Impact of Laser Power on Electrochemical Performance of CeO2/Al6061 Alloy Through Selective Laser Melting (SLM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Process Parameters
2.2. Surface Roughness and Porosity Measurement
2.3. Self-Corrosion Test
2.4. Electrochemical Test
3. Results and Discussion
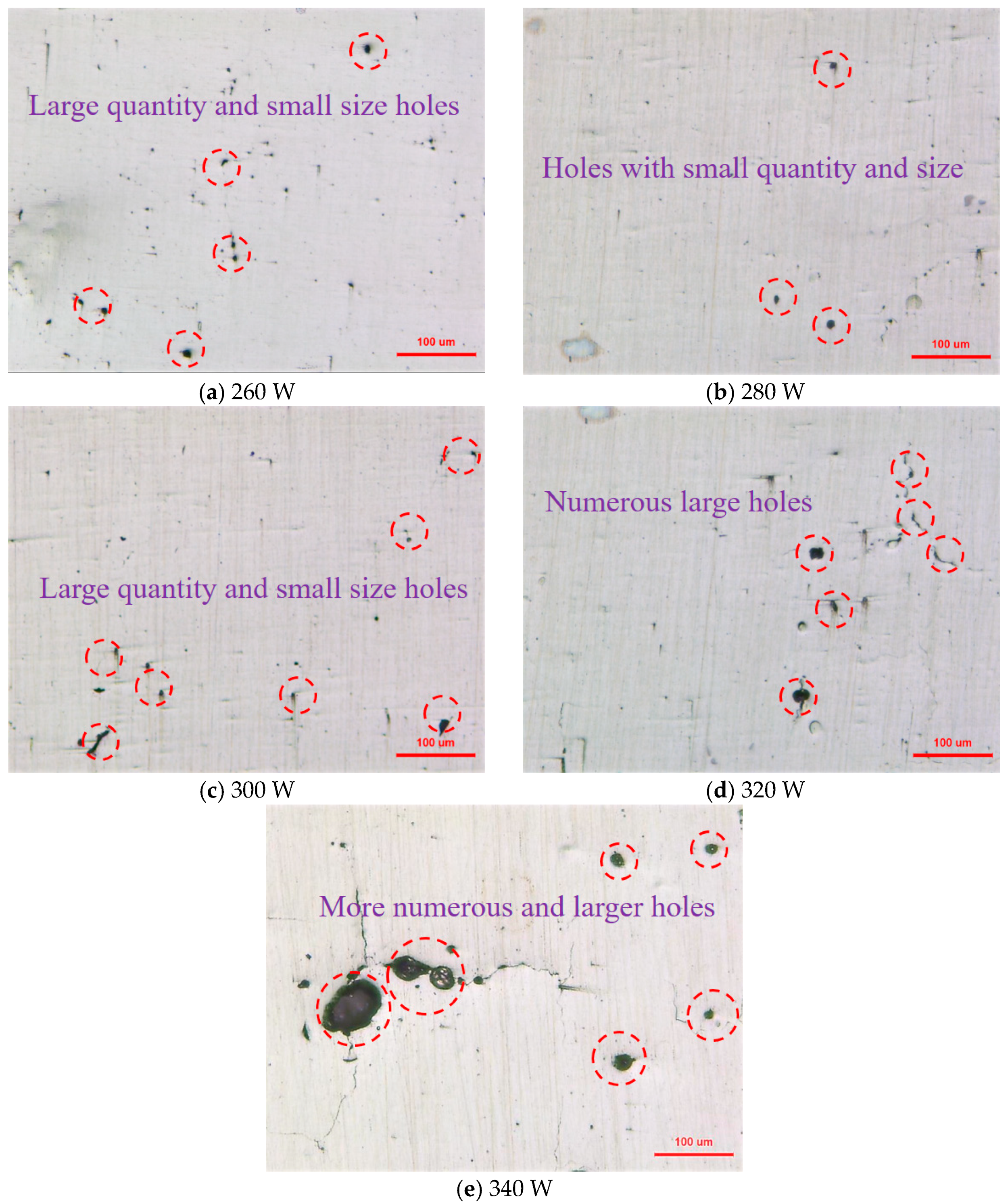
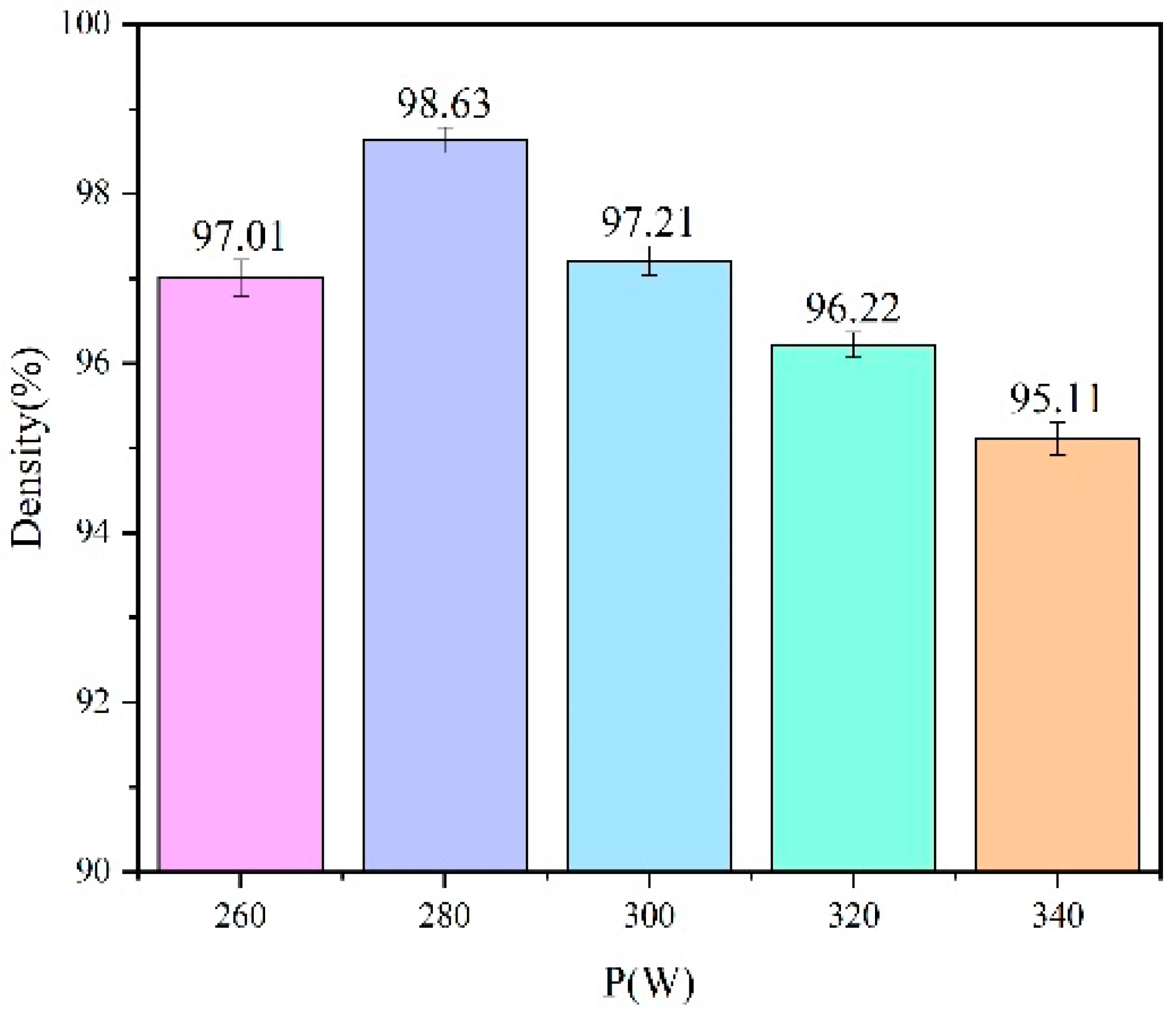
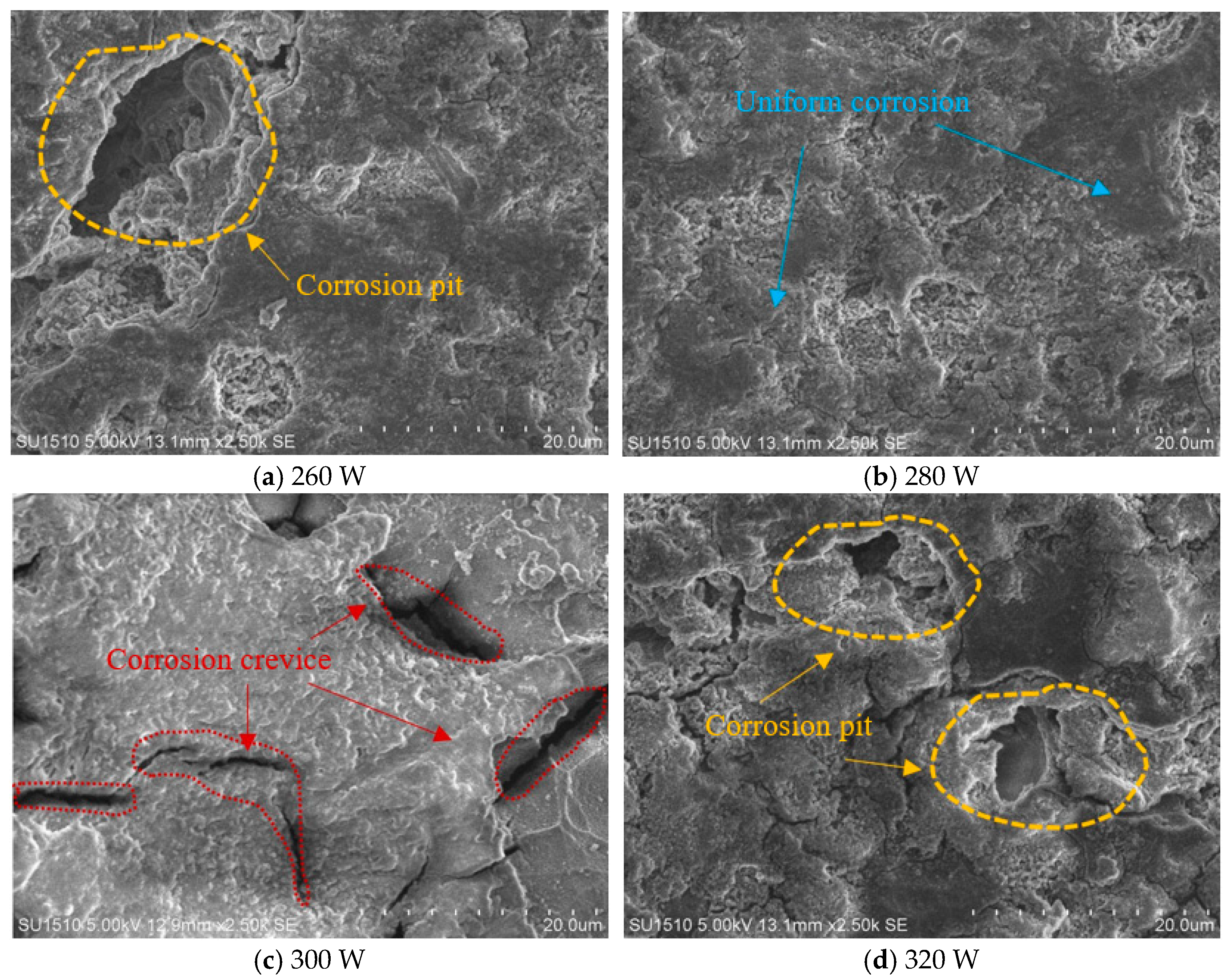
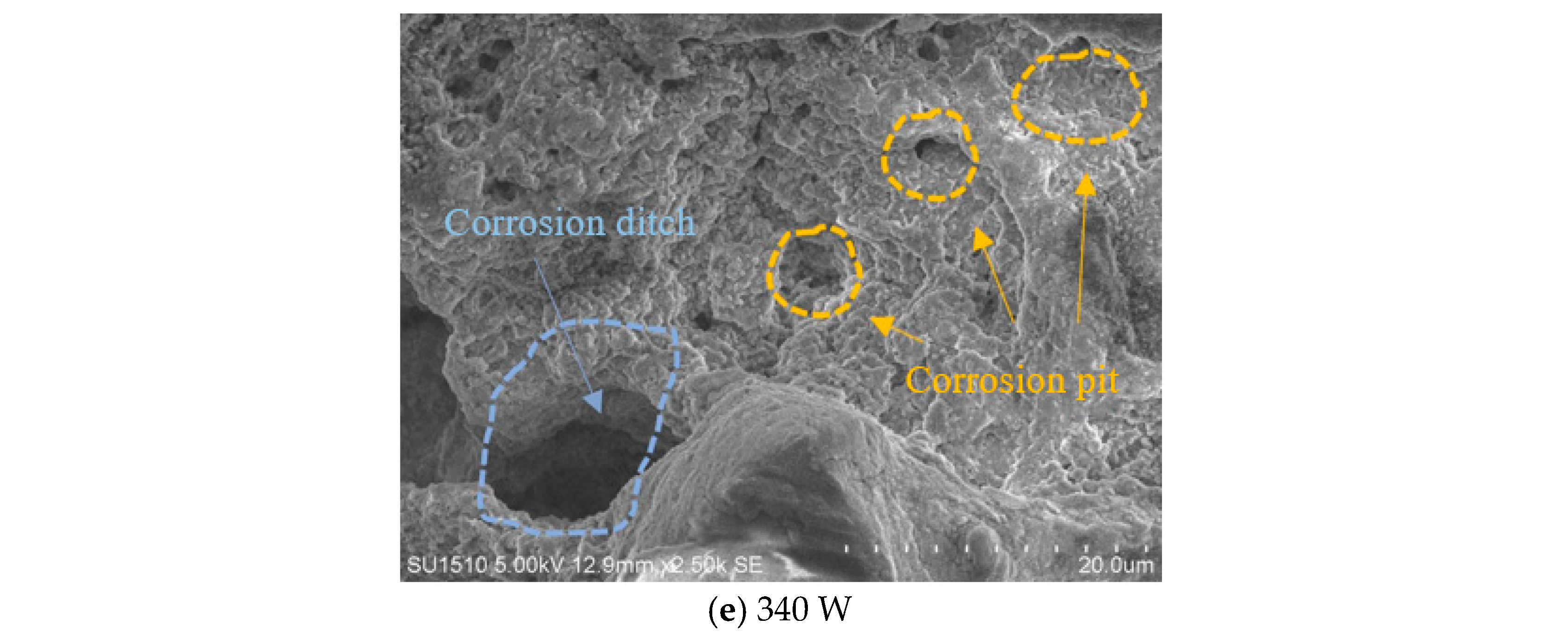
3.1. Surface Morphology and Density
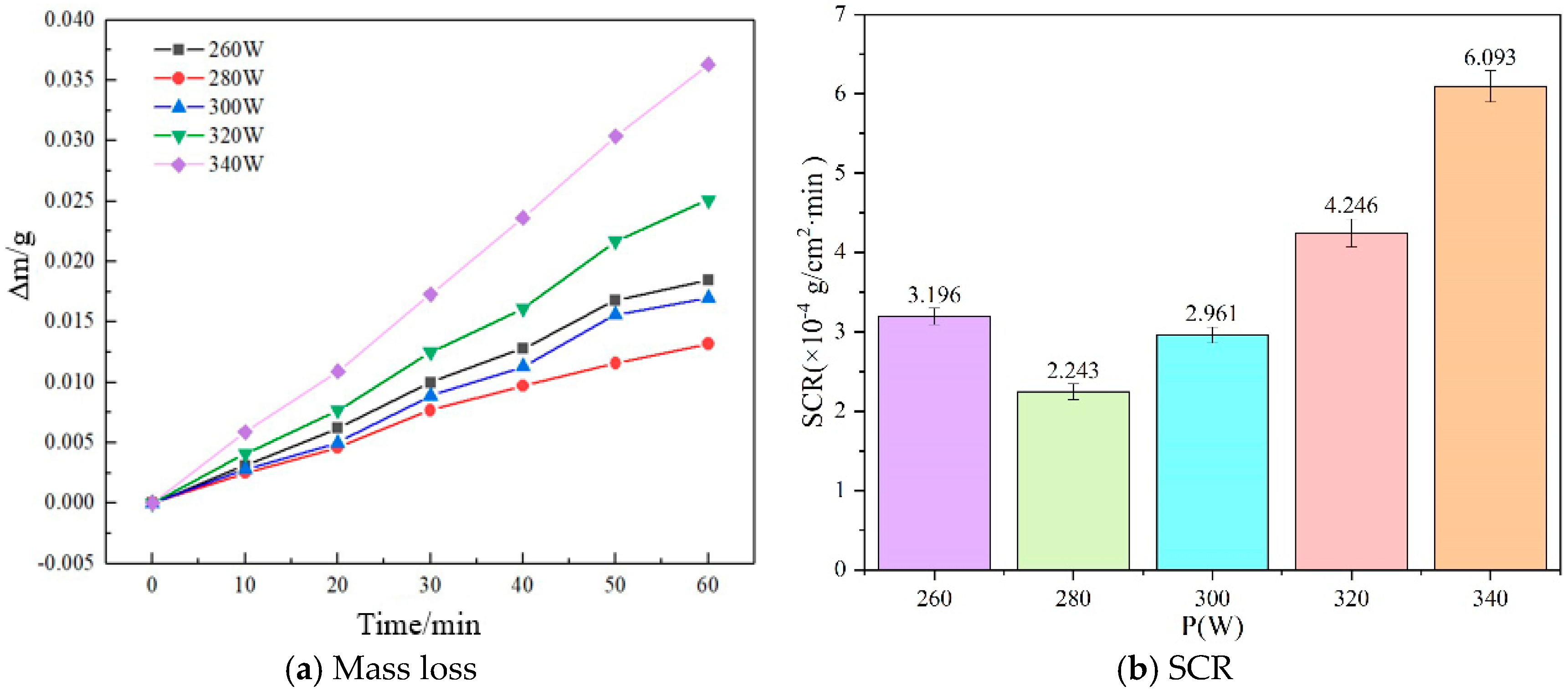
3.2. Self-Corrosion Rate
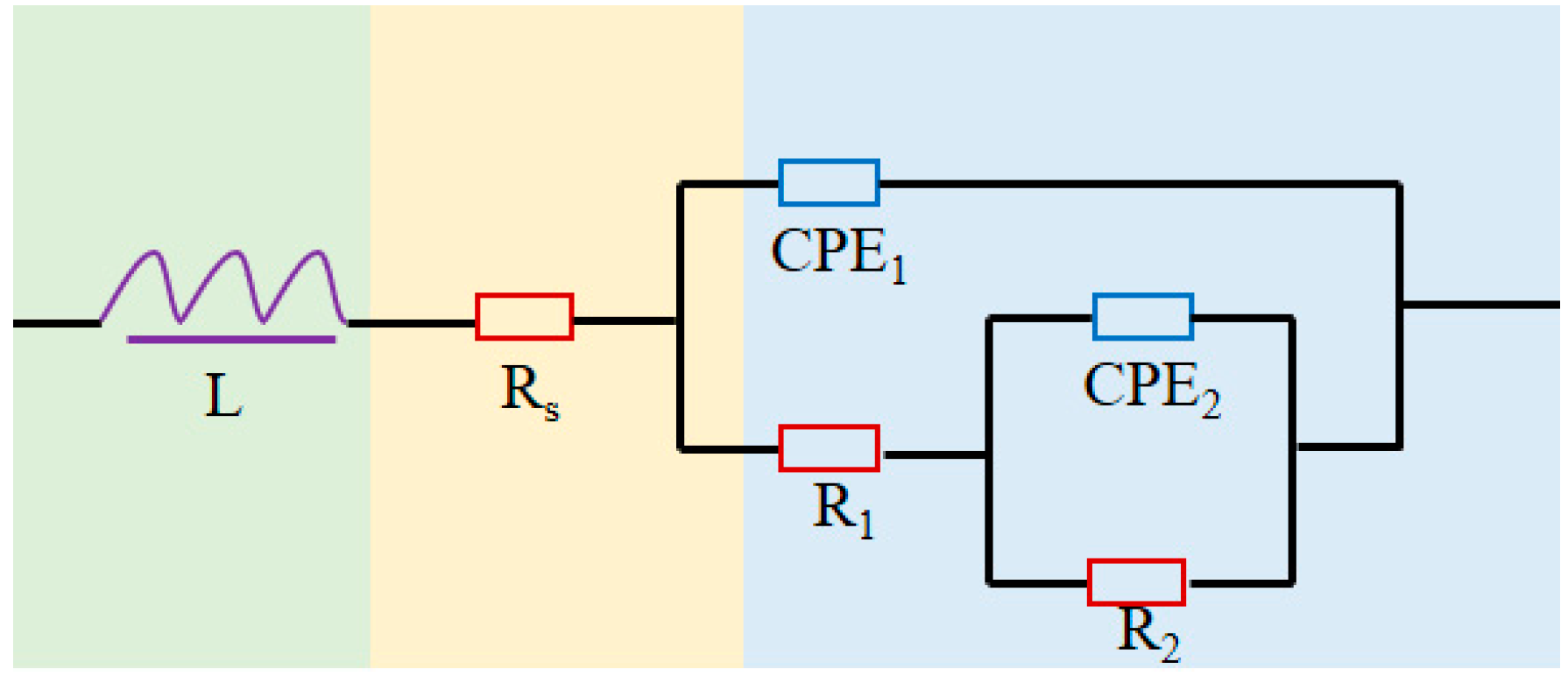
3.3. Electrochemical Behavior
4. Conclusions
- (1)
- As the laser power rises, the surface roughness of the sample initially decreases but then increases, and correspondingly, the sample’s density exhibits a trend of first rising and then falling. At a laser power of 280 W, the sample achieves optimal forming quality, with a surface roughness of 10.31 μm and a density of 98.63%, respectively.
- (2)
- As the laser power is increased, the self-corrosion rate of the sample exhibits a trend of initially declining and then rising. This is related to the surface quality of the sample. Under appropriate laser power, the surface of the sample has fewer pores and higher density, resulting in improved corrosion resistance.
- (3)
- Under different laser powers, the electrochemical behavior exhibits the same trend as the self-corrosion rate. As the laser power increases, OCP shows a trend of first negative shift and then positive shift. At a laser power of 280 W, the sample attains the most negative corrosion potential and exhibits the lowest corrosion current density, suggesting a high level of electrochemical activity.
- (4)
- In summary, variations in laser power influence the forming quality of the sample, resulting in alterations to its self-corrosion rate and electrochemical characteristics. Specifically, when the laser power is set to 280 W, the sample achieves the best forming quality, accompanied by a reduced self-corrosion rate and more stable electrochemical behavior.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nandhakumar, R.; Venkatesan, K. A process parameters review on selective laser melting-based additive manufacturing of single and multi-material: Microstructure, physical properties, tribological, and surface roughness. Mater. Today Commun. 2023, 35, 105538. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, K.; Pang, C.Y. Study on the effects of the processing parameters on the single tracks and the block support structures fabricated by selective laser melting. J. Laser Appl. 2024, 36, 012024. [Google Scholar] [CrossRef]
- Liu, W.; Huang, S.; Du, S.; Gao, T.; Zhang, Z.; Chen, X.; Huang, L. The effects of heat treatment on microstructure and mechanical properties of selective laser melting 6061 aluminum alloy. Micromachines 2022, 13, 1059. [Google Scholar] [CrossRef]
- Jerrard, P.; Hao, L.; Dadbakhsh, S.; Evans, K. Consolidation behaviour and microstructural characteristics of Al and a mixture of Al-Cu alloy powders following selective laser melting processing. Laser Eng. 2012, 22, 371. [Google Scholar]
- Carter, L.N.; Martin, C.; Withers, P.J. The influence of the laser scan strategy on grain structure and cracking behaviour in SLM powder-bed fabricated nickel superalloy. J. Alloys Compd. 2014, 615, 338. [Google Scholar] [CrossRef]
- Gürkan, K.; Fatih, Y.; Ilyas, H. Characterization of the structural and tribological properties of medical Ti6Al4V alloy produced in different production parameters using selective laser melting. 3D Print. Addit. Manuf. 2019, 6, 253–261. [Google Scholar]
- Xiao, X.; Liu, Y.; Cheng, W.; Liu, X. Microstructure and mechanical property of titanium-containing 6061 aluminum alloy prepared by selective laser melting. J. Mater. Eng. 2023, 51, 36. [Google Scholar]
- Zhang, X.Z.; Chen, L.; Zhou, J.; Ren, N.F. Simulation and experimental studies on process parameters, microstructure and mechanical properties of selective laser melting of stainless steel 316L. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 402. [Google Scholar] [CrossRef]
- Niaraki, M.; Torabian, H.; Rousta, S. Experimental Investigation on low cycle Fatigue Behavior of Al6061/SiC composite Fabricated by Friction Stir Processing. Technol. Aerosp. Eng. 2023, 14, 157. [Google Scholar]
- Bisht, M.; Gaur, V.; Singh, I. On mechanical properties of SLM Al-Si alloy: Role of heat treatment-induced evolution of silicon morphology. Mater. Sci. Eng. A 2022, 10, 144157. [Google Scholar] [CrossRef]
- Desai, V.; Badheka, V.; Zala, A. Fabrication of Al6061/Ti3AlC2 MAX phase surface composite by friction stir processing and investigation of wear properties. Tribol. Int. 2024, 14, 195. [Google Scholar] [CrossRef]
- Nanjunath, B.; Mishra, A.; Jayaprakash, H.S.; Karthik, D.V.; Kiran, N. Studies on Inconel 625 and Al6061 bimetallic joints produced by CMT-based welding process. Mater. Lett. 2024, 355, 1355431. [Google Scholar]
- Beal, M.; Azzougagh, M.N.; Pourchez, J.; Bertrand, P.; Cabrol, E.; Si-Mohand, H.; Keller, F.X. Effect of powder reuse on physical, chemical and toxicological properties of 6061-Zr aluminum alloy processed by laser powder bed fusion (L-PBF). Master Tech. 2023, 111, 103. [Google Scholar] [CrossRef]
- Carluccio, D.; Bermingham, M.J.; Zhang, Y.; StJohn, D.H.; Yang, K.; Rometsch, P.A.; Wu, X.; Dargusch, M.S. Grain refinement of laser remelted Al-7Si and 6061 aluminium alloys with Tibor and scandium additions. J. Manuf. Process. 2018, 35, 715. [Google Scholar] [CrossRef]
- Ramoni, M.; Shanmugam, R.; Ross, N.S. An experimental investigation of hybrid manufactured SLM based Al-Si10-Mg alloy under mist cooling conditions. J. Manuf. Process. 2021, 08, 045. [Google Scholar] [CrossRef]
- Aridi, R.; DiNova, V.; Zhang, T.Y.; Karna, S.; Yuan, L.; Krentz, T.; Hitchcock, D.; Gross, A.J. Characterization of defects in additively manufactured materials from mechanical properties. Mater. Sci. Eng. A 2024, 898, 146390. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Duan, W. Effect of laser power on the forming quality of Al6061 alloy manufactured via selective laser melting. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2024, 1, 238. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Debnath, D.; Pawar, M.D. Laser welding of additively manufactured AlSi10Mg and conventionally manufactured Al6061 alloy. Weld World 2024, 68, 1731. [Google Scholar] [CrossRef]
- O’Keeffe, C.; Kotlarz, M.; Gonalves, I.F. Chemical etching of Ti-6Al-4V biomaterials fabricated by selective laser melting enhances mesenchymal stromal cell mineralization. J. Biomed. Mater. Res. A 2024, 112, 9. [Google Scholar] [CrossRef]
- Jeong, J.U.; Lee, K.K.; Ahn, D.G. Study of surface roughness change of AlSi10Mg based on selective laser melting (SLM) using electro-polishing technique. J. Mech. Sci. Technol. 2024, 38, 9. [Google Scholar] [CrossRef]
- Arvieu, C.; Galy, C.; Le, G.E. Relative density of SLM-produced aluminum alloy parts: Interpretation of results. J. Manuf. Mater Process 2020, 4, 4030083. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Lu, J. Enhanced corrosion resistance and biofilm inhibition of functionally graded TC4/TC4-5Cu fabricated by selective laser melting against streptococcus mutans. Acta Metall. Sin. Engl. 2023, 36, 1961. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, M.; Li, J. Compressive behavior of hollow triply periodic minimal surface cellular structures manufactured by selective laser melting. Rapid Prototyp. J. 2023, 29, 569. [Google Scholar] [CrossRef]
- Li, Y.; Liao, H. Selective laser melting of novel SiC and TiC strengthen 7075 aluminum powders for anti-cracks application. J. Mater. Sci. Chem. Eng. 2024, 12, 136. [Google Scholar] [CrossRef]
- Tang, H.; Geng, Y.; Bian, S. An ultra-high strength over 700 MPa in Al-Mn-Mg-Sc-Zr alloy fabricated by selective laser melting. Acta Metall. Sin. 2022, 35, 466. [Google Scholar] [CrossRef]
- Wen, X.L.; Wang, C.B.; Liu, W.B.; Ren, H.W. Experimental study on the microstructure of FeCoNiCr high-entropy alloys by selective laser melting. J. N. Univ. 2023, 44, 536. [Google Scholar]
- Al, H.; Hiba, J.; Kassis, J.; Zaidani, W.B.T. The effect of repeated baking of porcelain on its bonding strength to a Co-Cr alloy 3D-printed by selective laser melting. Saudi Dental J. 2024, 36, 296. [Google Scholar]
- Wang, G.; Zhang, S.; Liu, T. The intrinsically quasi-isotropic tensile properties of as-fabricated Al-Mg-Si-Sc-Zr alloy produced by selective laser melting. J. Alloys Compd. 2023, 947, 169707. [Google Scholar] [CrossRef]
- Sathishkumar, A.; Soundararajan, R.; Sivasankaran, S. Effect of direct aging on the microstructure and mechanical behavior of AlSi10Mg alloy: Casting versus selective laser melting. J. Mater. Eng. Perform. 2023, 32, 3215. [Google Scholar] [CrossRef]
- Yan, X.; Tang, X. A novel method for early fatigue damage diagnosis in 316 L stainless steel formed by selective laser melting technology. Materials 2023, 16, 3363. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.; Xiang, Y.; Liu, P.; Ho, J.Y. Micro/nanostructuring of metal additively manufactured aluminum alloy for enhanced pool boiling of dielectric fluids. Int. J. Heat Mass Tran. 2024, 10, 221. [Google Scholar] [CrossRef]
- You, S.; Jiang, C.; Wang, L.; Zhan, K. Effect of CeO2 nanoparticles on the microstructure and properties of the NiCo-CeO2 composite coatings. Vacuum 2022, 196, 196. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, V.; Joshi, R.S. Investigation of rare earth particulate on tribological and mechanical properties of Al-6061 alloy composites for aerospace application. J. Mater. Res. Technol. 2019, 4, 3504. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, S.; Angra, S. Synergistic corrosion protection of stir-cast hybrid aluminum composites reinforcing CeO2 and GNPs nano-particulates. Aircr. Eng. Aerosp. Technol. 2023, 95, 1706–1715. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Udayashankar, N.K.; Nayak, J. Effect of heat treatment on pitting corrosion resistance of 6061 Al/SiCP composite coated by the cerium oxide film in 3.5 N NaCl solution. Surf. Eng. Appl. Electrochem. 2011, 47, 176. [Google Scholar] [CrossRef]
- Peng, G.; Niu, C.; Geng, Y.; Duan, W.; Cao, S. Multi-objective optimization for the forming quality of a CeO2/Al6061 alloy as an aluminum–air battery anode manufactured via selective laser melting. Crystals 2024, 14, 784. [Google Scholar] [CrossRef]
- Li, Y.; Duan, W. The effect of hatch spacing on the electrochemistry and discharge performance of a CeO2/Al6061 anode for an Al-air battery via selective laser melting. Crystals 2024, 14, 797. [Google Scholar] [CrossRef]
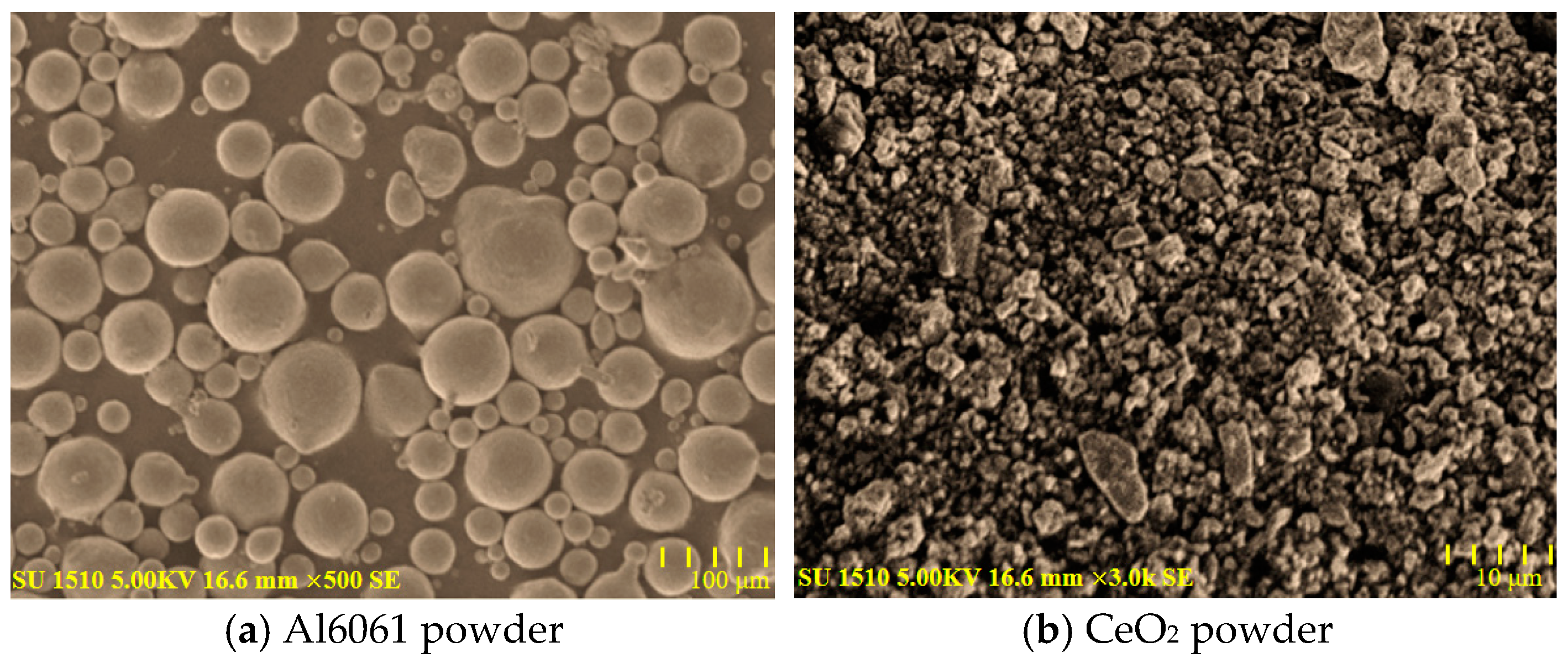




| Laser Power (W) | Scan Speed (mm/s) | Scan Spacing (mm) | Powder Thickness (mm) |
|---|---|---|---|
| 260, 280, 300, 320, 340 | 1000 | 0.13 | 0.03 |
| Laser Power | Electrochemical Parameters | ||
|---|---|---|---|
| E(v) | Icorr (A/cm2) | Rp (Ω∙cm2) | |
| 260 W | −1.615 | 2.345 × 10−2 | 1.8 |
| 280 W | −1.632 | 2.090 × 10−2 | 2.1 |
| 300 W | −1.626 | 2.258 × 10−2 | 2.0 |
| 320 W | −1.626 | 2.390 × 10−2 | 2.0 |
| 340 W | −1.624 | 2.567 × 10−2 | 1.7 |
| Laser Power | 260 W | 280 W | 300 W | 320 W | 340 W |
|---|---|---|---|---|---|
| L/Ω·cm2 | 8.204 × 10−7 | 7.827 × 10−20 | 5.625 × 10−7 | 7.900 × 10−7 | 7.352 × 10−5 |
| Rs/Ω·cm2 | 1.172 | 1.469 | 6.687 × 10−1 | 1.174 | 1.507 |
| CPE1/F·cm−2 | 2.835 × 10−4 | 3.540 × 10−4 | 5.190 × 10−6 | 2.119 × 10−4 | 1.716 × 10−4 |
| R1/Ω·cm2 | 5.021 × 10−1 | 8.048 × 10−1 | 7.892 × 10−1 | 6.472 × 10−1 | 4.752 × 10−1 |
| CPE2/F·cm−2 | 9.729 × 10−1 | 2.867 | 2.729 × 10−3 | 4.978 × 10−2 | 2.613 × 10−4 |
| R2/Ω·cm2 | 2.264 × 10−2 | 1.939 × 10−1 | 4.829 × 10−1 | 2.035 × 10−1 | 2.641 × 10−1 |
| χ2 | 4.818 × 10−4 | 6.143 × 10−4 | 3.890 × 10−4 | 5.298 × 10−4 | 5.902 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Han, J. Impact of Laser Power on Electrochemical Performance of CeO2/Al6061 Alloy Through Selective Laser Melting (SLM). Crystals 2025, 15, 84. https://doi.org/10.3390/cryst15010084
Sun F, Han J. Impact of Laser Power on Electrochemical Performance of CeO2/Al6061 Alloy Through Selective Laser Melting (SLM). Crystals. 2025; 15(1):84. https://doi.org/10.3390/cryst15010084
Chicago/Turabian StyleSun, Fengyong, and Jitai Han. 2025. "Impact of Laser Power on Electrochemical Performance of CeO2/Al6061 Alloy Through Selective Laser Melting (SLM)" Crystals 15, no. 1: 84. https://doi.org/10.3390/cryst15010084
APA StyleSun, F., & Han, J. (2025). Impact of Laser Power on Electrochemical Performance of CeO2/Al6061 Alloy Through Selective Laser Melting (SLM). Crystals, 15(1), 84. https://doi.org/10.3390/cryst15010084





