 | 2 | 4-1 | 4-2 |
|---|---|---|---|---|---|---|---|---|
| Descriptions | Blue | White | ||||||
| Mg | 10,518.17 | 12,220.16 | 11,007.03 | 9782.20 | 7408.06 | 806.51 | 304.38 | 2471.81 |
| Ca | 19,664.29 | 19,212.02 | 17,929.73 | 16,066.03 | 15,992.17 | 3313.97 | 2679.11 | 3174.62 |
| Ti | 1232.93 | 1062.32 | 1080.19 | 1414.18 | 700.31 | 221.48 | 64.11 | 286.68 |
| V | 42.42 | 25.85 | 20.99 | 11.18 | 17.76 | 17.88 | 14.16 | 11.34 |
| Cr | 111.26 | 67.65 | 56.35 | 29.66 | 46.52 | 47.58 | 37.85 | 29.94 |
| Mn | 71.82 | 45.35 | 47.79 | 25.08 | 33.13 | 33.75 | 26.67 | 29.94 |
| Fe | 3964.46 | 6445.96 | 6836.04 | 12,960.80 | 22,757.70 | 1783.82 | 6465.05 | 1330.49 |
| Ni | 578.21 | 363.49 | 307.70 | 182.34 | 316.18 | 280.02 | 257.78 | 177.36 |
| Cu | 49.03 | 28.38 | 25.78 | 13.48 | 22.94 | 22.56 | 17.14 | 13.44 |
| La | 0.300 | 0.199 | 0.289 | 0.105 | 0.194 | 0.182 | 0.114 | 9.380 |
| Ce | 0.290 | 0.157 | 0.258 | 0.116 | 0.094 | 0.121 | 0.142 | 27.160 |
| Pr | 0.168 | 0.081 | 0.063 | 0.104 | 0.076 | 0.042 | 0.063 | 0.063 |
| Nd | 1.050 | 0.317 | 0.272 | 0.219 | 0.630 | 0.390 | 0.160 | 0.310 |
| Sm | 1.790 | 0.960 | 0.790 | 0.460 | 0.520 | 0.460 | 0.430 | 0.225 |
| Eu | 0.360 | 0.190 | 0.222 | 0.125 | 0.094 | 0.223 | 0.080 | 0.072 |
| Gd | 0.550 | 0.620 | 0.720 | 0.235 | 0.460 | 0.730 | 0.380 | 0.330 |
| Tb | 0.150 | 0.190 | 0.082 | 0.027 | 0.073 | 0.067 | 0.066 | 0.053 |
| Dy | 0.720 | 0.540 | 0.255 | 0.146 | 0.156 | 0.260 | 0.240 | 0.450 |
| Ho | 0.160 | 0.120 | 0.080 | 0.024 | 0.033 | 0.077 | 0.061 | 0.052 |
| Er | 0.730 | 0.270 | 0.390 | 0.065 | 0.113 | 0.259 | 0.107 | 0.103 |
| Tm | 0.163 | 0.056 | 0.063 | 0.013 | 0.017 | 0.048 | 0.049 | 0.012 |
| Yb | 0.980 | 0.420 | 0.350 | 0.078 | 0.098 | 0.288 | 0.264 | 0.162 |
| Lu | 0.350 | 0.193 | 0.077 | 0.038 | 0.024 | 0.064 | 0.034 | 0.072 |
| Ion Type | CN | Ionic Radius (Å) |
|---|---|---|
| Ca2+ | 6 | 1 |
| Fe2+ | 6 | 0.78 |
| Ti4+ | 6 | 0.605 |
| V4+ | 6 | 0.58 |
| Mg2+ | 6 | 0.72 |
| Al3+ | 6 | 0.535 |
| Na+ | 6 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, S.; Zhang, Y.; Shi, G.; Long, T. Color Mechanism of Blue Myanmar Jadeite Jade: The Role of Trace Elements and Mineralogical Characteristics. Crystals 2025, 15, 843. https://doi.org/10.3390/cryst15100843
Dai S, Zhang Y, Shi G, Long T. Color Mechanism of Blue Myanmar Jadeite Jade: The Role of Trace Elements and Mineralogical Characteristics. Crystals. 2025; 15(10):843. https://doi.org/10.3390/cryst15100843
Chicago/Turabian StyleDai, Shangzhan, Yu Zhang, Guanghai Shi, and Taafee Long. 2025. "Color Mechanism of Blue Myanmar Jadeite Jade: The Role of Trace Elements and Mineralogical Characteristics" Crystals 15, no. 10: 843. https://doi.org/10.3390/cryst15100843
APA StyleDai, S., Zhang, Y., Shi, G., & Long, T. (2025). Color Mechanism of Blue Myanmar Jadeite Jade: The Role of Trace Elements and Mineralogical Characteristics. Crystals, 15(10), 843. https://doi.org/10.3390/cryst15100843







