Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker
Abstract
1. Introduction
2. Materials and Methods
2.1. General
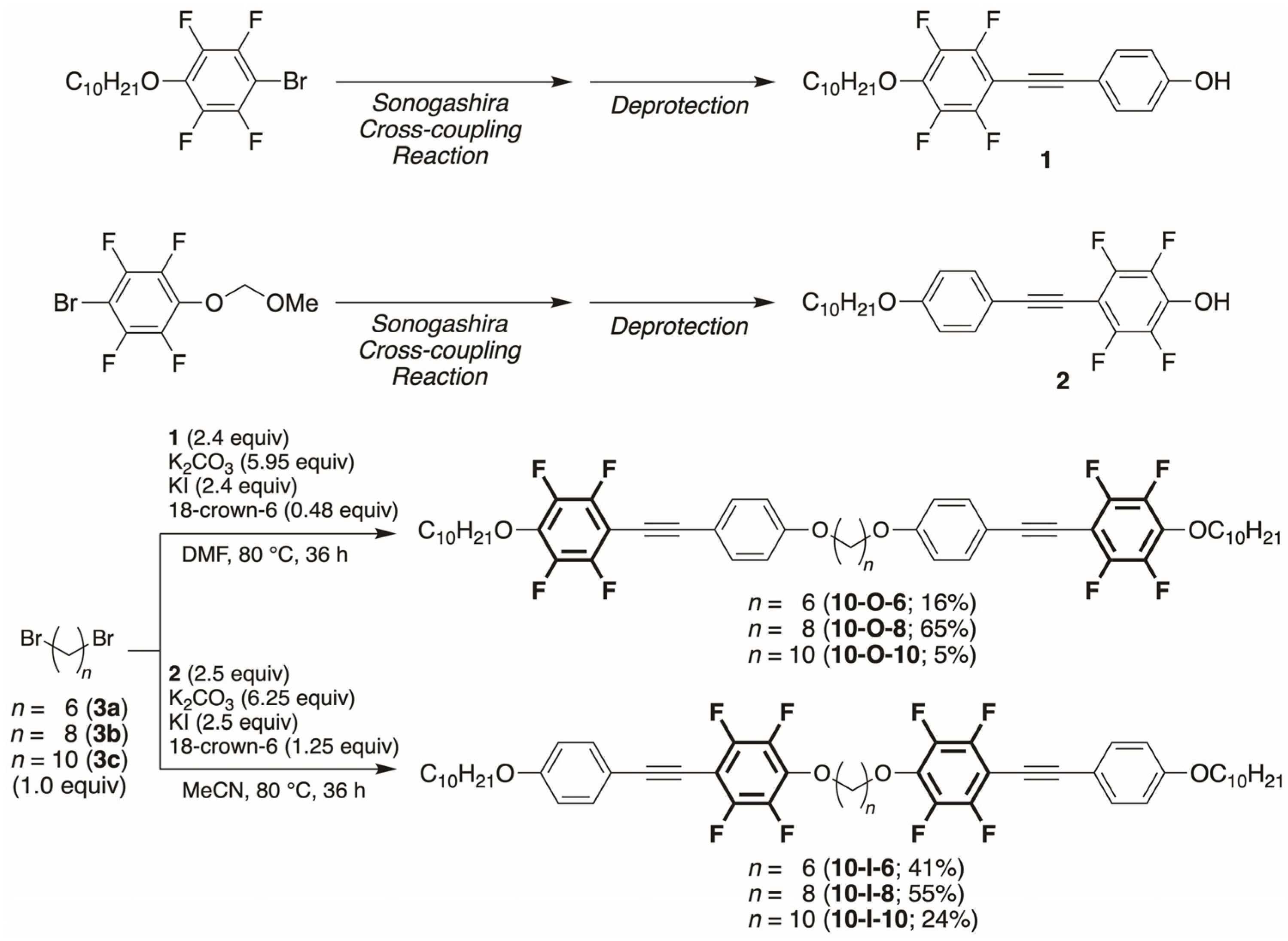
2.2. Typical Synthetic Procedure of Outer Aromatic Ring-Fluorinated Mesogen Dimer 10-O-6 with Decyloxy Chains at Both Ends
2.2.1. 1,6-Bis(4-[2-(2,3,5,6-tetrafluoro-4-decyloxyphenyl)ethyn-1-yl]phenoxy)hexane (10-O-6)
2.2.2. 1,8-Bis(4-[2-(2,3,5,6-tetrafluoro-4-decyloxyphenyl)ethyn-1-yl]phenoxy)octane (10-O-8)
2.2.3. 1,10-Bis(4-[2-(2,3,5,6-tetrafluoro-4-decyloxyphenyl)ethyn-1-yl]phenoxy)decane (10-O-10)
2.3. Typical Synthetic Procedure of Inner Aromatic Ring-Fluorinated Mesogen Dimer 10-I-6 with Decyloxy Chains at Both Ends
2.3.1. 1,6-Bis(4-[2-(4-decyloxyphenyl)ethyn-1-yl]2,3,5,6-tetrafluorophenoxy)hexane (10-I-6)
2.3.2. 1,8-Bis(4-[2-(4-decyloxyphenyl)ethyn-1-yl]2,3,5,6-tetrafluorophenoxy)octane (10-I-8)
2.3.3. 1,10-Bis(4-[2-(4-decyloxyphenyl)ethyn-1-yl]2,3,5,6-tetrafluorophenoxy)decane (10-I-10)
2.4. Density Functional Theory (DFT) Calculation
2.5. Phase-Transition Behavior
2.6. Photophysical Behavior
3. Results and Discussion
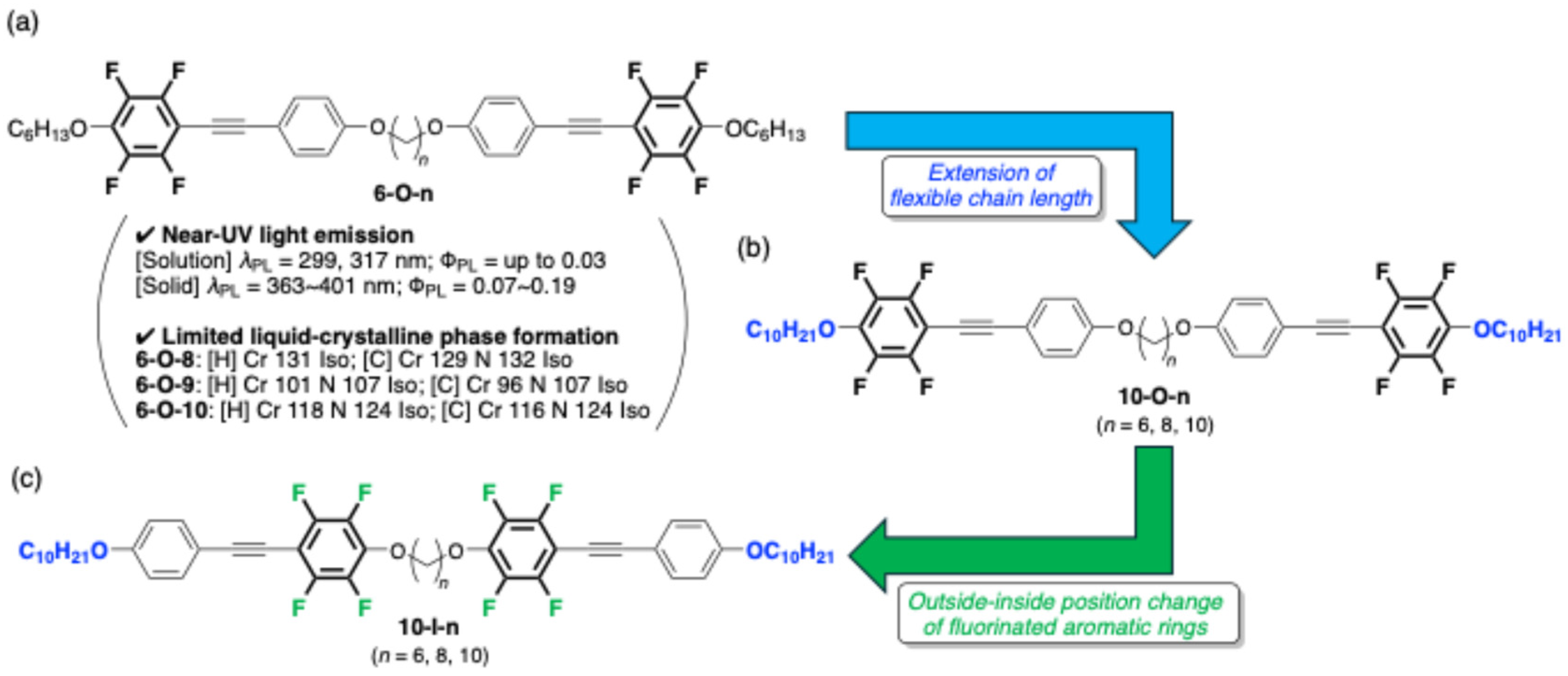
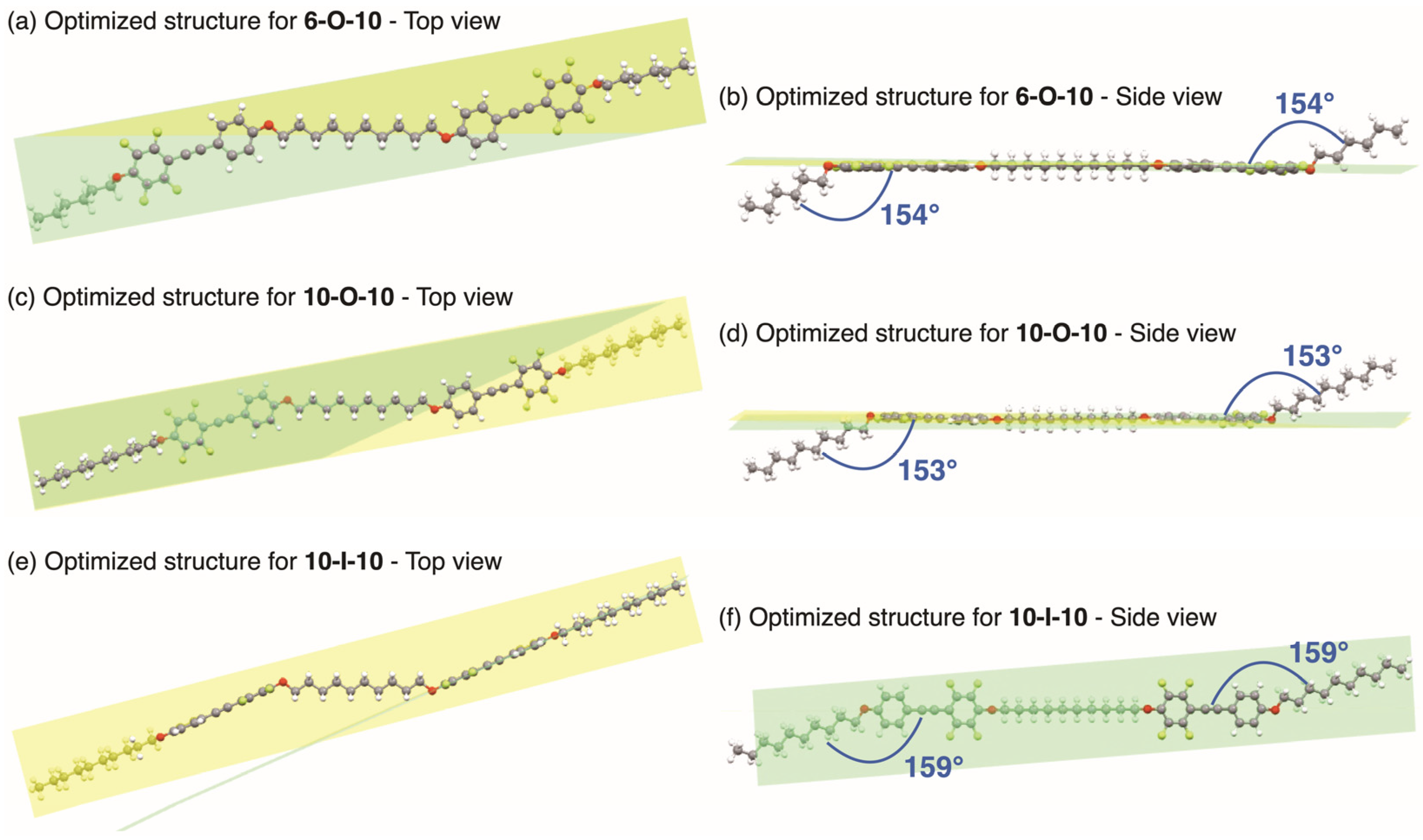
3.1. Molecular Design
3.2. Thermophysical Properties
3.3. Photophysical Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimizu, M.; Sakurai, T. Organic fluorophores that emit ultraviolet light in the aggregated state. Aggregate 2022, 3, e144. [Google Scholar] [CrossRef]
- Chen, S.; Xu, H. Electroluminescent materials toward near ultraviolet region. Chem. Soc. Rev. 2021, 50, 8639–8668. [Google Scholar] [CrossRef]
- Wang, M.; Gu, X.; Chen, J.; Yang, X.; Cheng, P.; Xu, K. A novel near-infrared colorimetric-fluorescent probe for hydrogen sulfide and application in bioimaging. J. Photochem. Photobiol. A 2023, 437, 114438. [Google Scholar] [CrossRef]
- Leem, Y.C.; Myoung, N.; Hong, S.H.; Jeong, S.; Seo, O.; Park, S.J.; Yim, S.Y.; Kim, J.H. Near-UV light emitting diode with on-chip photocatalysts for purification applications. Nanoscale Adv. 2022, 4, 3585–3591. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Zhang, F.Q.; Zhang, X.M. Single component lanthanide hybrids based on metal–organic framework for near-ultraviolet white light LED. ACS Appl. Mater. Interfaces 2016, 8, 24123–24130. [Google Scholar] [CrossRef]
- Li, H.G.; Wu, G.; Chen, H.Z.; Wang, M. Polymer/ZnO hybrid materials for near-UV sensors with wavelength selective response. Sens. Actuators B 2011, 160, 1136–1140. [Google Scholar] [CrossRef]
- Yamada, S.; Uto, E.; Sakurai, T.; Konno, T. Development of thermoresponsive near-ultraviolet photoluminescent liquid crystals using hexyloxy-terminated fluorinated tolane dimers connected with an alkylene spacer. J. Mol. Liq. 2022, 362, 119755. [Google Scholar] [CrossRef]
- Chen, R.; Tang, J.; Mao, Z.; Chen, X.; Chen, P.; An, Z. High-Δn tolane-liquid crystal diluters with low melting point and low rotational viscosity. J. Mol. Liq. 2024, 398, 124312. [Google Scholar] [CrossRef]
- Hamdi, R.; Khalfallah, C.B.; Soltani, T. Synthesis and study of physicochemical properties of relatively high birefringence liquid crystals: Tolane-type with symmetric alkoxy side groups. J. Mol. Liq. 2020, 310, 113205. [Google Scholar] [CrossRef]
- Takatsu, H. Development and industrialization of liquid crystalline tolane. J. Syn. Org. Chem. Jpn. 1999, 57, 629–632. [Google Scholar] [CrossRef]
- Ferrante, C.; Kensy, U.; Dick, B. Does diphenylacetylene (tolan) fluoresce from its second excited singlet state? Semiempirical MO calculations and fluorescence quantum yield measurements. J. Phys. Chem. 1993, 97, 13457–13463. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Lim, E.C. Nature of the ‘dark’ state in diphenylacetylene and related molecules: State switch from the linear ππ* state to the bent πσ* state. Chem. Phys. Lett. 2004, 387, 352–355. [Google Scholar] [CrossRef]
- Saltiel, J.; Kumar, V.K.R. Photophysics of diphenylacetylene: Light from the “dark state”. J. Phys. Chem. A 2012, 116, 10548–10558. [Google Scholar] [CrossRef]
- Menning, S.; Krämer, M.; Duckworth, A.; Rominger, F.; Beeby, A.; Dreuw, A.; Bunz, U.H.F. Bridged tolanes: A twisted tale. J. Org. Chem. 2014, 79, 6571–6578. [Google Scholar] [CrossRef]
- Kozhemyakin, Y.; Krämer, M.; Rominger, F.; Dreuw, A.; Bunz, U.H.F. A tethered tolane: Twisting the excited state. Chem. Eur. J. 2018, 24, 15219–15222. [Google Scholar] [CrossRef]
- Tong, J.; Wang, Y.J.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Crystallization-induced emission enhancement of a simple tolane-based mesogenic luminogen. J. Phys. Chem. C 2015, 119, 21875–21881. [Google Scholar] [CrossRef]
- Zang, Y.; Li, Y.; Li, B.; Li, H.; Yang, Y. Light emission properties and self-assembly of a tolane-based luminogen. RSC Adv. 2015, 5, 38690–38695. [Google Scholar] [CrossRef]
- Yamada, S.; Kobayashi, K.; Morita, M.; Konno, T. D–π–A-type fluorinated tolanes witha diphenylamino group: Crystal polymorphism formation and photophysical behavior. CrystEngComm 2022, 4, 936–941. [Google Scholar] [CrossRef]
- Yamada, S.; Kobayashi, K.; Konno, T. Development of yellow-to-orange photoluminescence molecules based on alterations in the donor units of fluorinated tolanes. Molecules 2022, 27, 5782. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yamada, S.; Morita, M.; Sakurai, T.; Yasui, M.; Konno, T. Solid-state red fluorescence of intramolecularly ring-fused donor–π–acceptor-type fluorinated diphenylacetylenes achieved by enhancing intramolecular charge transfer properties. J. Mater. Chem. C 2025, 13, 1369–1377. [Google Scholar] [CrossRef]
- Sorai, M.; Saito, K. Alkyl chains acting as entropy reservoir in liquid crystalline materials. Chem. Rec. 2003, 3, 29–39. [Google Scholar] [CrossRef]
- Wight, C.D.; Xiao, Q.; Wagner, H.R.; Hernandez, E.A.; Martin, E.; Lynch, V.M.; Iverson, B.L. Influence of the alkyl side chain length on the assembly and thermochromic solid-state properties in symmetric and asymmetric monoalkoxynaphthalene–naphthalimide donor–acceptor dyads. Cryst. Growth Des. 2022, 22, 7363–7373. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16, Revision B.01; Gaussian, Incorporated: Wallingford, CT, USA, 2016. [Google Scholar]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF density functional for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jensen, J.H. Improving the efficiency and convergence of geometry optimization with the polarizable continuum model: New energy gradients and molecular surface tessellation. J. Comput. Chem. 2004, 25, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Gerber, R.B.; Buch, V.; Ratner, M.A. Time-dependent self-consistent field approximation for intramolecular charge transfer. I. Formulation and application to dissociation of van der Waals molecules. J. Chem. Phys. 1982, 77, 3022–3030. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
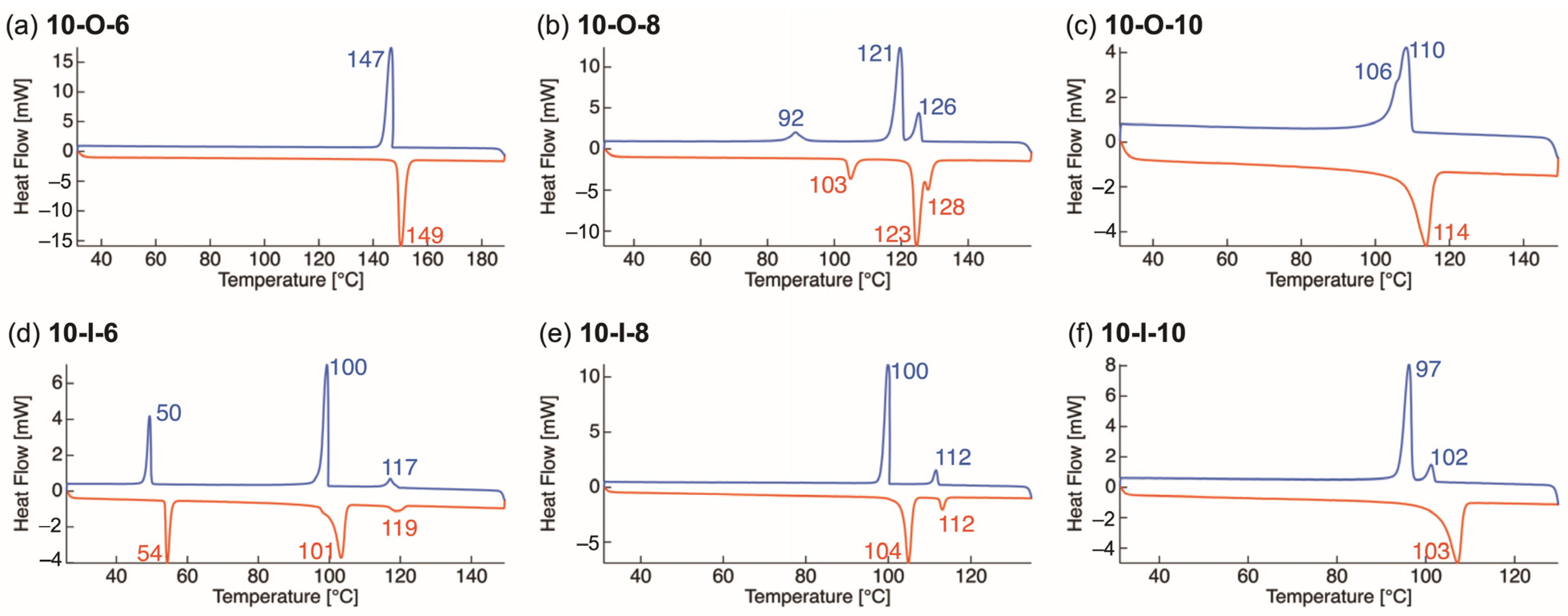


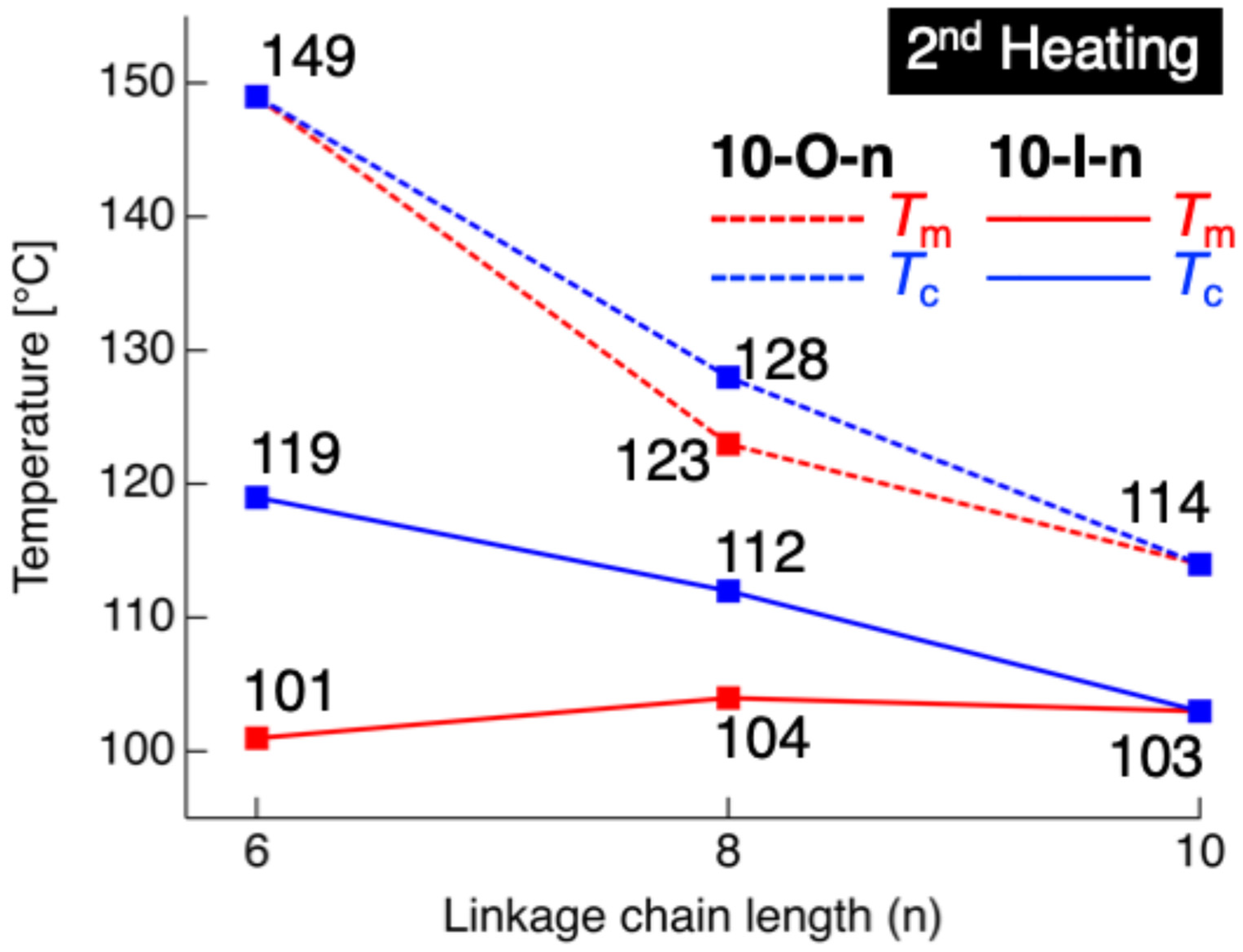


| Compd. | Phase Sequence and Temperature [°C] a (Phase-Transition Enthalpy [kJ mol−1]) a | Compd. | Phase Sequence and Temperature [°C] a (Phase-Transition Enthalpy [kJ mol−1]) a |
|---|---|---|---|
| 10-O-6 | [H] Cr 149 (80.2) Iso | 10-I-6 | [H] Cr1 54 (20.1) Cr2 101 (46.4) N 119 (4.3) Iso |
| [C] Cr 147 (−83.4) Iso | [C] Cr1 50 (−19.7) Cr2 100 (−49.6) N 117 (−5.0) Iso | ||
| 10-O-8 | [H] Cr1 103 (10.4) Cr2 123 (64.4) SmA 128 (–) Iso | 10-I-8 | [H] Cr 104 (44.7) N 112 (4.8) Iso |
| [C] Cr1 92 (−8.7) Cr2 121 (−51.3) SmA 126 (−13.9) Iso | [C] Cr 100 (−47.8) N 112 (−5.6) Iso | ||
| 10-O-10 | [H] Cr1 114 (72.1) Iso | 10-I-10 | [H] Cr 103 (71.2) Iso |
| [C] Cr1 106 (–) b Cr2 110 (−71.4) Iso | [C] Cr 97 (−56.8) N 102 (−7.1) Iso |
| Compound | λabs [nm] a (ε [103, L mol−1 cm−1]) | λPL [nm] b (ΦPL) c | τave [ns] | τ1 [ns] | τ2 [ns] | kr [ns−1] d | knr [ns−1] e | knr/kr |
|---|---|---|---|---|---|---|---|---|
| 10-O-6 | 300 (80.6), 316 (74.0) | 358 (0.033) | 1.28 | 0.83 | 2.43 | 0.026 | 0.755 | 29.0 |
| 10-O-8 | 300 (63.9), 316 (58.3) | 359 (0.032) | 0.77 | – | – | 0.041 | 1.26 | 30.7 |
| 10-O-10 | 299 (64.9), 316 (58.7) | 358, 418sh (0.035) | 0.85 | – | – | 0.041 | 1.14 | 27.8 |
| 10-I-6 | 300 (24.7), 317 (24.0) | 360, 384sh (0.028) | 1.06 | – | – | 0.026 | 0.917 | 35.3 |
| 10-I-8 | 300 (35.8), 316 (32.4) | 358 (0.034) | 0.79 | – | – | 0.043 | 1.22 | 28.3 |
| 10-I-10 | 300 (61.9), 316 (55.7) | 357 (0.033) | 0.86 | – | – | 0.038 | 1.12 | 29.5 |
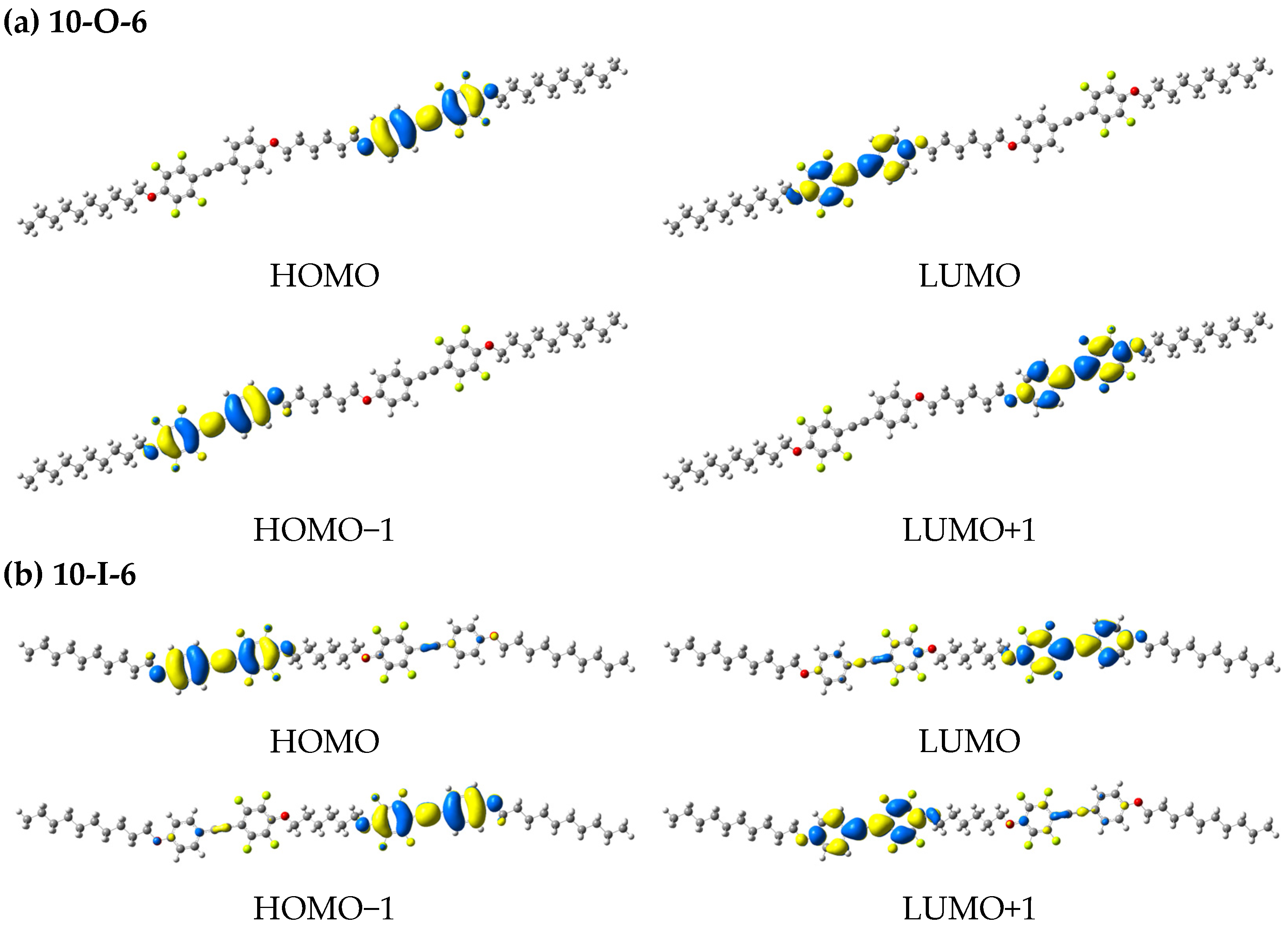
| Compound | HOMO−1/ HOMO [eV] | LUMO/ LUMO+1 [eV] | ΔEH-L [eV] | Theoretical Electronic Transition (Population) | Calculated λabs [nm] | Oscillator Strength (f) |
|---|---|---|---|---|---|---|
| 10-O-6 | −7.19/−7.19 | −0.96/−0.96 | 6.23 | HOMO−1 → LUMO (47.3%) HOMO → LUMO+1 (47.5%) | 295 | 3.3737 |
| 10-I-6 | −7.20/−7.19 | −0.97/−0.96 | 6.23 | HOMO−1 → LUMO (34.9%) HOMO−1 → LUMO+1 (10.7%) HOMO → LUMO (11.0%) HOMO → LUMO+1 (38.4%) | 295 | 3.3645 |
| 10-O-10 | −7.32/−7.32 | −0.93/−0.93 | 6.39 | HOMO−1 → LUMO+1 (42.5%) HOMO → LUMO (51.1%) | 284 | 3.2167 |
| 10-I-10 | −7.32/−7.31 | −0.93/−0.93 | 6.39 | HOMO−1→ LUMO+1 (47.0%) HOMO → LUMO (47.1%) | 284 | 3.2227 |
| Compd. | λPL [nm] | ΦPL a | τave [ns] | τ1 [ns] | τ2 [ns] | kr [ns–1] b | knr [ns–1] c | knr/kr |
|---|---|---|---|---|---|---|---|---|
| 10-O-6 | 358 | 0.14 | 0.75 | – | – | 0.187 | 1.15 | 6.15 |
| 10-O-8 | 358, 377, 396sh | 0.28 | 1.23 | – | – | 0.228 | 0.585 | 2.56 |
| 10-O-10 | 360, 376, 398sh | 0.14 | 1.49 | – | – | 0.094 | 0.577 | 6.14 |
| 10-I-6 | 355, 372sh | 0.11 | 1.25 | 0.82 | 2.60 | 0.088 | 0.715 | 8.12 |
| 10-I-8 | 355, 372sh | 0.051 | 0.83 | – | – | 0.061 | 1.14 | 18.7 |
| 10-I-10 | 372 | 0.091 | 2.36 | 1.46 | 3.80 | 0.038 | 0.385 | 10.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inui, S.; Kitaoka, H.; Eguchi, Y.; Yasui, M.; Konno, T.; Yamada, S. Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker. Crystals 2025, 15, 840. https://doi.org/10.3390/cryst15100840
Inui S, Kitaoka H, Eguchi Y, Yasui M, Konno T, Yamada S. Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker. Crystals. 2025; 15(10):840. https://doi.org/10.3390/cryst15100840
Chicago/Turabian StyleInui, Sorato, Hayato Kitaoka, Yuto Eguchi, Motohiro Yasui, Tsutomu Konno, and Shigeyuki Yamada. 2025. "Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker" Crystals 15, no. 10: 840. https://doi.org/10.3390/cryst15100840
APA StyleInui, S., Kitaoka, H., Eguchi, Y., Yasui, M., Konno, T., & Yamada, S. (2025). Design of Near-UV Photoluminescent Liquid-Crystalline Dimers: Roles of Fluorinated Aromatic Ring Position and Flexible Linker. Crystals, 15(10), 840. https://doi.org/10.3390/cryst15100840







